Thrombin Production and Human Neutrophil Elastase Sequestration by Modified Cellulosic Dressings and Their Electrokinetic Analysis
Abstract
:1. Introduction
1.1. Hemostasis
1.2. The Relation of Material Surface Charge to Hemostatic Activity
1.3. Hemorrhage Control Dressings
1.4. Inflammation and Chronic Wounds
1.5. Negatively Charged Materials for Chronic Wounds
1.6. Biocompatibility of Cellulosics and other Materials and the Role of Charge
1.7. Modified Cotton Dressings for Hemostasis and Chronic Wounds
1.8. Electrokinetic Assessment of Material Surface Charge
2. Materials and Methods
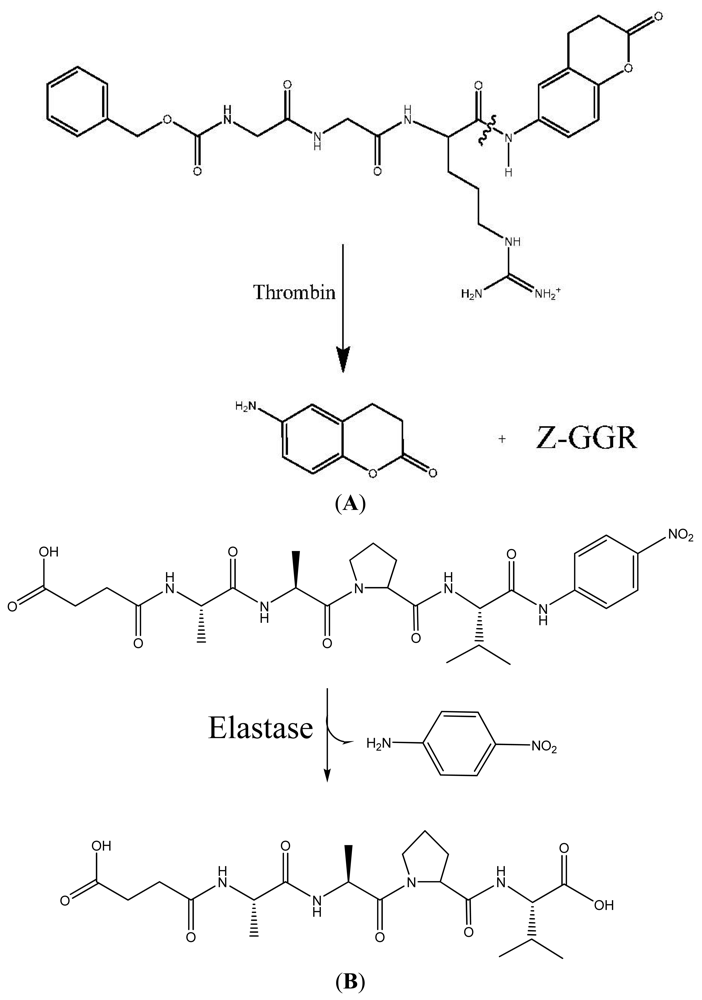
2.1. Thrombin Assay
2.2. Streaming Zeta Potential
2.3. Elastase Assay
3. Results and Discussion
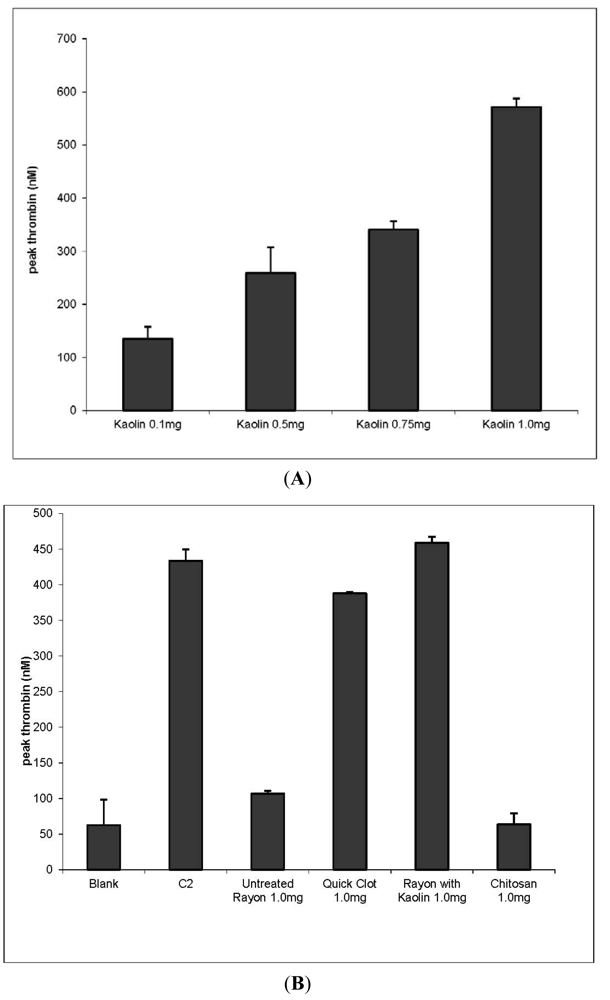
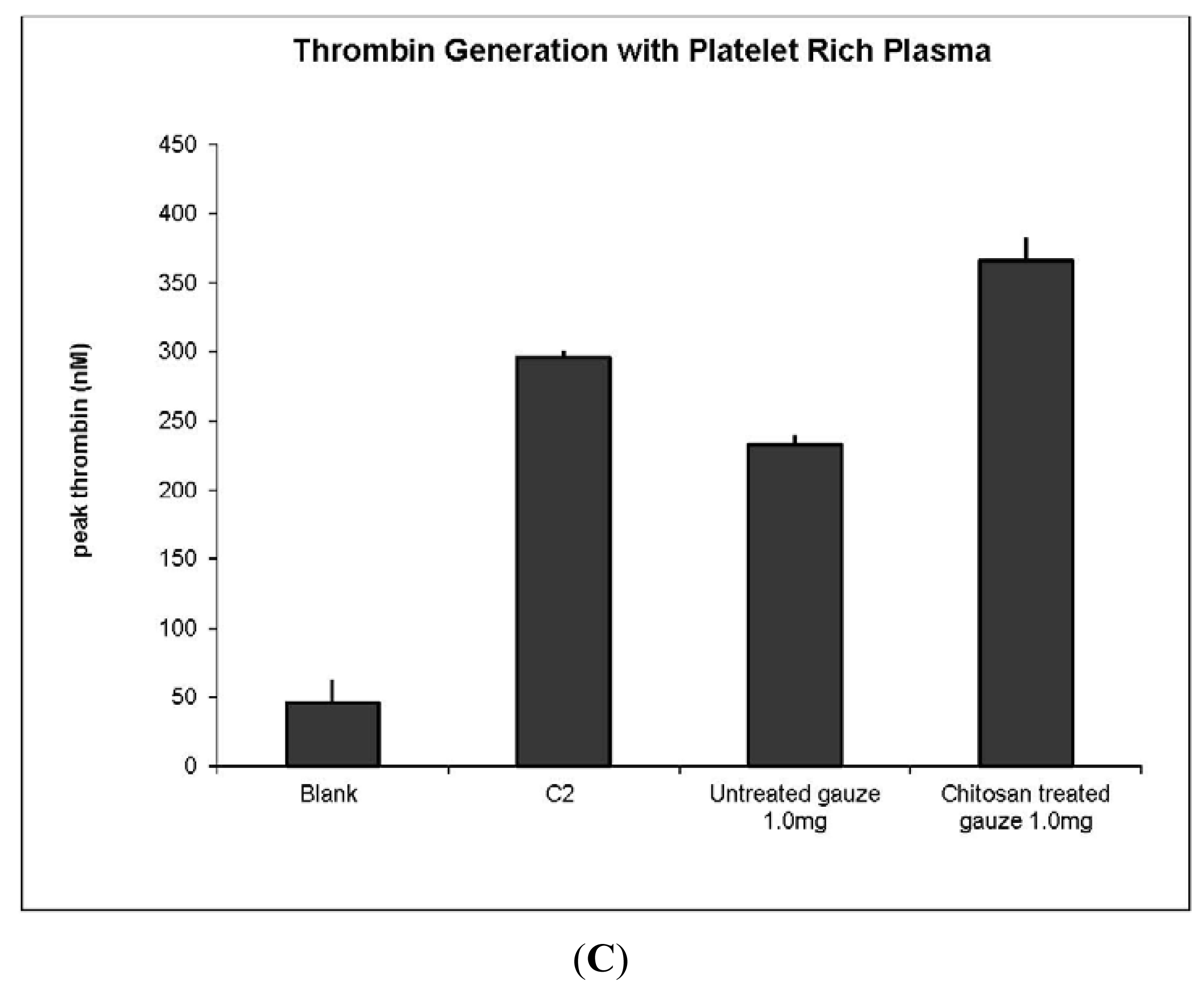
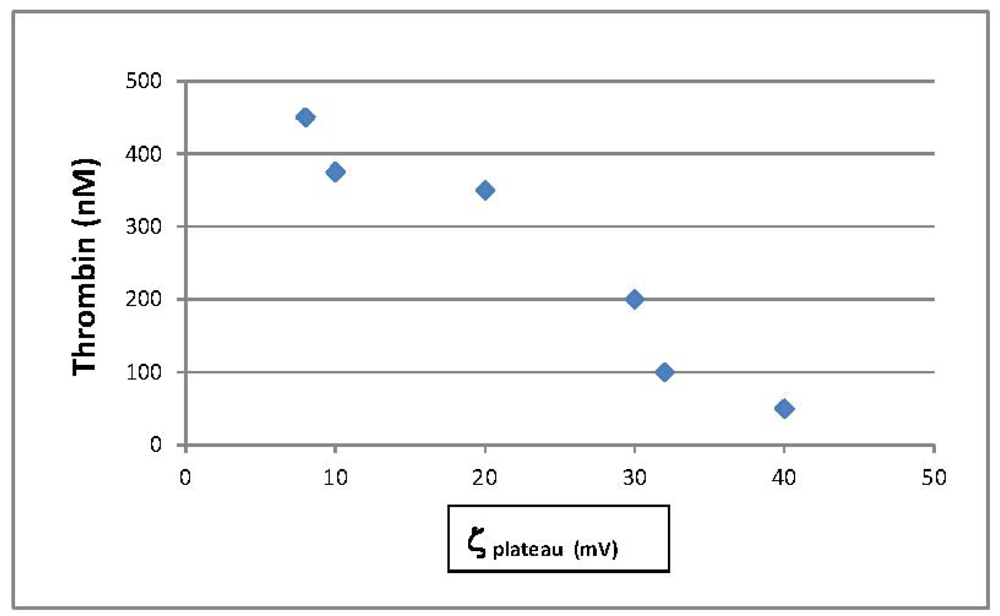
3.1. Thrombin Production to Assess Hemostatic Materials
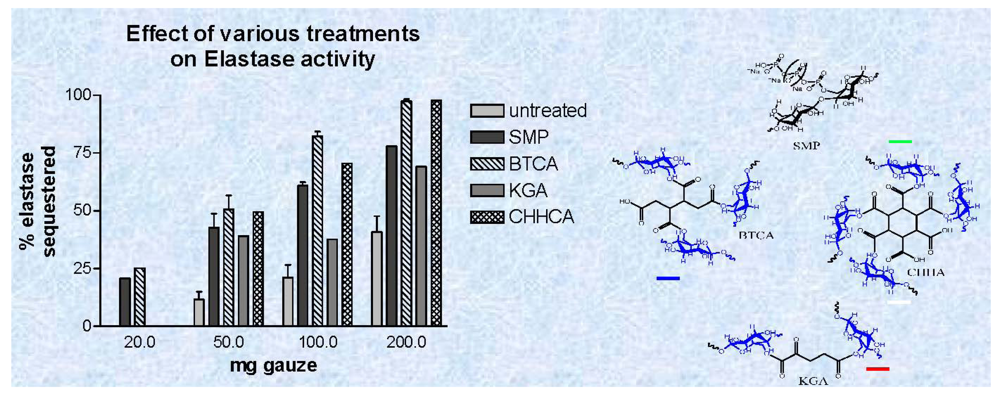
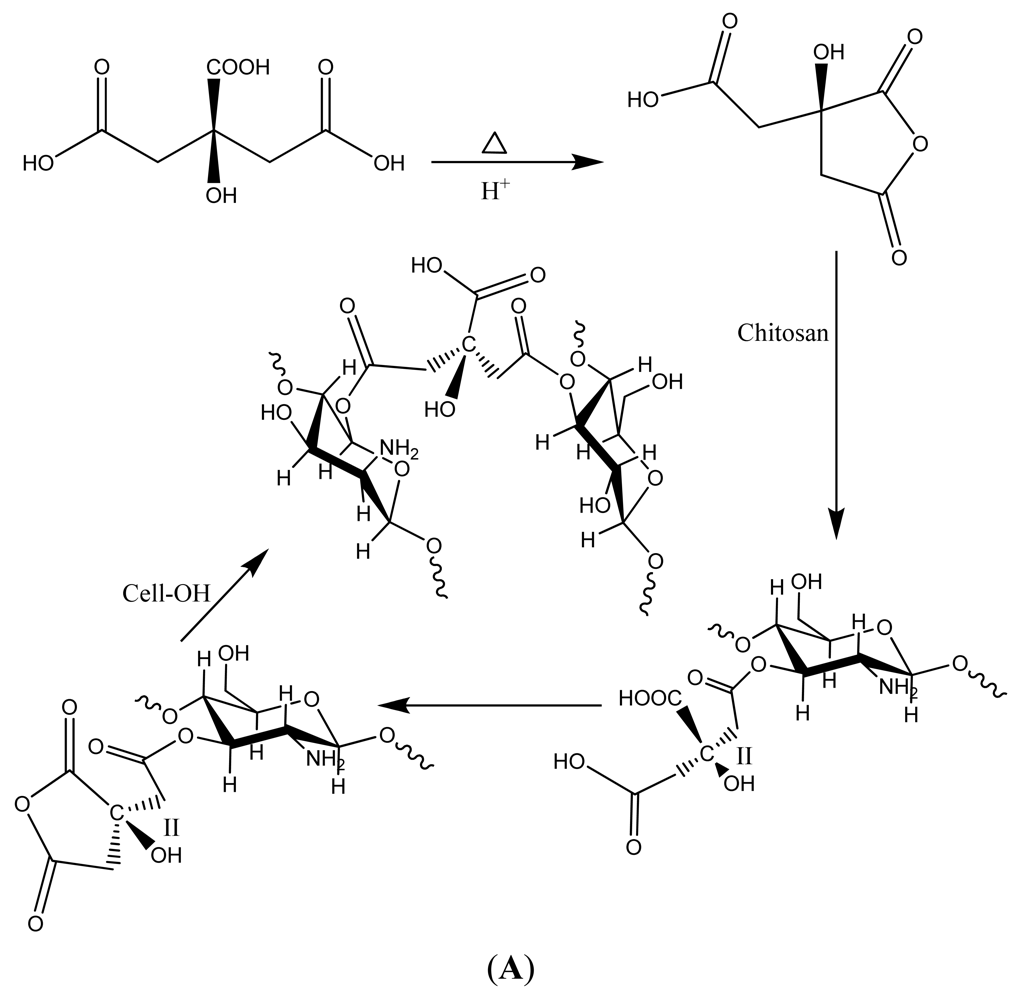
3.2. Elastase Sequestration by Negatively Charged Cotton Dressings
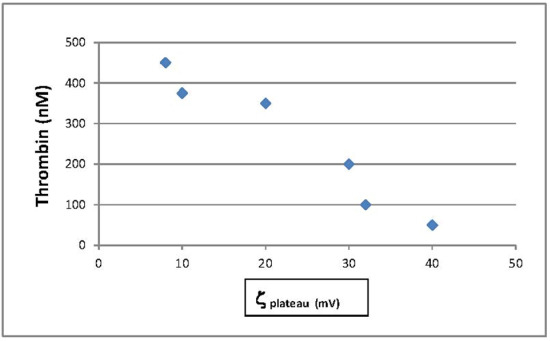
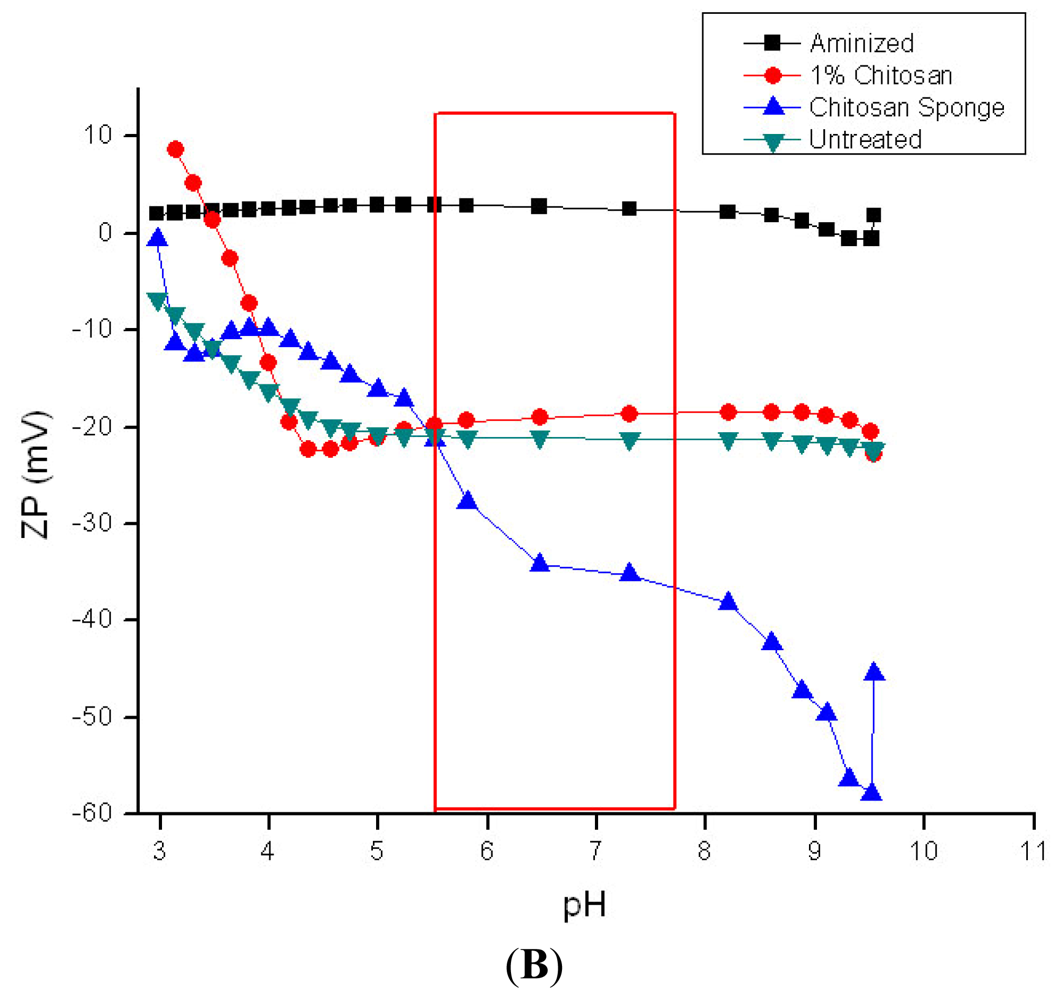
3.4. Zeta Potential Measurements on Hemostatic and Chronic Wound Dressings
 QuickClot Combat Gauze (4A);
QuickClot Combat Gauze (4A);
 Rayon/polyester, 50/50 gauze; (B) ■ aminized cotton gauze;
Rayon/polyester, 50/50 gauze; (B) ■ aminized cotton gauze;
 chitosan-citric acid grafted cotton gauze;
chitosan-citric acid grafted cotton gauze;
 chitosan-lactic acid composite sponge;
chitosan-lactic acid composite sponge;
 untreated cotton gauze (4B).
untreated cotton gauze (4B).
 QuickClot Combat Gauze (4A);
QuickClot Combat Gauze (4A);
 Rayon/polyester, 50/50 gauze; (B) ■ aminized cotton gauze;
Rayon/polyester, 50/50 gauze; (B) ■ aminized cotton gauze;
 chitosan-citric acid grafted cotton gauze;
chitosan-citric acid grafted cotton gauze;
 chitosan-lactic acid composite sponge;
chitosan-lactic acid composite sponge;
 untreated cotton gauze (4B).
untreated cotton gauze (4B).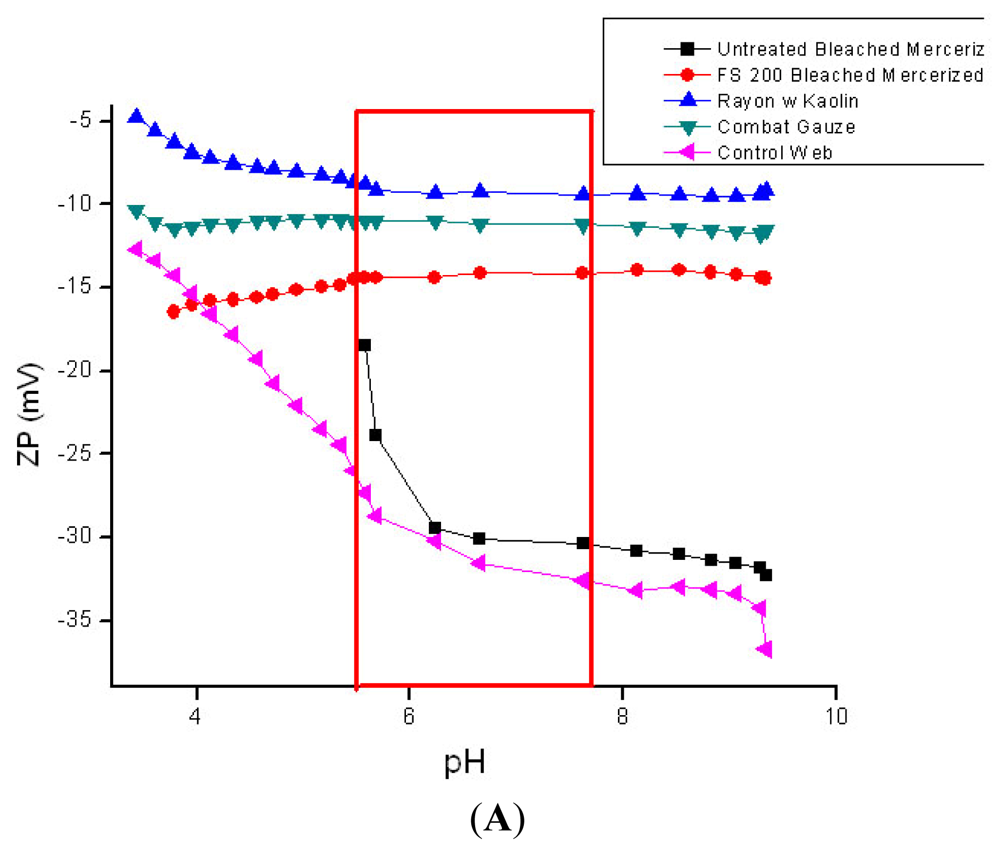
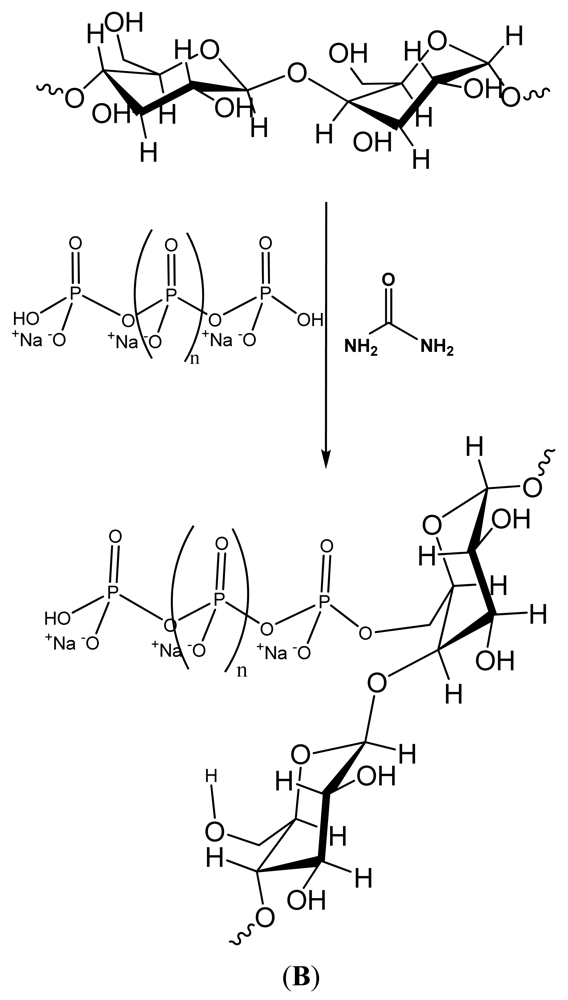
 and
and
 Phosphoryoated cotton gauze;
Phosphoryoated cotton gauze;
 1,2,3,4-butanetetracarboxylic acid (BTCA),
1,2,3,4-butanetetracarboxylic acid (BTCA),
 2-ketoglutaric acid (KGA;
2-ketoglutaric acid (KGA;
 1,2,3,4,5,6,-cyclohexanehexacarboxylic acid.
1,2,3,4,5,6,-cyclohexanehexacarboxylic acid.
 and
and
 Phosphoryoated cotton gauze;
Phosphoryoated cotton gauze;
 1,2,3,4-butanetetracarboxylic acid (BTCA),
1,2,3,4-butanetetracarboxylic acid (BTCA),
 2-ketoglutaric acid (KGA;
2-ketoglutaric acid (KGA;
 1,2,3,4,5,6,-cyclohexanehexacarboxylic acid.
1,2,3,4,5,6,-cyclohexanehexacarboxylic acid.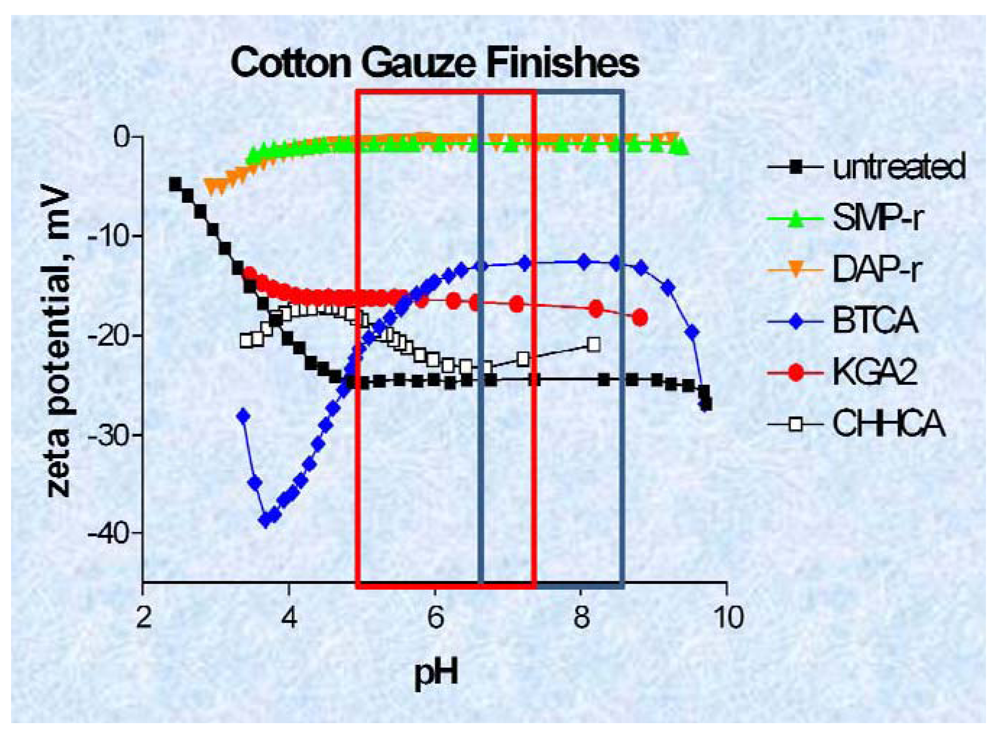
4. Summary
References
- Clark, R.A.F. Wound repair: Overview and general considerations. In The Molecular and Cellular Biology of Wound Repair; Richard, A.F., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 3–35. [Google Scholar]
- Davie, E.W. A brief historical review of the waterfall/cascade of blood coagulation. J. Biol. Chem. 2003, 278, 50819–50832. [Google Scholar]
- Tanaka, K.A.; Key, N.S.; Levy, J.H. Blood coagulation: Hemostasis and thrombin regulation. Anesth. Analg. 2009, 108, 1433–1446. [Google Scholar]
- Lu, G.; Broze, G.J.; Krishnaswamy, S. Formation of factors IX and Xa by the extrinsic pathway: Differential regulation by tissue factor pathway inhibitor and antithrombin III. J. Biol. Chem. 2004, 279, 17241–17249. [Google Scholar]
- Peerschke, E.I.; Zucker, M.B.; Grant, R.A.; Egan, J.J.; Johnson, M.M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood 1980, 55, 841–847. [Google Scholar]
- Hornyak, T.J.; Shafer, J.A. Interactions of factor XIII with fibrin as substrate and cofactor. In Biochemistry; 1992; Volume 31, pp. 423–429. [Google Scholar]
- White, G.C. The partial thromboplastin time: Defining an era in coagulation. J. Thromb. Haemost. 2003, 1, 2267–2270. [Google Scholar]
- Margolis, J. Initiation of blood coagulation by glass and related surfaces. J. Physiol. 1957, 137, 95–109. [Google Scholar]
- Ratnoff, O.D.; Rosenblum, J.M. The role of Hageman factor in the initiation of clotting by glass. J. Lab. Clin. Med. 1957, 50, 941–942. [Google Scholar]
- Smith, S.A.; Morrissey, J.H. Polyphosphate enhances fibrin clot structure. Blood 2008, 112, 2810–2816. [Google Scholar]
- Bjorn, S.; Thim, L. Activation of coagulation factor VII to VIIa. Res. Disclosure 1986, 269, 564–565. [Google Scholar]
- Pedersen, A.H.; Lundhansen, T.; bisgaardfrantzen, H.; Olsen, F.; Petersen, L.C. Autoactivation of human recombinant coagulation factor VII. Biochemistry 1989, 28, 9331–9336. [Google Scholar]
- Marcum, J.A.; Rosenbuerg, R.D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry 1984, 23, 1730–1737. [Google Scholar]
- Colman, R.W. Hemostatic complications of cardiopulmonary bypass. Am. J. Hematol. 1995, 48, 267–272. [Google Scholar]
- Blezer, R.; Fouache, B.; Willems, G.M.; Lindhout, T. Activation of blood coagulation at heparin-coated surfaces. J. Biomed Mater. Res. 1997, 37, 108–113. [Google Scholar]
- Lavaud, S.; Paris, B.; Maheut, H.; Randoux, C.; Renaux, J.-L.; Rieu, P.; Chanard, J. Assessment of the heparin-binding AN69 ST hemodialysis membrane: II. Clnical studies without heparin administration. ASAIO 2005, 51, 348–351. [Google Scholar]
- Naito, K.; Fujikawa, K. Activation of human blood coagulation factor XI independent of factor XII. J. Biol. Chem. 1991, 266, 7353–7356. [Google Scholar]
- Harding, I.S.; Rashid, N.; Hing, K.A. Surface charge and the effect of excess calcium ions on the hydroxyapatite surface. Biomaterials 2005, 26, 6818–6826. [Google Scholar]
- Rodenas, L.G.; Palacios, J.M.; Apella, M.C.; Morando, P.J.; Blesa, M.A. Surface properties of various powdered hydroxyapatites. J. Colloid Interface Sci. 2005, 290, 145–154. [Google Scholar]
- Kikuchi, L.; Park, J.Y.; Victor, C.; Davies, J.E. Platelet interactions with calcium-phosphate-coated surfaces. Biomaterials 2005, 26, 5285–5295. [Google Scholar]
- Ostomel, T.A.; Shi, Q.; Stoimenov, P.K.; Stucky, G.D. Metal oxide surface charge mediated hemostasis. Langmuir 2007, 23, 11233–11238. [Google Scholar]
- Ostomel, T.A.; Shi, Q.; Stucky, G.D. Oxide hemostatic activity. J. Am. Chem. Soc. 2006, 128, 8384–8385. [Google Scholar]
- Pusateri, A.E.; Holcomb, J.B.; Kheirabadi, B.S.; Alam, H.B.; Wade, C.E.; Ryan, K.L. Making sense of the preclinical literature on advanced hemostatic products. J. Trauma 2006, 60, 674–682. [Google Scholar]
- Acheson, E.M.; Kheirabadi, B.S.; Deguzman, R.; Dick, E.J., Jr.; Holcomb, J.B. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J. Trauma 2005, 59, 865–874, discussion 874-875. [Google Scholar]
- Ward, K.R.; Tiba, M.H.; Holbert, W.H.; Blocher, C.R.; Draucher, G.T.; Proffitt, E.K.; Bowlin, G.L.; Ivatury, R.R.; Diegelmann, R.F. Comparison of a new hemostatic agent to current combat hemostatic agents in a swine modelof lethal extremity arterial hemorrhage. J. Trauma 2007, 63, 276–283. [Google Scholar]
- QuickClot Product History; Z-Medica Corporation: Wallingford, CT, USA, 2008. Available online: http://www.z-medica.com/healthcare/About-Us/QuikClot-Product-History.aspx (accessed on 26 September 2011).
- Thomas, A.; Harding, K.G.; Moore, K. Alginates from wound dressings activate human macrophages to secrete tumor necrosis factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar]
- Christoforou, C.; Lin, X.; Bennett, S.; Connors, D.; Skalla, W.; Mustoe, T.; Linehan, J.; Arnold, F.; Guskin, E. Biodegradable positively charged ion exchange beads: A novel biomaterial for enhancing soft tissue repair. J. Biomed. Mater. Res. 1998, 42, 376–386. [Google Scholar]
- Connors, D.; Gies, D.; Lin, H.; Gruskin, E.; Mustoe, T.A.; Tawil, N.J. Increase in wound breaking strength in rats in the presence of positively charged dextran beads correlates with an increase in endogenous transforming growth factor-β 1 and its receptor TGF-βR1 in close proximity to the wound. Wound Rep. Reg. 2000, 8, 292–303. [Google Scholar]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med. 1999, 92, 283–285. [Google Scholar]
- Tonks, A.J.; Cooper, R.A.; Jones, K.P.; Blair, S.; Parton, J.; Tonks, A. Honey stimulated inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar]
- Byeon, S.W.; Pelley, R.P.; Ullrich, S.E.; Waller, T.A.; Bucana, C.D.; Strickland, F.M. Aloe barbadensis extracts reduce the production of interleukin-10 after exposure to ultraviolet radiation. J. Investig. Dermatol. 1998, 110, 811–817. [Google Scholar]
- Yager, D.; Nwomeh, B. The proteolytic environment of chronic wounds. Wound Reg. Rep. 1999, 7, 433–441. [Google Scholar]
- Cullen, B.; Smith, R.; McCulloch, E.; Silcock, D.; Morrison, L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Reg. Rep. 2002, 10, 16–25. [Google Scholar]
- Edwards, J.V.; Yager, D.R.; Cohen, I.K.; Diegelmann, R.F.; Montante, S.; Bertoniere, N.; Bopp, A.F. Modified cotton gauze dressings that selectively absorb neutrophil elastase activity in solution. Wound Rep. Reg. 2001, 9, 50–58. [Google Scholar]
- Edwards, J.V.; Batiste, S.L.; Gibbins, E.M.; Goheen, S.C. Synthesis and activity of NH2- and COOH-terminal elastase recognition sequences on cotton. J. Peptide Res. 1999, 54, 536–543. [Google Scholar]
- Moseley, R.; Leaver, M.; Walker, M.; Waddington, R.J.; Parsons, D.; Chen, W.Y.I.; Embery, G. Comparison of the antioxidant properties of HYAFF-11p75, AQUACEL and hyaluronan towards reactive oxygen species in vitro. Biomaterials 2002, 23, 2255–2264. [Google Scholar]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar]
- Edwards, J.V.; Howley, P.S. Human neutrophil elastase and collagenase sequestration with phosphorylated cotton wound dressings. J. Biomed. Mater. Res. Part A 2007, 83A, 446–454. [Google Scholar]
- Edwards, J.V.; Howley, P.; Davis, R.; Mashchak, A.; Goheen, S.C. Protease inhibition by oleic acid transfer from chronic wound dressing to albumin. Int. J. Pharm. 2007, 340, 42–51. [Google Scholar]
- Vachon, D.J.; Yager, D.R. Novel sulfonated hydrogel composite with the ability to inhibit proteases and bacterial growth. J. Biomed. Mater. Res. 2006, 76A, 35–43. [Google Scholar]
- Meddahi, A.; Hassan, L.; Caruelle, J.-P.; Barritault, D.; Hornbeck, W. FGF protection and inhibition of human neutrophil elastase by carboxymethylbenzylamide sulfonate dextran derivatives. Int. J. Biol. Macromol. 1996, 18, 141–145. [Google Scholar]
- Andrade, J.D. Interfacial phenomena and biomaterials. Med. Instrum. 1973, 7, 110–120. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Hanosn, S.R.; Harker, L.A.; Whiffen, J.D. Blood-compatibilty-water-content relationships for radiation-grafted hydrogels. J. Polym. Sci. Symp. 1979, 66, 363–375. [Google Scholar]
- Raepple, E.; Hill, H.U.; Loos, M. Model of interaction of different polyanions with the first (C1,C1), the second (C2) and the fourth (C4) component of complement-1. Effect of fluid phase C1 and on C1 bond to EA or EAC4. Immunochemistry 1976, 13, 251–255. [Google Scholar]
- Ferruti, P.; Knobloch, S.; Ranucci, E.; Gianasi, E.; Duncan, R. A novel chemical modification of poly-L-lysine reducing toxicity while preserving cationic properties. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1997, 24, 45–46. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar]
- Puelo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar]
- Brodbeck, W.G.; Shive, M.S.; Colton, E.; Makayama, Y.; Matsuda, T.; Anderson, J.M. Influence of biomaterials surface chemistry on the apoptosis of adherent cells. J. Biomed. Mater. Res. 2001, 55, 661–668. [Google Scholar]
- Chapman, R.C.; Ostuni, E.; Yan, L.; Whitesides, G.M. Preparation of mixed self assembled monolayers (SAMS) that resist adsorption of proteins using the reaction of amines with SAM that present interchain carboxylic anhydride groups. Langmuir 2000, 16, 6927–6936. [Google Scholar]
- Kiuchi, L.; Park, J.Y.; Victor, C.; Davies, J.E. Platelet interactions with calcium-phosphate surfaces. Biomaterials 2005, 26, 5285–5295. [Google Scholar]
- Baltazat-y-Jimenez, A.; Bismarck, A. Wetting behavior, moisture up-take and electrokinetic properties of lignocellulosic fibres. Cellulose 2007, 14, 115–127. [Google Scholar]
- Stana-Kleinschek, K.S.; Strnad, S.; Ribitsch, V. Surface characterization and adsorption abilities of cellulose fibers. Polym. Eng. Sci. 1999, 39, 1412–1424. [Google Scholar]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and Nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar]
- Edwards, J.V. Future structure and properties of mechanism-based wound dressings. In Modified Fibers with Medical and Specialty Applications; Edwards, J.V., Buschle-Diller, G., Goheen, S.C., Eds.; Springer: Nieuwerkerk, The Netherlands, 2006; pp. 11–35. [Google Scholar]
- Clark, W.R.; Rocha, E.; Ronco, C. Solute removal by hollow-fiber dialyzers. In Hemodiafiltration. (contributions to Nephrology 158); Ronco, C., Canaud, B., Aljama, P., Eds.; Karger: Basel, Switzerland, 2007; pp. 20–33. [Google Scholar]
- Yue, Z.; Wen, F.; Gao, S.; Ang, M.Y.; Pallathadka, P.K.; Liu, L.; Yu, H. Preparation of three-dimensional interconnected macroporous cellulosic hydrogels for soft tissue engineering. Biomaterials 2010, 31, 8141–8152. [Google Scholar]
- Wiegand, C.; Elsner, P.; Hipler, U.-C.; Klemm, D. Protease and ROS activities influenced by a composite of bacterial cellulose and collagen type I in vitro. 2006, 13, 689–696. [Google Scholar]
- Guillaume, C.; Senet, P.; Vaneau, M.; Martel, P.; Guillqume, J.-C.; Meaume, S.; Teot, L.; Debure, D.; Dompmartin, A.; Bachelet, H.; et al. Dressings for acute and chronic wounds. Arch. Dermatol. 2007, 143, 1297–1304. [Google Scholar]
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rousselle, M.-A.; Goynes, W.R.; Edwards, J.V.; Hunter, L.; McAlister, D.D.; et al. Chemical Composition of Cotton in, Cotton Fiber Chemistry and Technology; Lewin, M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–21. [Google Scholar]
- Thomas, S. Wound Management and Dressings 1990, 1–197.
- Rothwell, S.W.; Reid, T.J.; Dorsey, J.; Flournoy, W.S.; Bodo, M.; Jammey, P.A.; Sawyer, E. A salmon thrombin-fibrin bandage controls arterial bleeding in a swine aortotomy model. J. Trauma 2005, 59, 143–149. [Google Scholar]
- Edwards, J.V.; Howley, P.; Prevost, N.; Condon, B.; Arnold, J.; Diegelmann, R. Positively and negatively charged ionic modifications to cellulose assessed as cotton-based protease-lowering and hemostatic wound agents. Cellulose 2009, 16, 911–921. [Google Scholar]
- Stana-Kleinschek, K.; Ribitsch, V. Electrokinetic properties of processed cellulose fibers. Colloids Surf. A 1998, 140, 127–138. [Google Scholar]
- Pothan, L.A.; Bellman, Kailas, L.; Thomas, S. Influence of chemical treatments on the electrokinetic properties of cellulose fibres. J. Adhes. Sci. Technol. 2002, 16, 157–178. [Google Scholar]
- Grahme, D.C. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 1947, 47, 441–501. [Google Scholar]
- McLaughlin, S. Electrostatic potentials at membrane-solution interfaces. In Current Topics in Membranes and Transport; Akleinzeller, F.B., Ed.; Academic Press: New York, NY, USA, 2011; Volume 9, pp. 71–144. [Google Scholar]
- Nir, S. A model for cation adsorption in closed systems: Application to calcium binding to phospholipid vesicles. J. Colloid Interface Sci. 1984, 102, 313–321. [Google Scholar]
- Yang, C.Q.; Wang, X. Formation of cyclic anhydride intermediates and esterification of cotton cellulose by multifunctional carboxylic acids: An infrared spectroscopy study. Textile Res. J. 1996, 66, 595–603. [Google Scholar]
- Edwards, J.V.; Eggleston, E.; Yager, D.R.; Cohen, I.K.; Diegelmann, R.F.; Bopp, A.F. Design, preparation, and assessment, of citrate-linked monosaccharide cellulose conjugates with elastase-lowering activity. Carbohydr. Polym. 2002, 50, 305–314. [Google Scholar]
- About Zeta Potential; Zeta News: San Ysidro, CA, USA, November 2009. Available online: http://zeta-potential.sourceforge.net/zeta-potential.shtml#SmoluchowskiEquation (accessed on 12 December 2011).
- Ramjee, M.J. The use of fluorogenic substrates to monitor thrombin generation for the analysis of plasma and whole blood coagulation. Anal. Biochem. 2000, 277, 11–18. [Google Scholar]
- Schneider, L.A.; Korber, A.; Grabbe, S. Dissemond, influence of pH on wound-healing: A new perspective for wound therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar]
- Kheirabadi, B.S.; Mace, J.E.; Irasema, B.; Terrazas, M.S.; Fedyk, C.G.; Estep, J.S.; Dubick, M.A.; Blackbourne, L.H. Safety evaluation of new hemostatic agents, smectite granules, and kaolin-coated gauze in a vascular injury wound model in swine. J. Trauma 2010, 68, 269–278. [Google Scholar]
- Pusateri, A.E.; McCarthy, S.J.; Gregory, K.W.; Harris, R.A.; Mc Manus, A.T.; Goodwin, C.W. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of sever venous hemorrhage and hepatic injury in swine. J. Trauma 2003, 54, 177–182. [Google Scholar]
- Edwards, J.V.; Parikh, D.V.; Howley, P.; Batiste, S.; Condon, B. Improving on army field gauze for lethal vascular injuries: Challenges in dressing development. Proceedings of the Beltwide Cotton Conferences, Nashville, TN, USA, 8–11 January 2008; pp. 1859–1862.
- Edwards, J.V.; Condon, B.; Howley, P.S.; Batiste, S. Improving on army field gauze for lethal vascular injuries: A progress report, Proceedings of the 2009 Beltwide Cotton Conferences, San Antonio, TX, USA, 5–8 January 2009; pp. 1432–1435.
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar]
- Fischer, T.H.; Bode, A.P.; Demcheva, M.; Vournakis, J.N. Hemostatic properties of glucosamine-based materials. J. Biomed. Mater. 2007, 80A, 167–174. [Google Scholar]
- Thatte, H.S.; Zagarins, S.; Khuri, S.F.; Fischer, T.H. Mechanism of poly-N-acetyl glucosamine polymer-mediated hemostasis: Platelet interactions. J. Trauma 2004, 57, S13–S21. [Google Scholar]
- Fischer, T.H.; Valeri, C.R.; Smith, C.J.; Scull, C.M.; Merricks, E.P.; Nichols, T.C.; Demcheva, M.; Vournakis, J.N. Non-classical processes in surface hemostasis: Mechanisms for the poly-N-acetyl glucosamine-induced alteration of red blood cell morphology and surface prothrombogenicity. Biomed. Mater. 2008, 3, 65–73. [Google Scholar]
- Edwards, J.V.; Howley, Yachmenev, V.; Lambert, A.; Condon, B. Development of a continuous finishing chemistry process for manufacture of a phosphorylated cotton chronic wound dressing. J. Ind. Text. 2009, 39, 27–42. [Google Scholar]
- Remijn, J.A.; da Costa, M.P.; Ijsseldijk, M.J.; Sixma, J.J.; de Groot, P.G.; Zwaginga, J.J. Impaired platelet adhesion to lysed fbrin whereas neutrophil adhesion remains intact under conditions of flow. Blood Coagul. Fibrinolysis 2006, 17, 421–424. [Google Scholar]
- Connors, D.; Gies, D.; Lin, H.; Gruskin, E.; Mustoe, T.A.; Tawil, N.J. Increase in wound breaking strength in rats in the presence of positively charged dextran beads correlates with an increase in endogenous transforming growth factor-β1 and its receptor TGF-βR1 in close proximity to the wound. Wound Repair Regen. 2000, 8, 292–303. [Google Scholar]
- Cardoso, C.R.; Favoreto, S.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Edwards, J.V.; Prevost, N. Thrombin Production and Human Neutrophil Elastase Sequestration by Modified Cellulosic Dressings and Their Electrokinetic Analysis. J. Funct. Biomater. 2011, 2, 391-413. https://doi.org/10.3390/jfb2040391
Edwards JV, Prevost N. Thrombin Production and Human Neutrophil Elastase Sequestration by Modified Cellulosic Dressings and Their Electrokinetic Analysis. Journal of Functional Biomaterials. 2011; 2(4):391-413. https://doi.org/10.3390/jfb2040391
Chicago/Turabian StyleEdwards, Judson Vincent, and Nicolette Prevost. 2011. "Thrombin Production and Human Neutrophil Elastase Sequestration by Modified Cellulosic Dressings and Their Electrokinetic Analysis" Journal of Functional Biomaterials 2, no. 4: 391-413. https://doi.org/10.3390/jfb2040391