An Extensive Method for Maintenance of Sterility in Mammalian Cell Culture Laboratory Routine
Abstract
:1. Introduction
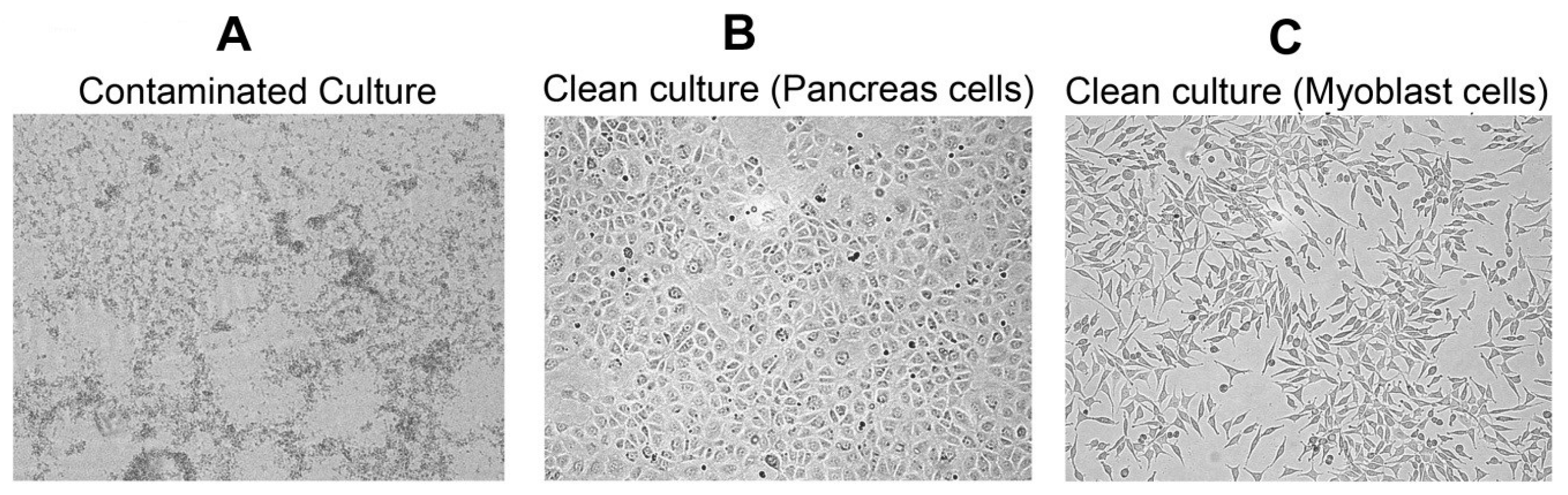
2. Results
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Blood Agar Plate Preparations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richmond, J.Y.; McKinney, R.W. Biosafety in Microbiological and Biomedical Laboratories, 5th ed.; U.S. Department of Health and Human ServicesPublic Health Service, Public Health Service Centers for Disease Control and Prevention, National Institutes of Health: Atlanta, GA, USA, 2009.
- Bykowski, T.; Stevenson, B. Aseptic Technique. Curr. Protoc. Microbiol. 2008. [Google Scholar] [CrossRef]
- Cote, R.J. Aseptic Technique for Cell Culture. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Franklin, NJ, USA, 2001; Chapter 1, Unit 1.3. [Google Scholar]
- Cohen, S.; Samadikuchaksaraei, A.; Polak, J.M.; Bishop, A.E. Antibiotics Reduce the Growth Rate and Differentiation of Embryonic Stem Cell Cultures. Tissue Eng. 2006, 12, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use Antibiotics in Cell Culture with Caution: Genome-Wide Identification of Antibiotic-Induced Changes in Gene Expression and Regulation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and Microbial Decontamination of Pool Water Using Activated Potassium Peroxymonosulfate. Water Res. 2008, 42, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Su, X.W.; D’Souza, D.H. Inactivation of Human Norovirus Surrogates by Benzalkonium Chloride, Potassium Peroxymonosulfate, Tannic Acid, and Gallic Acid. Foodborne Pathog. Dis. 2012, 9, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, J.; So, B.; Lee, K.; Yun, S.; Lee, M.; Choe, N. Evaluation of Changes Induced by Temperature, Contact Time, and Surface in the Efficacies of Disinfectants against Avian Influenza Virus. Poult. Sci. 2014, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Mainous, M.E.; Smith, S.A. Efficacy of Common Disinfectants against Mycobacterium Marinum. J. Aquat. Anim. Health 2005, 17, 284–288. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çelik-Uzuner, S.; Uzuner, U. An Extensive Method for Maintenance of Sterility in Mammalian Cell Culture Laboratory Routine. Challenges 2017, 8, 26. https://doi.org/10.3390/challe8020026
Çelik-Uzuner S, Uzuner U. An Extensive Method for Maintenance of Sterility in Mammalian Cell Culture Laboratory Routine. Challenges. 2017; 8(2):26. https://doi.org/10.3390/challe8020026
Chicago/Turabian StyleÇelik-Uzuner, Selcen, and Uğur Uzuner. 2017. "An Extensive Method for Maintenance of Sterility in Mammalian Cell Culture Laboratory Routine" Challenges 8, no. 2: 26. https://doi.org/10.3390/challe8020026




