Remotely Monitoring Cancer-Related Fatigue Using the Smart-Phone: Results of an Observational Study
Abstract
:1. Introduction
- is inexpensive and integrated in daily life of patients, since it is based on smartphones,
- is accepted by patients suffering from cancer related fatigue,
- allows to collect subjective data in terms of patients’ self-reports and objective behavioral data,
- is designed for long-term usage.
2. Materials and Methods
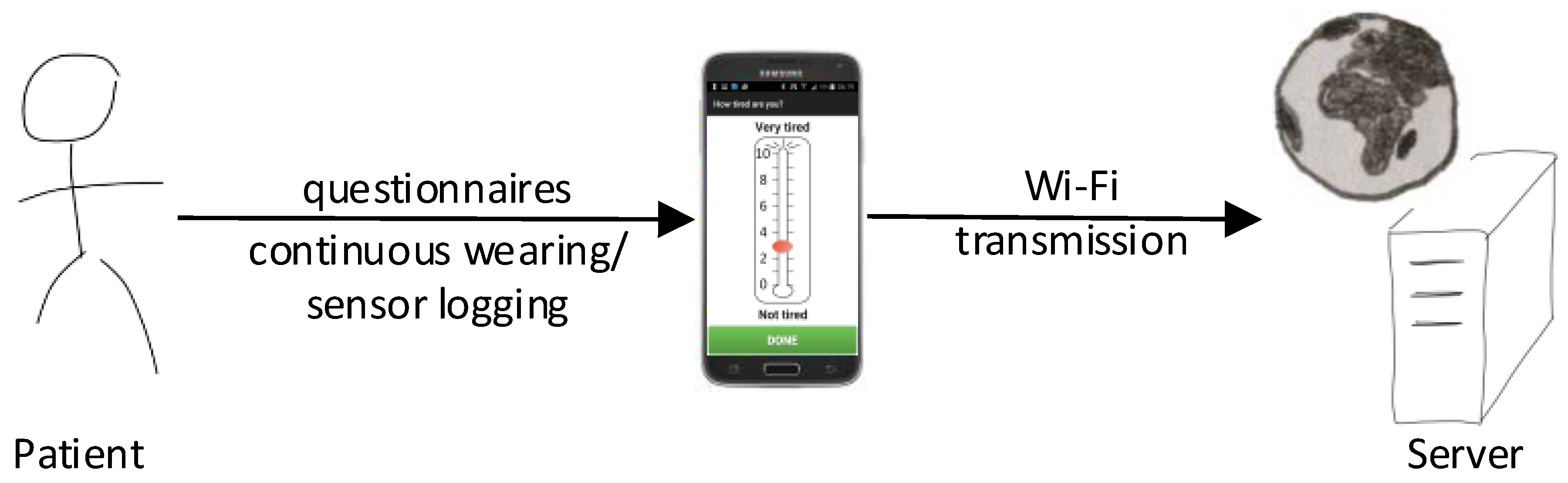
2.1. Monitoring System
Android App
2.2. Data Transmission
Web-Server
2.3. Study Protocol
2.3.1. Patient Recruitment
- Aged years,
- mild to severe fatigue, i.e., FACIT-F [28],
- willingness to use the provided smartphone,
- successful briefing how to use the smartphone and activity monitoring app,
- signed informed consent.
- explained the study concept and goal
- asked to participate in the study
- tested for eligibility, i.e., tested for fatigue FACIT-F and depression (M.I.N.I.)
2.3.2. Baseline
2.3.3. The Study Period
2.3.4. The Study End
2.4. Data Analysis
- Is activity monitoring by the use of smartphones feasible in these patients that are often not capable to achieve their daily tasks?
- Can we generate patient-specific activity data with the goal to support research and interventions for CRF?
2.4.1. Feasibility, Retention and Wearability
- completeness of answering the digital self-reports,
- logging time of the smartphone,
- wearability index WI, denoting the percentage of time per day during which the phone was on-body.
2.4.2. Smartphone On-/Off-Body Detection
2.4.3. Descriptive Statistics and Exploratory Data Analysis
3. Results
3.1. Patients’ Demographics
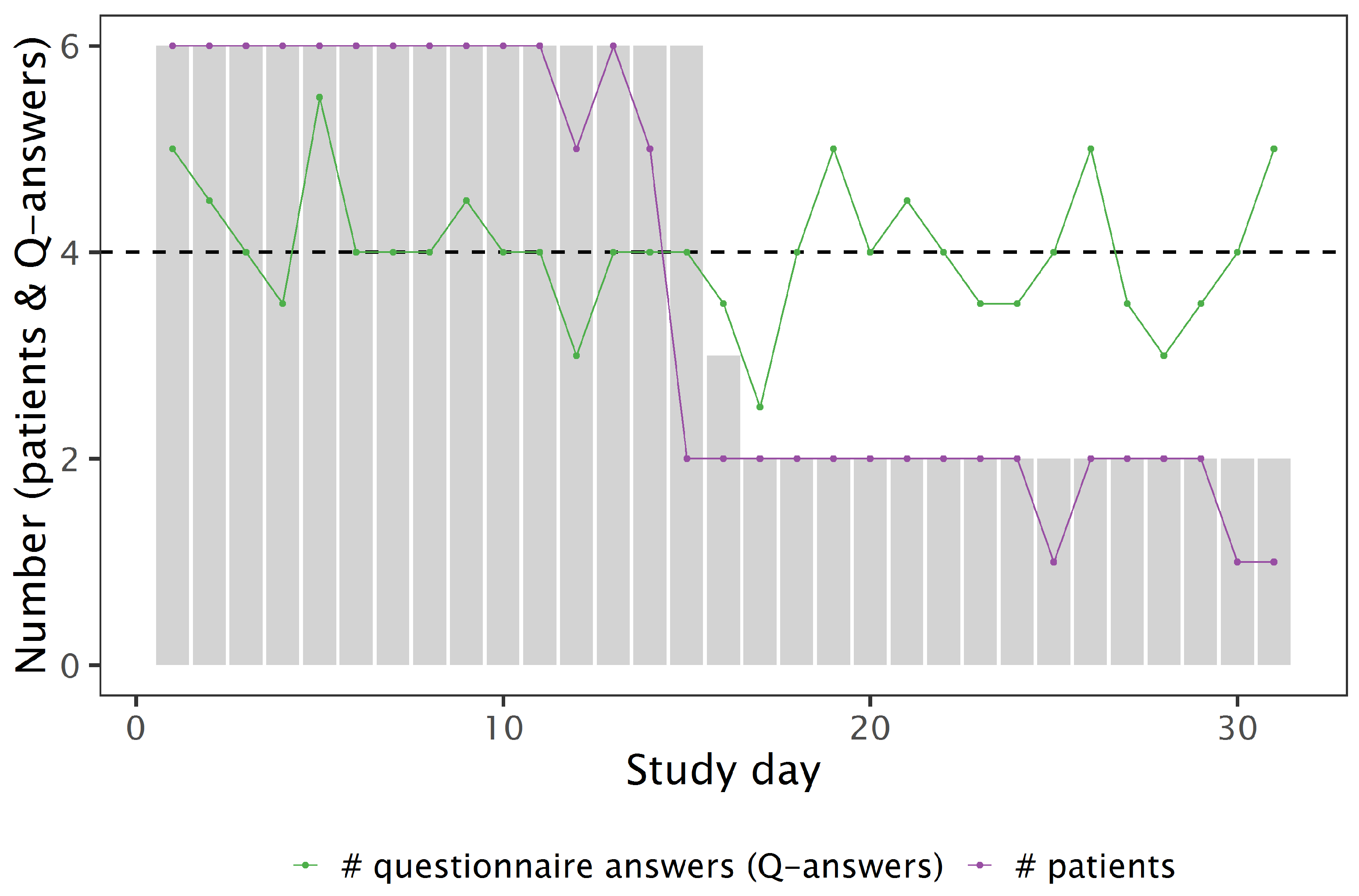
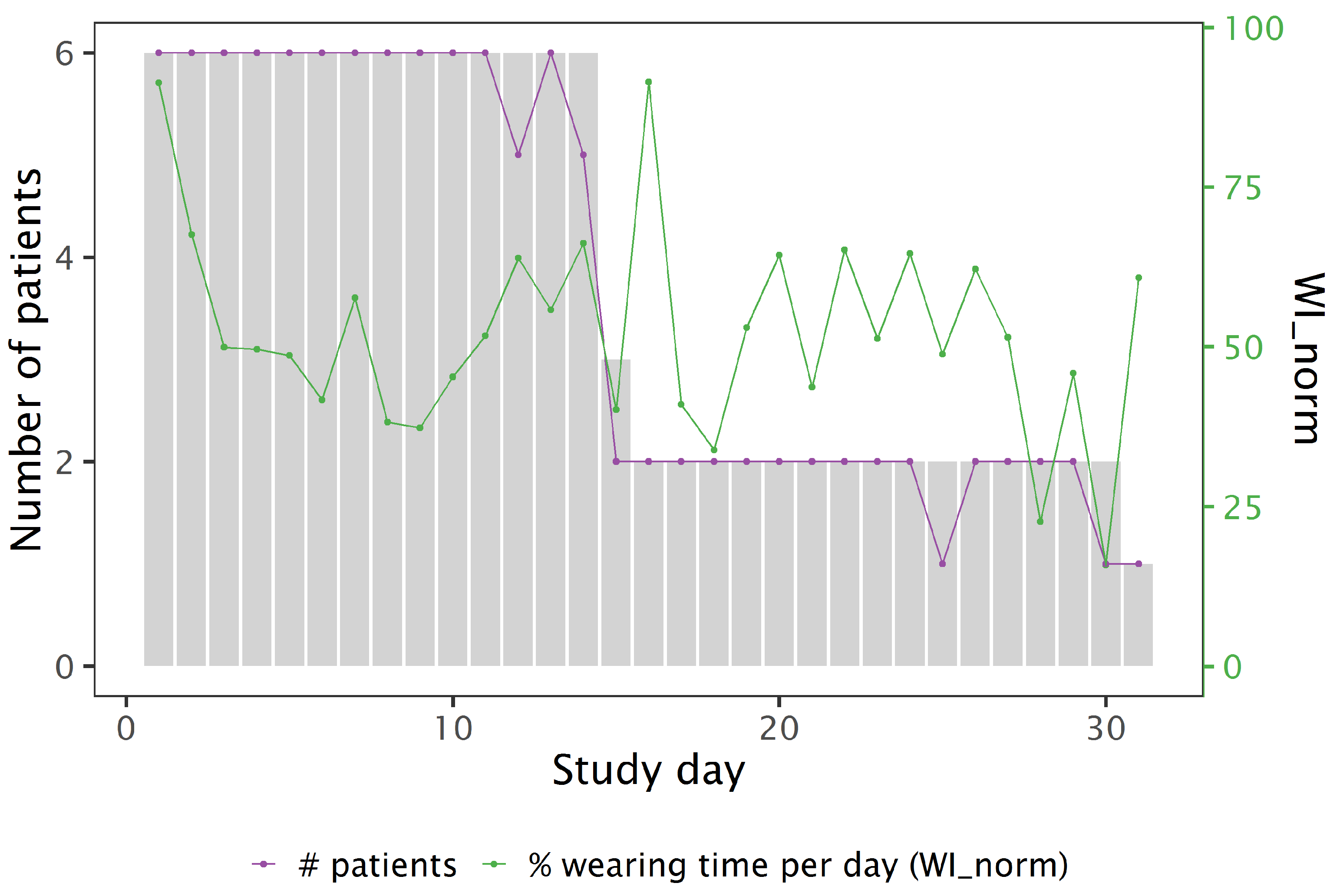
3.2. Feasibility and Retention
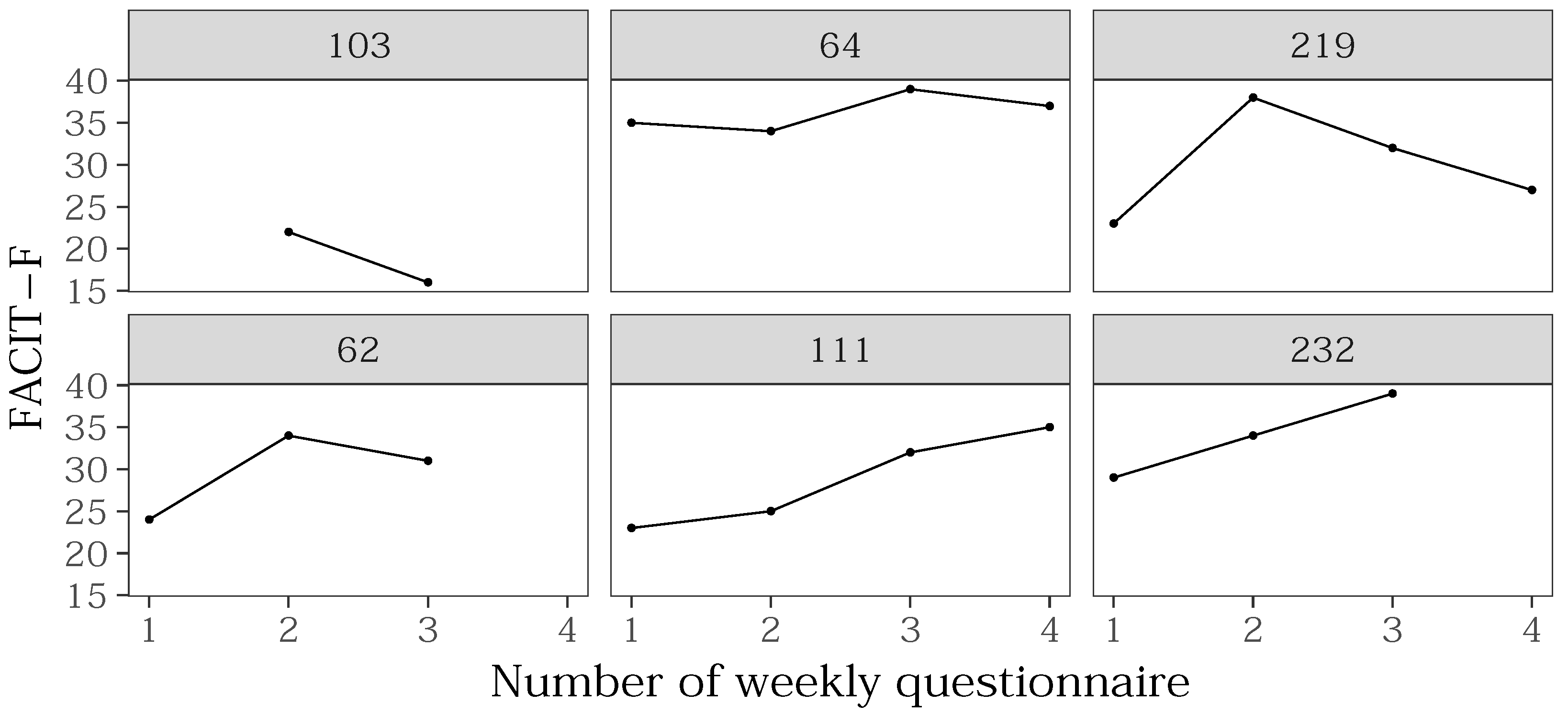
3.3. Analysis of FACIT-F Questionnaire Answers
3.4. Analysis of Digital Questionnaires: Fatigue and Interference
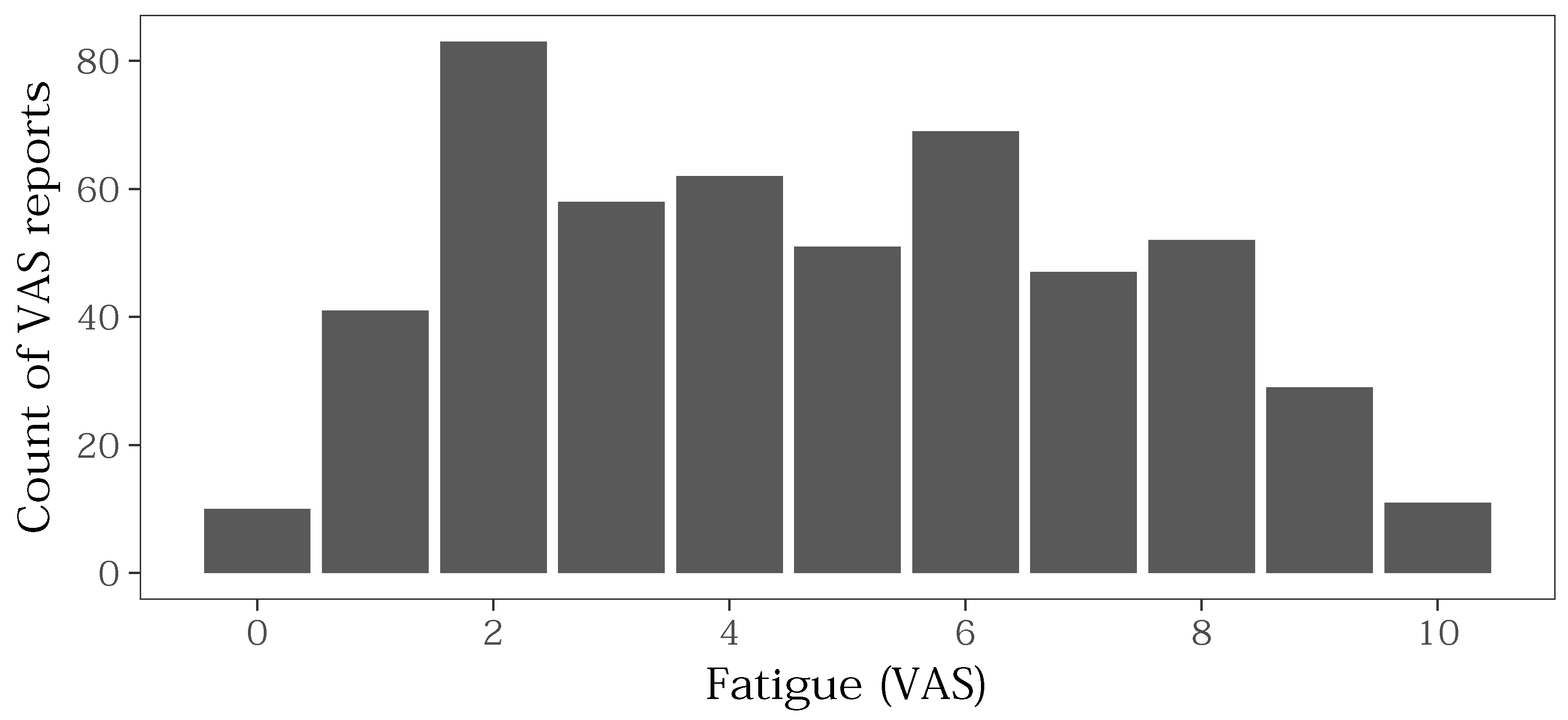
3.4.1. Distribution of VAS Values
3.4.2. Relation between Fatigue and Interference of Fatigue
- The higher the fatigue, the more impact on daily activities. If one is less tired, it is probably easier to ignore the lack of energy during activities of daily life. However, literature gives no clear conclusions to confirm this hypothesis. Franke et al. have observed a similar behaviour in fatigued patients suffering from Hepatitis C [33]. They found out that interference is a significant predictor for depression. On the other hand, depressed patients were more likely to report a severe fatigue.
- The patients could not well distinguish between the two questionnaires. To investigate this hypothesis, a future study can collect data from the digital questionnaires and correlate them with validated instruments such as the Brief Fatigue Inventory that measures intensity and interference of fatigue at the same time. Considering the result of [33], also depression should be controlled.
3.4.3. Intra-Day Course of Fatigue
- late night
- 2–6 o’clock,
- morning
- 6–10 o’clock,
- midday
- 10–14 o’clock,
- afternoon
- 14–18 o’clock,
- evening
- 18–22 o’clock,
- night
- 22–2 o’clock.
3.5. Analysis of Activity Data
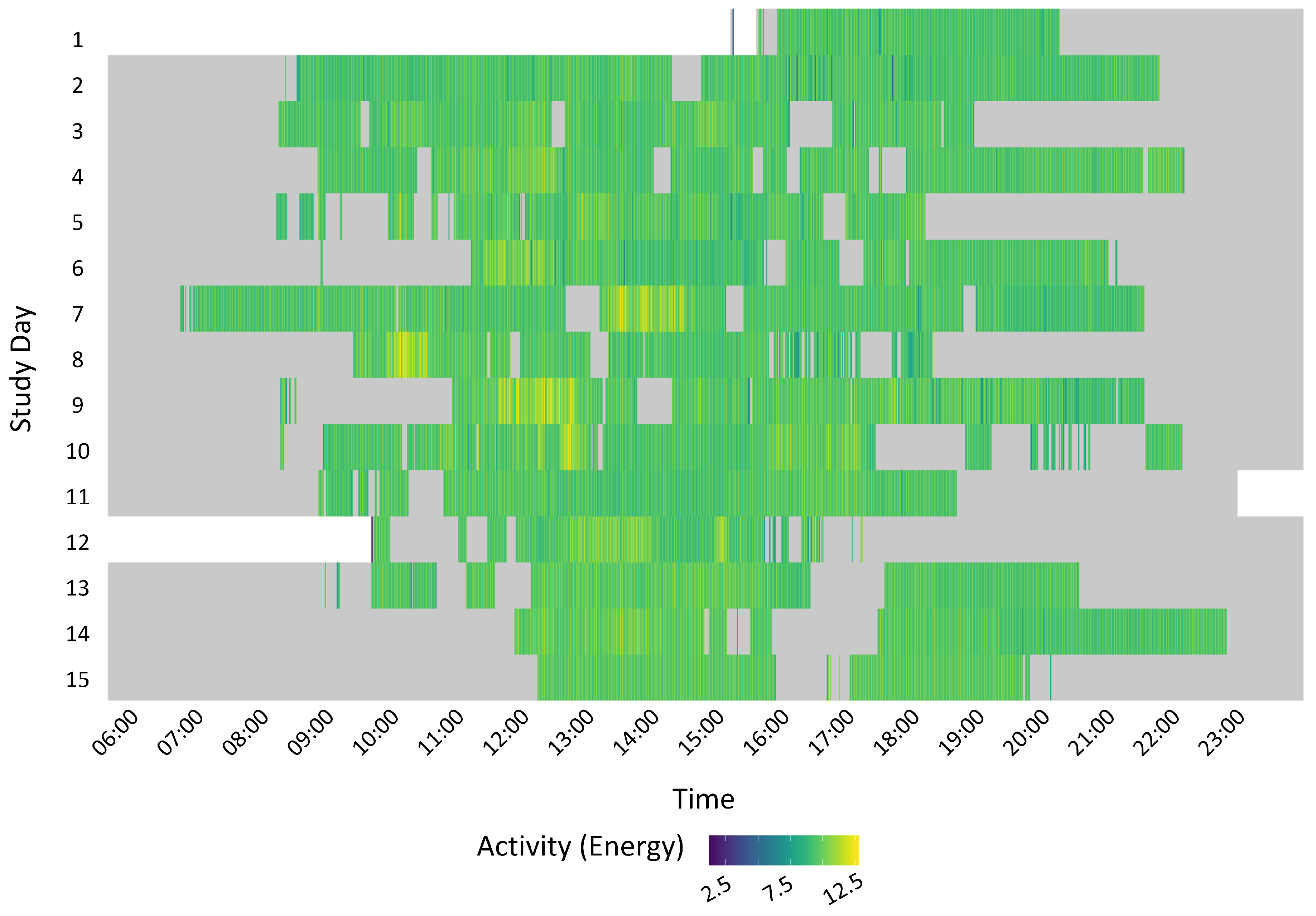
3.5.1. Visualisation of Sedentary Behaviour
3.5.2. Correlation Analysis
4. Discussion
4.1. Summary of Results
4.2. Advantages and Limits of Mobile Health
4.3. Limitations
4.4. Future Work
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRF | cancer related fatigue |
| QoL | quality of life |
| app | smartphone application |
| VAS | visual analogue scale |
| SSL | secure sockets layer |
| FACIT-F | functional assessment of chronic illness therapy-fatigue |
| M.I.N.I. | mini international neuropsychiatric interview |
| ADL | activities of daily life |
| min | minute |
| SVM | support vector machine |
| RBF | radial basis function |
| IQR | inter quartile range |
| ESM | experienced-based sampling method |
References
- Stasi, R.; Abriani, L.; Beccaglia, P.; Terzoli, E.; Amadori, S. Cancer-Related Fatigue: Evolving Concepts in Evaluation and Treatment. Cancer 2003, 98, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue: Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Curt, G.A. The Impact of Fatigue on Patients with Cancer: Overview of FATIGUE 1 and 2. Oncologist 2000, 5, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A.M.; Gerber, L.H.; Mayer, D.K. Cancer-related fatigue: Implications for breast cancer survivors. Cancer 2012, 118, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.L.; Garssen, B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: A treatment study. Psychooncology 2012, 21, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Sadja, J.; Mills, P.J. Effects of Yoga Interventions on Fatigue in Cancer Patients and Survivors: A Systematic Review of Randomized Controlled Trials. Explore 2013, 9, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minton, O.; Berger, A.; Barsevick, A.; Cramp, F.; Goedendorp, M.; Mitchell, S.A.; Stone, P.C. Cancer-related fatigue and its impact on functioning. Cancer 2013, 119, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Exercise and cancer-related fatigue in adults: A systematic review of previous systematic reviews with meta-analyses. BMC Cancer 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhimaraj, A. Remote monitoring of heart failure patients. Methodist Debakey Cardiovasc. J. 2013, 9, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Urrea, B.; Misra, S.; Plante, T.B.; Kelli, H.M.; Misra, S.; Blaha, M.J.; Martin, S.S. Mobile Health Initiatives to Improve Outcomes in Primary Prevention of Cardiovascular Disease. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Grunerbl, A.; Muaremi, A.; Osmani, V.; Bahle, G.; Ohler, S.; Troester, G.; Mayora, O.; Haring, C.; Lukowicz, P. Smart-Phone Based Recognition of States and State Changes in Bipolar Disorder Patients. IEEE J. Biomed. Health Informat. 2014, 19, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Faurholt-Jepsen, M.; Vinberg, M.; Christensen, E.M.; Frost, M.; Bardram, J.; Kessing, L.V. Daily electronic self-monitoring of subjective and objective symptoms in bipolar disorder–the MONARCA trial protocol (MONitoring, treAtment and pRediCtion of bipolAr disorder episodes): A randomised controlled single-blind trial. BMJ Open 2013, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mayora, O.; Arnrich, B.; Bardram, J.; Drager, C.; Finke, A.; Frost, M.; Giordano, S.; Grunerbl, A.; Raring, C.; Raux, R.; et al. Mobile Health Systems for Bipolar Disorder. Proc. MindCare 2013, 5, 424–429. [Google Scholar]
- Saeb, S.; Zhang, M.; Karr, C.J.; Schueller, S.M.; Corden, M.E.; Kording, K.P.; Mohr, D.C. Mobile Phone Sensor Correlates of Depressive Symptom Severity in Daily-Life Behavior: An Exploratory Study. J. Med. Internet Res. 2015, 17, e175. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zeev, D.; Wang, R.; Abdullah, S.; Brian, R.; Scherer, E.A.; Mistler, L.A.; Hauser, M.; Kane, J.M.; Campbell, A.; Choudhury, T. Mobile Behavioral Sensing for Outpatients and Inpatients With Schizophrenia. Psychiatr. Serv. 2016, 67, 558–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazilu, S.; Blanke, U.; Calatroni, A.; Gazit, E.; Hausdorff, J.M.; Tröster, G. The role of wrist-mounted inertial sensors in detecting gait freeze episodes in Parkinson’s disease. Pervasive Mob. Comput. 2016. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiter, J.; Feese, S.; Arnrich, B.; Tröster, G.; Amft, O.; Macrea, L.; Maurer, K. Activity monitoring in daily life as an outcome measure for surgical pain relief intervention using smartphones. In Proceedings of the 2013 International Symposium on Wearable Computers, Zurich, Switzerland, 8–12 September 2013; p. 127. [Google Scholar]
- Burton, C.; McKinstry, B.; Szentagotai Tatar, A.; Serrano-Blanco, A.; Pagliari, C.; Wolters, M. Activity monitoring in patients with depression: A systematic review. J. Affect. Disord. 2013, 145, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.H. Big data analysis for personalized health activities: Machine learning processing for automatic keyword extraction approach. Symmetry 2018, 10, 93. [Google Scholar] [CrossRef]
- Timmerman, J.; Weering, M.D.V.; Tönis, T.; Hermens, H.; Vollenbroek-Hutten, M. Relationship between patterns of daily physical activity and fatigue in cancer survivors. Eur. J. Oncol. Nurs. 2015, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Hasan, S.; Dubey, D.; Sarangi, S. Smartphone apps as a source of cancer information: Changing trends in health information-seeking behavior. J. Cancer Educ. 2013, 28, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Karargyris, A.; Karargyris, O.; Pantelopoulos, A. DERMA/Care: An advanced image-processing mobile application for monitoring skin cancer. Proc. Int. Conf. Tools Artif. Intell. 2012, 2, 1–7. [Google Scholar]
- Davis, S.W.; Oakley-Girvan, I. mHealth Education Applications Along the Cancer Continuum. J. Cancer Educ. 2015, 30, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Klaas, V.; Tröster, G.; Fagundes, C.P. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: A systematic review and meta-analysis. Psychooncology 2017, 26, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Klaas, V.V.C.; Calatroni, A.; Hardegger, M.; Guckenberger, M.; Theile, G.; Tröster, G. Monitoring Patients in Ambulatory Palliative Care: A Design for an Observational Study. In Proceedings of the 6th International Conference on Wireless Mobile Communication and Healthcare, Milan, Italy, 14–16 November 2016. [Google Scholar]
- FACIT. FACIT-Erschöpfung (Fassung 4); FACIT: Elmhurst, IL, USA, 2012. [Google Scholar]
- Csikszentmihalyi, M.; Larson, R. Validity and Reliability of the Experience-Sampling Method. In Flow and the Foundations of Positive Psychology; Collected Works Mihaly Csikszentmihalyi; Springer: Dordrecht, The Netherlands, 2014; pp. 35–54. [Google Scholar]
- Bossola, M.; Di Stasio, E.; Giungi, S.; Rosa, F.; Tazza, L. Fatigue is associated with serum interleukin-6 levels and symptoms of depression in patients on chronic hemodialysis. J. Pain Symptom Manag. 2015, 49, 578–585. [Google Scholar] [CrossRef] [PubMed]
- DiCicco-Bloom, B.; Crabtree, B.F. The qualitative research interview. Med. Educ. 2006, 40, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Franke, L.; Therstappen, E.; Schlosser, B.; Biermer, M.; Berg, T.; Schäfer, M.; Arck, P.; Uebelhack, R.; Friebe, A. A preliminary study on the relationship between platelet serotonin transporter functionality, depression, and fatigue in patients with untreated chronic hepatitis C. Depress. Res. Treat. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gresham, G.K.; Piantadosi, S.; Drye, L.; Figlin, R.A.; Shinde, A.M. Applications of wearable activity monitors (WAM) in cancer clinical trials (CT): A review of the literature. J. Clin. Oncol. 2016, 34, e21598. [Google Scholar] [CrossRef]
- Giunti, G.; Fernández, E.G.; Zubiete, E.D.; Romero, O.R. Supply and demand in mHealth apps for persons with multiple sclerosis: Systematic search in app stores and scoping literature review. J. Med. Internet Res. 2018, 20. [Google Scholar] [CrossRef] [PubMed]




| Modality | Description | Sampling Rate |
|---|---|---|
| Questionnaires | two simple to use visual analogue scales allow to rate the level of perceived fatigue and interference in a range from 0 (not tired/no interference)–10 (extremely tired/extreme interference). The third question provides a selection of 7 daily activities, e.g., relaxing, housework, multimedia, and a blank card (“Other”). | 4 random times per day (based on ESM) |
| Physical Activity | The sampling rate of sensor measurements is controlled by Android and varies depending on usage. | |
| Accelerometer | 40 Hz | |
| Barometer | 2 Hz | |
| Magnetometer | 10 Hz |
| Parameter Name | Value |
|---|---|
| windows size | 256 samples |
| step width | 1 |
| C | 31.62 |
| gamma | 0.0025 |
| Feature | Description |
|---|---|
| with of over 1 h window | |
| with of over 1 h window | |
| WEAR | wearability index calculated over 1 h window |
| ENERGY | |
| ENERGY.WEAR |
| Pat. Id | Age (Year) | Gender | FACIT-F (BL) |
|---|---|---|---|
| 103 | 32 | f | 22 ** |
| 64 | 58 | m | 35 |
| 219 | 73 | f | 23 |
| 62 | 37 | f | 24 |
| 111 | 33 | f | 23 |
| 232 | 52 | f | 29 |
| 66 * | 56 | f | 24 |
| Pat. Id | Participation (d) | LT/d (h) | h | q/d | WI | WI |
|---|---|---|---|---|---|---|
| 103 | 32 | 23.6 | 1 | 4 | ||
| 64 | 16 | 22.0 | 2 | 3.3 | ||
| 219 | 31 | 23.9 | 0 | 3.9 | ||
| 62 | 15 | 23.1 | 1 | 4.1 | ||
| 111 | 15 | 21.6 | 3 | 4.1 | ||
| 232 | 15 | 23.0 | 1 | 6.6 |
| Pat. Id | Correlation | Significance |
|---|---|---|
| 103 | r(97) = 0.62 | p < 2.2 |
| 64 | r(60) = 0.98 | p < 2.2 |
| 219 | r(59) = 0.57 | p = 7.505 |
| 62 | r(119) = 0.78 | p = 5.87 |
| 111 | r(51) = 0.95 | p < 2.2 |
| 232 | r(125) = 0.87 | p = 1.211 |
| Correlation Sign | |||
|---|---|---|---|
| VAR | pos | 0.78–0.91 (2) | 0.61 (1) |
| neg | −0.8 (1) | −0.76 (1) | |
| RMS | pos | 0.6–0.76 (2) | 0.61–0.63 (2) |
| WEAR | pos | 0.55 (1) | 0.61 (1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaas, V.C.; Troster, G.; Walt, H.; Jenewein, J. Remotely Monitoring Cancer-Related Fatigue Using the Smart-Phone: Results of an Observational Study. Information 2018, 9, 271. https://doi.org/10.3390/info9110271
Klaas VC, Troster G, Walt H, Jenewein J. Remotely Monitoring Cancer-Related Fatigue Using the Smart-Phone: Results of an Observational Study. Information. 2018; 9(11):271. https://doi.org/10.3390/info9110271
Chicago/Turabian StyleKlaas, Vanessa Christina, Gerhard Troster, Heinrich Walt, and Josef Jenewein. 2018. "Remotely Monitoring Cancer-Related Fatigue Using the Smart-Phone: Results of an Observational Study" Information 9, no. 11: 271. https://doi.org/10.3390/info9110271





