Ceramic Identity Contributes to Mechanical Properties and Osteoblast Behavior on Macroporous Composite Scaffolds
Abstract
:1. Introduction
2. Experimental Section
2.1. Scaffold Preparation
2.2. Scaffold Characterization
2.3. Cell Culture
2.4. Osteogenic Potential
2.5. Statistical Analysis
3. Results and Discussion
3.1. Scaffold Characterization
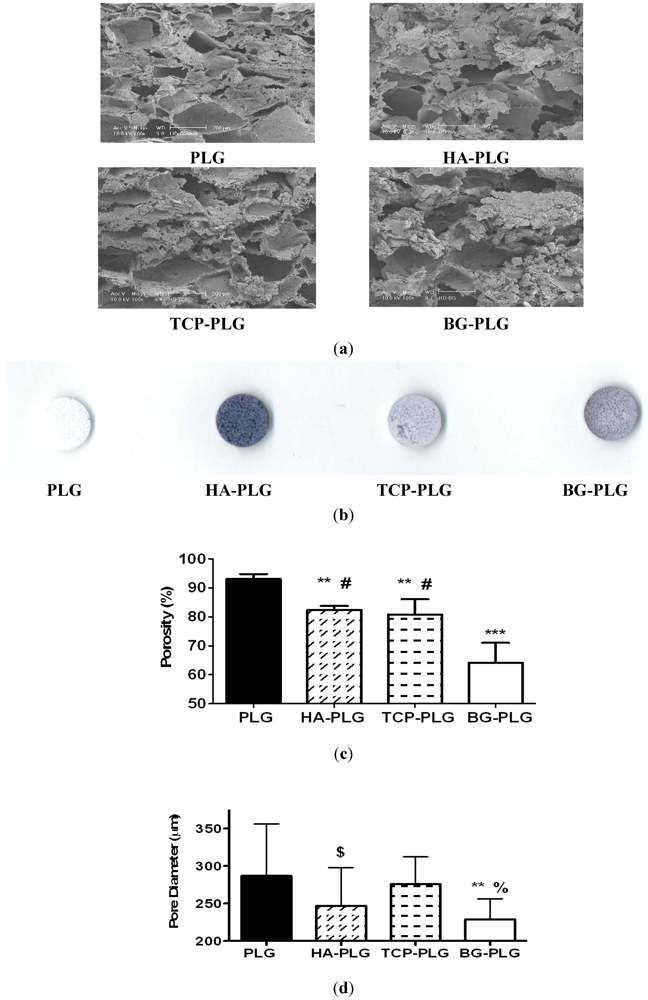
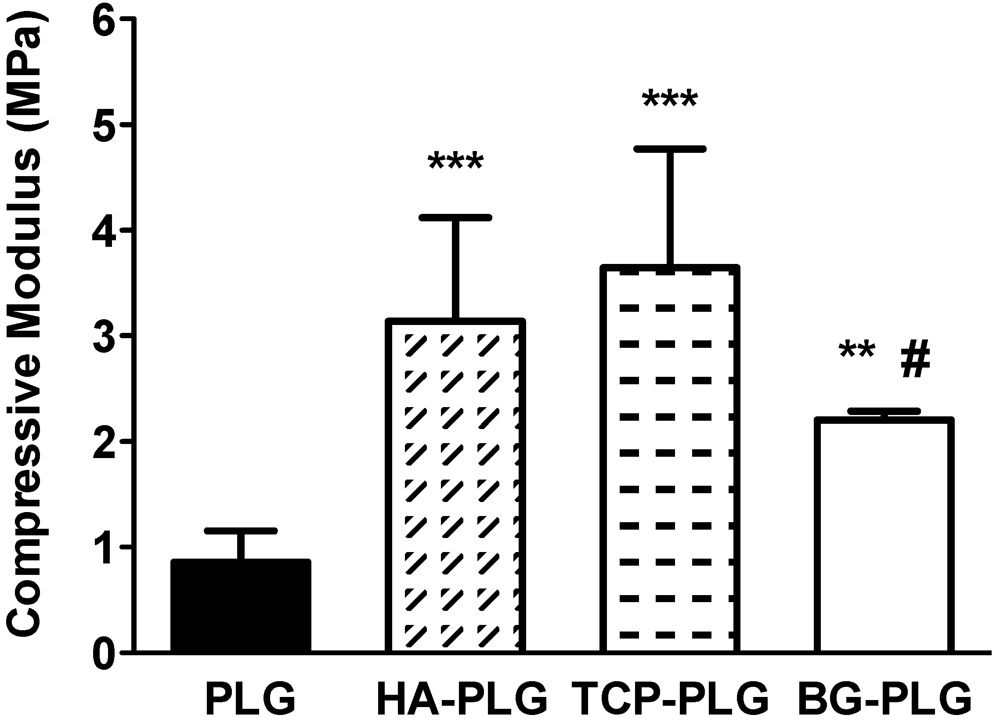
3.2. Osteoconductive Potential of Composite Scaffolds
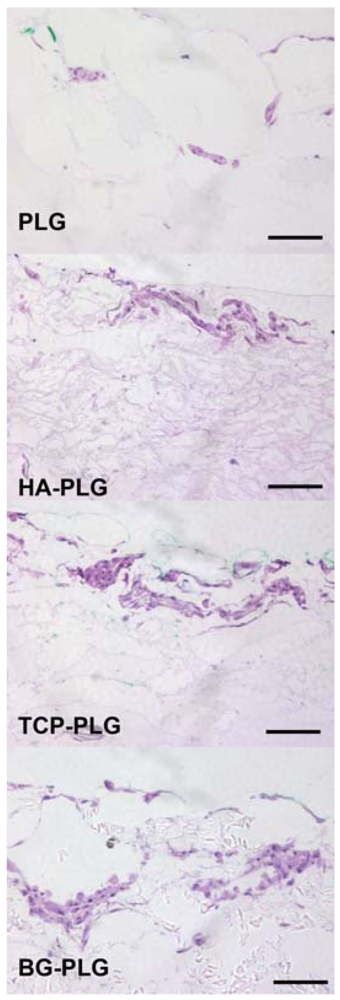
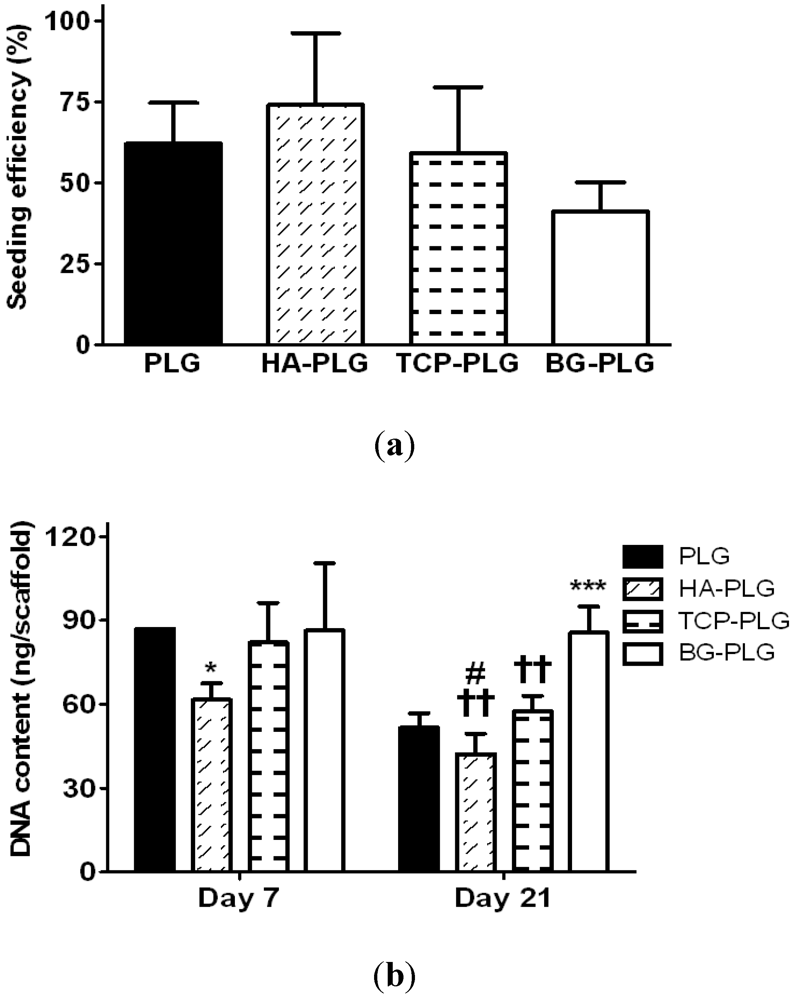
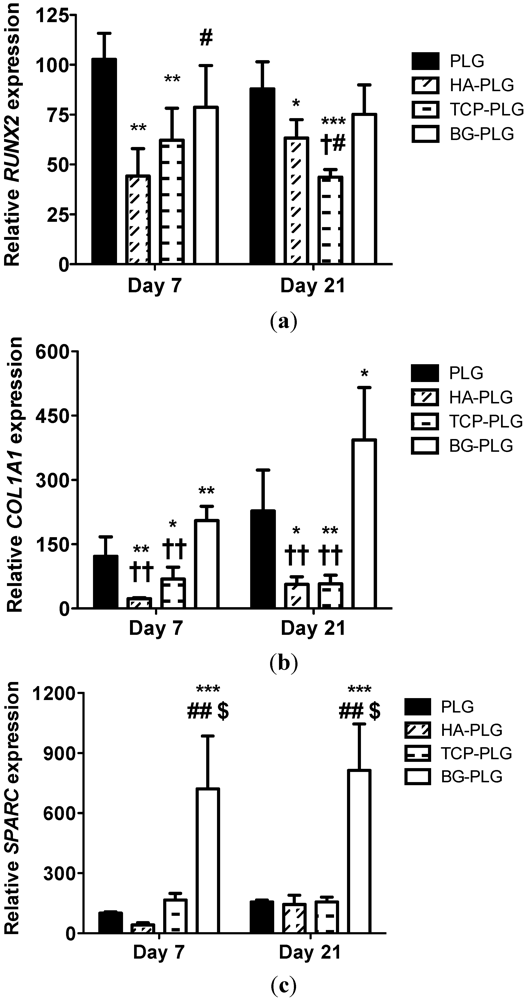
3.3. Osteogenic Response of NHOsts

4. Conclusions
Acknowledgments
References
- Moroni, A.; Larsson, S.; Hoang Kim, A.; Gelsomini, L.; Giannoudis, P.V. Can we improve fixation and outcomes? Use of bone substitutes. J. Orthop. Trauma 2009, 23, 422–425. [Google Scholar]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef]
- Loo, S.C.; Moore, T.; Banik, B.; Alexis, F. Biomedical applications of hydroxyapatitenanoparticles. Curr.Pharm. Biotechnol. 2010, 11, 333–342. [Google Scholar]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Keating, J.F.; McQueen, M.M. Substitutes for autologous bone graft in orthopaedic trauma. J. Bone Joint Surg. Br. 2001, 83, 3–8. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirose, M.; Kotobuki, N.; Tadokoro, M.; Ohgushi, H.; Fukuchi, T.; Sato, J.; Seto, K. Bone augmentation by bone marrow mesenchymal stem cells cultured in three-dimensional biodegradable polymer scaffolds. J. Biomed. Mater. Res. A 2009, 91, 428–435. [Google Scholar]
- Frohlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: Biological requirements, tissue culture and clinical relevance. Curr.Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef]
- Ge, Z.; Tian, X.; Heng, B.C.; Fan, V.; Yeo, J.F.; Cao, T. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed. Mater. 2009, 4, 021001:1–021001:7. [Google Scholar]
- Davis, H.E.; Rao, R.R.; He, J.; Leach, J.K. Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. J. Biomed. Mater. Res. A 2009, 90, 1021–1031. [Google Scholar]
- Lu, H.H.; El-Amin, S.F.; Scott, K.D.; Laurencin, C.T. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2003, 64, 465–474. [Google Scholar]
- Kim, S.S.; Ahn, K.M.; Park, M.S.; Lee, J.H.; Choi, C.Y.; Kim, B.S. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J. Biomed. Mater. Res. A 2007, 80A, 206–215. [Google Scholar] [CrossRef]
- Daculsi, G.; Goyenvalle, E.; Cognet, R.; Aguado, E.; Suokas, E.O. Osteoconductive properties of poly(96L/4D-lactide)/beta-tricalcium phosphate in long term animal model. Biomaterials 2011, 32, 3166–3177. [Google Scholar] [Green Version]
- Yao, J.; Radin, S.; Leboy, P.S.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar]
- He, J.; Decaris, M.L.; Leach, J.K. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng. 2012, in press. [Google Scholar]
- He, J.; Genetos, D.C.; Leach, J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng. Part A 2010, 16, 127–137. [Google Scholar]
- Day, R.M.; Maquet, V.; Boccaccini, A.R.; Jerome, R.; Forbes, A. In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J. Biomed. Mater. Res. A 2005, 75, 778–787. [Google Scholar]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar]
- Kim, S.S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Davis, H.E.; Case, E.M.; Miller, S.L.; Genetos, D.C.; Leach, J.K. Osteogenic response to BMP-2 of hMSCs grown on apatite-coated scaffolds. Biotechnol. Bioeng. 2011, 108, 2727–2735. [Google Scholar]
- Leu, A.; Leach, J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm. Res. 2008, 25, 1222–1229. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar]
- Ge, C.; Xiao, G.; Jiang, D.; Yang, Q.; Hatch, N.E.; Roca, H.; Franceschi, R.T. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J. Biol. Chem. 2009, 284, 32533–32543. [Google Scholar]
- Drissi, H.; Luc, Q.; Shakoori, R.; Chuva De Sousa Lopes, S.; Choi, J.Y.; Terry, A.; Hu, M.; Jones, S.; Neil, J.C.; Lian, J.B.; et al. Transcriptional autoregulation of the bone related CBFA1/RUNX2 gene. J. Cell. Physiol. 2000, 184, 341–350. [Google Scholar]
- Charles, P.; Mosekilde, L.; Risteli, L.; Risteli, J.; Eriksen, E.F. Assessment of bone remodeling using biochemical indicators of type I collagen synthesis and degradation: Relation to calcium kinetics. Bone Miner 1994, 24, 81–94. [Google Scholar] [CrossRef]
- Termine, J.D.; Kleinman, H.K.; Whitson, S.W.; Conn, K.M.; McGarvey, M.L.; Martin, G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981, 26, 99–105. [Google Scholar]
- Carter, D.R.; Hayes, W.C. Bone compressive strength: The influence of density and strain rate. Science 1976, 194, 1174–1176. [Google Scholar]
- Sicchieri, L.G.; Crippa, G.E.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Pore size regulates cell and tissue interactions with PLGA-CaP scaffolds used for bone engineering. J. Tissue Eng. Regen. Med. 2012, 6, 155–162. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar]
- Hoppe, A.; Guldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar]
- Scaglione, S.; Lazzarini, E.; Ilengo, C.; Quarto, R. A composite material model for improved bone formation. J. Tissue Eng. Regen. Med. 2010, 4, 505–513. [Google Scholar]
- Smith, L.L.; Niziolek, P.J.; Haberstroh, K.M.; Nauman, E.A.; Webster, T.J. Decreased fibroblast and increased osteoblast adhesion on nanostructuredNaOH-etched PLGA scaffolds. Int. J. Nanomedicine 2007, 2, 383–388. [Google Scholar]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar]
- Leu, A.; Stieger, S.M.; Dayton, P.; Ferrara, K.W.; Leach, J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng. Part A 2009, 15, 877–885. [Google Scholar]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Lopes, M.A.; Monteiro, F.J.; Santos, J.D.; Serro, A.P.; Saramago, B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 1999, 45, 370–375. [Google Scholar]
- Anderson, S.I.; Downes, S.; Perry, C.C.; Caballero, A.M. Evaluation of the osteoblast response to a silica gel in vitro. J. Mater. Sci. Mater. Med. 1998, 9, 731–735. [Google Scholar] [CrossRef]
- Bosetti, M.; Zanardi, L.; Hench, L.; Cannas, M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J. Biomed. Mater. Res. A 2003, 64, 189–195. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Morales-Hernandez, D.G.; Genetos, D.C.; Working, D.M.; Murphy, K.C.; Leach, J.K. Ceramic Identity Contributes to Mechanical Properties and Osteoblast Behavior on Macroporous Composite Scaffolds. J. Funct. Biomater. 2012, 3, 382-397. https://doi.org/10.3390/jfb3020382
Morales-Hernandez DG, Genetos DC, Working DM, Murphy KC, Leach JK. Ceramic Identity Contributes to Mechanical Properties and Osteoblast Behavior on Macroporous Composite Scaffolds. Journal of Functional Biomaterials. 2012; 3(2):382-397. https://doi.org/10.3390/jfb3020382
Chicago/Turabian StyleMorales-Hernandez, Diana G., Damian C. Genetos, David M. Working, Kaitlin C. Murphy, and J. Kent Leach. 2012. "Ceramic Identity Contributes to Mechanical Properties and Osteoblast Behavior on Macroporous Composite Scaffolds" Journal of Functional Biomaterials 3, no. 2: 382-397. https://doi.org/10.3390/jfb3020382




