In Vivo Cytogenotoxicity of Electronic Waste Leachate from Iloabuchi Electronic Market, Diobu, Rivers State, Nigeria on Allium Cepa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Test Leachate
2.3. Determination of Physical and Chemical Parameters
3. Experiment
3.1. Allium cepa Assay
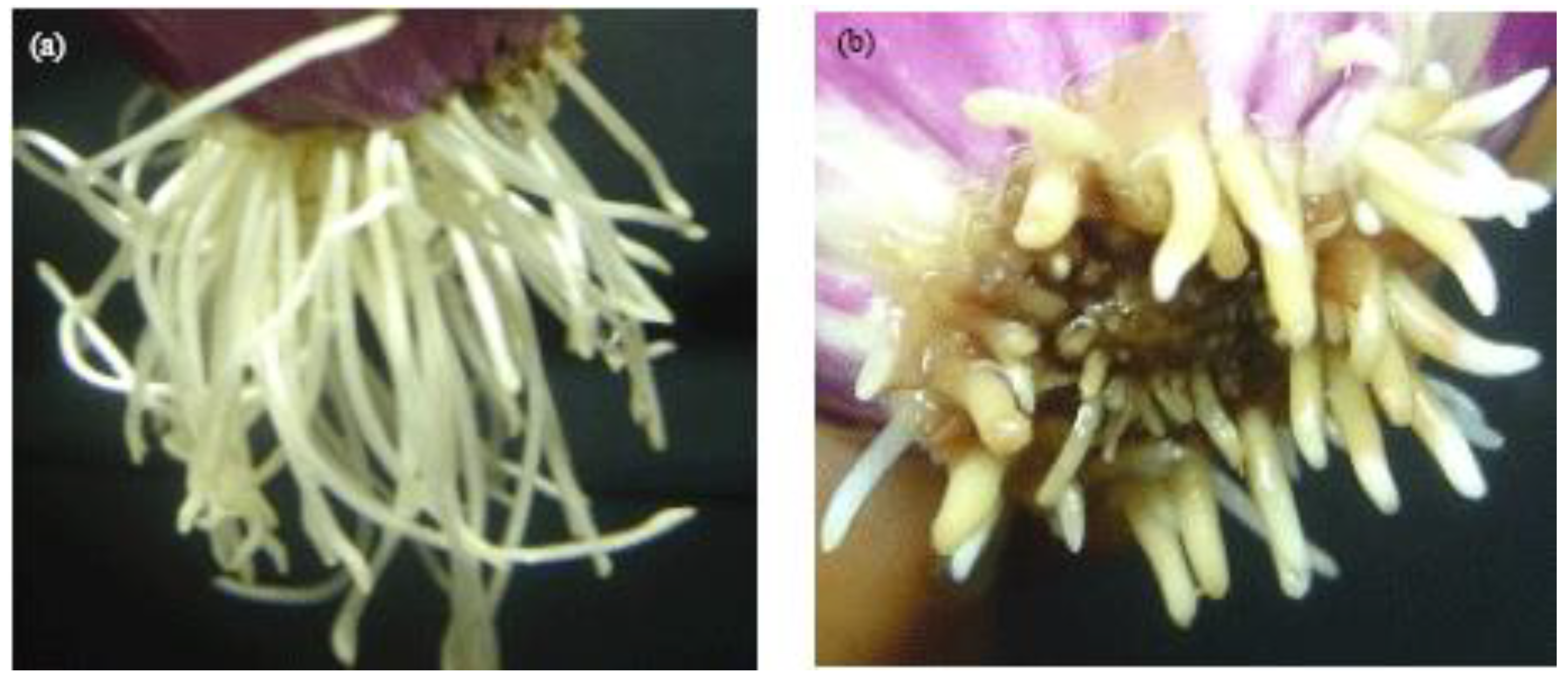
3.2. Toxicity of Samples to Onion Root Growth
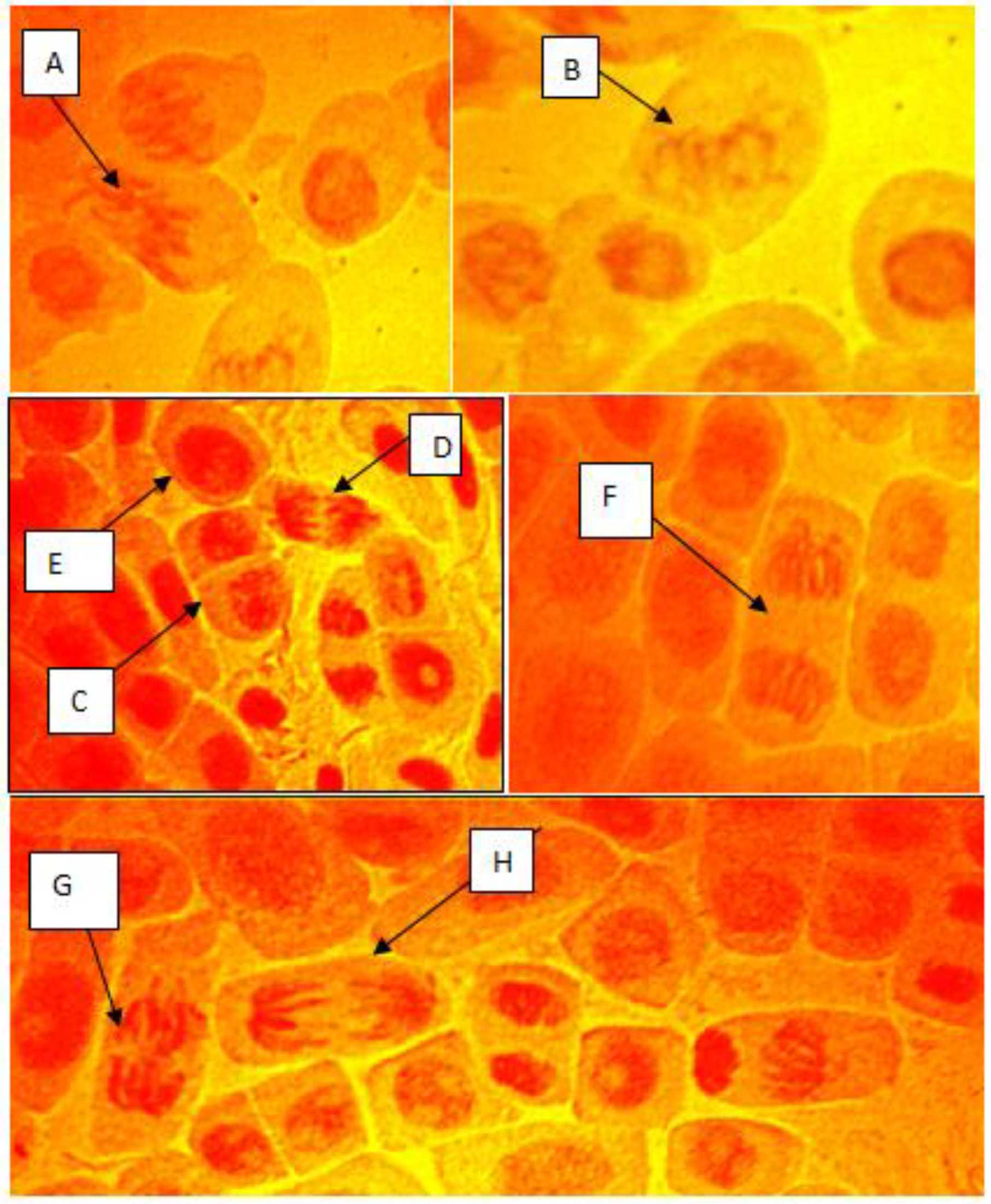
3.3. Chromosomal Aberrations in A. cepa
3.4. Statistical Analysis
4. Results
4.1. Physico-Chemical Characteristics
| Parameter | Unit | E-waste | Tap water | FEPA | USEPA |
|---|---|---|---|---|---|
| PH | - | 6.17 | 7.5 | 6.9 | 6.5–8.5 |
| colour | - | Milky white | Colourless | - | - |
| odour | - | unpleasant | Odourless | - | - |
| SALINITY | - | 120 | 1.4 | - | - |
| TEMPERATURE | oC | 29.2 | 26.1 | <40 oC | <40 oC |
| Dissolved oxygen | mg/L | 3.96 | - | - | |
| TURBIDITY | FTU | 90.3 | 0 | 10 | 10 |
| Total dissolved solid | mg/L | 175 | 78 | 2000 | 500 |
| Lead | mg/L | 1.35 | 0.04 | 0.01 | 0.015 |
| Cadmium | mg/L | 0.56 | 0.01 | 0.05 | 0.05 |
| Chromium | mg/L | 0.31 | 0.5 | 0.05 | 0.05 |
| Copper | mg/L | 5.27 | 1.4 | 0.30 | 1.00 |
| Iron | mg/L | 1.53 | 0.05 | 0.05 | 0.30 |
| Nickel | mg/L | 10.22 | 1.3 | - | - |
| Zinc | mg/L | 6.12 | 1.3 | 1.00 | 1.00 |
4.2. Toxicity of the Leachate to Root Growth in A. cepa
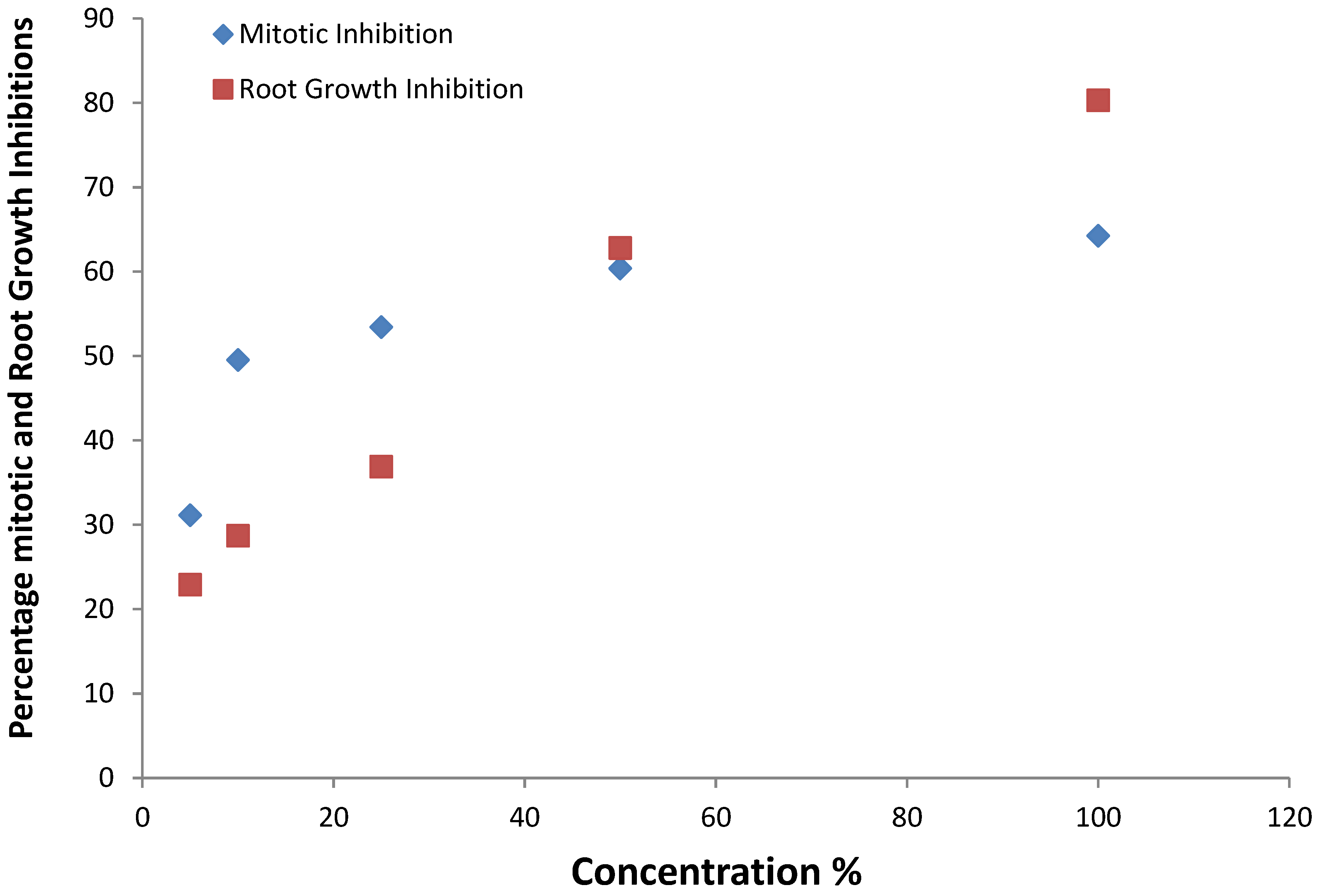
| Concentration (%) | Mean Length ± SE | Root growth inhibition (%) | P < 0.05 | 95% CL |
|---|---|---|---|---|
| 0 | 2.71 ± 0.11 | - | 0.6 | |
| 5 | 2.09 ± 0.17 | 22.9 | 0.0498 * | 0.34 |
| 10 | 1.94 ± 0.20 | 28.7 | 0.0346 * | 0.23 |
| 25 | 1.71 ± 0.20 | 36.9 | 0.0139 ** | 0.33 |
| 50 | 1.01 ± 0.06 | 62.8 | 0.0005 *** | 0.4 |
| 100 | 0.53 ± 0.10 | 80.3 | 0.0002 *** | 0.31 |
| EC50 = 37.5%` | ||||
| Concentration (%) | No. of classified cells | Total aberration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells at anaphase | Cells at prophase | Stiky chromosome | Distured spindle | Anaphase bridges | Chromosome fragment | Chromosome bridge | Laggard chromosome | Others | ||
| Control (Tap Water) | 34 | 60 | - | - | - | - | - | - | - | - |
| 5 | 69 | 47 | 3 | 3 | 1 | - | - | 2 | 9 | |
| 10 | 66 | 32 | 2 | 8 | 1 | 2 | - | - | - | 13 |
| 25 | 35 | 30 | 1 | - | 2 | - | 13 | 2 | 3 | 21 |
| 50 | 45 | 15 | 1 | 2 | 1 | - | 18 | 1 | 1 | 24 |
| 100 | 38 | 25 | 7 | 8 | - | - | 10 | 5 | 4 | 34 |
| TOTAL | 385 | 255 | 11 | 18 | 4 | 2 | 41 | 8 | 10 | 101 |
| %Total Aberration | 10.98 | 17.82 | 3.96 | 1.98 | 40.59 | 7.92 | 9.90 | |||
| Concentration (%) | No. of cells scored | Mitotic index | Mitotic inhibition |
|---|---|---|---|
| Control (Tap water) | 4000 | 14.13 | - |
| 5 | 4000 | 9.73 | 31.14 * |
| 10 | 4000 | 7.13 | 49.54 * |
| 25 | 4000 | 6.58 | 53.43 * |
| 50 | 4000 | 5.60 | 60.37 * |
| 100 | 4000 | 5.05 | 64.26 * |
4.3. Chromosomal Aberration in A. cepa
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sthiannopkao, S.; Wong, M.H. Handling e-waste in developed and developing countries: Initiatives, practices, and consequences. Sci. Total Environ. 2013, 463–464, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Arora, R. Best Practices for E-Waste Management in Developing Nations; GTZ-ASEM: New Delhi, India, 2008. [Google Scholar]
- Nnorom, I.C.; Osibanjo, O. Electronic waste (e-waste): Material flows and management practices in Nigeria. Waste Manag. 2008, 28, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Manhart, A.; Osibanjo, O.; Aderinto, A.; Prakash, S. Informal E-Waste Management in Lagos, Nigeria—Socio-Economic Impacts and Feasibility of International Recycling Co-Operations; Final Report of Component 3 of the UNEP SBC E-waste Africa Project; Öko-Institut e.V.: Freiburg, Germany, 2011. [Google Scholar]
- Orisakwe, O.E. Environmental pollution and blood lead levels in Nigeria: Who is unexposed? Int. J. Occup. Environ. Health. 2009, 15, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Alabi, O.A.; Bakare, A.A. Genotoxicity and mutagenicity of electronic waste leachates using animal bioassays. Toxicol. Environ. Chem. 2011, 93, 1073–1088. [Google Scholar] [CrossRef]
- Bakare, A.A.; Okunola, A.; Alabi, A.M.; Gbadebo, I.O.; Alimba, C.G. In vivo cytogenotoxicity and oxidative stress induced by electronic waste leachate and contaminated well water. Challenges 2013, 4, 169–187. [Google Scholar] [CrossRef]
- Rank, J.; Nielsen, M.H. Allium cepa anaphase-telophase root tip chromosome aberration assay on N-methyl-N-nitrosourea, maleic hydrazide, sodium azide, and ethyl methanesulfonate. Mutat. Res. 1997, 390, 121–127. [Google Scholar] [CrossRef]
- Babatunde, B.B.; Bakare, A.A. Genotoxicity screening of wastewaters from Agbara industrial estate, Nigeria evaluated with the Allium test. Pollut. Res. 2006, 25, 227–234. [Google Scholar]
- Konya, R.S.; Babatunde, B.B.; Iniefe, D. Assessment of e-waste statue in port Harcourt and its environs. Environ. Sci. 2013, 3, 45–66. [Google Scholar]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Waste Water, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- United States Environmental Protection Agency (USEPA). National recommended water quality criteria–Correction: EPA 822/Z-99-001; USEPA: Washington, DC, USA, 1999.
- Bakare, A.A.; Lateef, A.; Amuda, O.S.; Afolabi, R.O. The aquatic toxicity and characterization of chemical and microbiological constituents of water samples from Oba River, Odo-Oba, Nigeria. Asian J. Microbiol. Biotechnol. Environ. Sci. 2003, 5, 11–17. [Google Scholar]
- Fiskesjo, G. Allium test for screening chemicals: Evaluation of cytologic parameters. In Plants for Environmental Studies; Wang, W., Gorsuch, J.W., Hughes, J.S., Eds.; CRC Lewis Publishers: Boca Raton, NY, USA, 1997; pp. 308–333. [Google Scholar]
- ASTM. Standard Practice for Conducting Early Seedling Growth Tests 1, American Society for Testing and Material Designation E 1598-94; ASTM: Washington, DC, USA, 1994; pp. 1493–1499. [Google Scholar]
- Federal Environmental Protection Agency (FEPA). S1.8 National Environmental Protection (Leachate Limitations) Regulations; FEPA: Washington, DC, USA, 1991. [Google Scholar]
- World Health Organisation (WHO). Guideline for Drinking Water Quality—Recommendations 2; World Health Organisation: Geneva, Switzerland, 1996. [Google Scholar]
- Brigden, K.; Labunska, I.; Santillo, D.; Johnston, P. Chemical contamination at e-waste recycling and disposal sites in Accra and Korforidua, Ghana; Greenpeace International: Amsterdam, The Netherland, 2008. [Google Scholar]
- Haefliger, P.; Mathieu-Nolf, M.; Lociciro, S.; Ndiaye, C.; Coly, M.; Diouf, A.; Faye, A.L.; Sow, A.; Tempowski, J.; Pronczuk, J.; Filipe Junior, A.P.; Bertollini, R.; Neira, M. Mass lead intoxication from informal used lead-acid battery recycling in Dakar, Senegal. Environ. Health Perspect. 2009, 117, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, L.; Elinder, C.G.; Dahlberg, B.; Lundberg, M.; Jarup, L.; Persson, B.; Axelson, O. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report on Inventorization of E-Waste in Two Cities in Andhra Pradesh and Karnataka (Hyderabad And Bangalore); WHO: Geneva, Switzerland, 2010. [Google Scholar]
- National Toxicology Program, Public Health Service, U.S. Department of Health and Human Services (DHHS). Report on Carcinogens, 11th ed.DHHS: Research Triangle Park, NC, USA, 2008.
- Osibanjo, O.; Nnorom, I.O.; Bakare, A.A.; Alabi, O.A. Environmental and public health consequences of adopting crude recovery techniques in e-waste management in developing countries: An emerging global crisis. In Advances in Environmental Research; Daniels, J.A., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; Vol. 171. [Google Scholar]
- Fiskesjo, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Marcos, C.; Radetski, A.-M.V.; Ferard, J.-F. Ecotoxicological assessment of solid waste: A combined liquid- and solid-phase testing approach using a battery of bioassays and biomarkers. Environ. Toxicol. Chem. 1999, 18, 1195–1202. [Google Scholar]
- Bakare, A.A.; Wale-Adeyemo, A.R. The mutagenic and cytotoxic effects of leacheates from domestic solid wastes and Aba-Eku landfill, Nigeria on Allium cepa. Nat. Environ. Pollut. Technol. 2004, 3, 455–462. [Google Scholar]
- Alabi, O.A.; Bakare, A.A.; Filippin-Monteiro, F.B.; Sierra, J.A.; Creczynski-Pasa, T.B. Electronic waste leachate-mediated DNA fragmentation and cell death by apoptosis in mouse fibroblast (NIH/3T3) cell line. Ecotoxicol. Environ. Saf. 2013, 94, 87–93. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babatunde, B.; Anabuike, F. In Vivo Cytogenotoxicity of Electronic Waste Leachate from Iloabuchi Electronic Market, Diobu, Rivers State, Nigeria on Allium Cepa. Challenges 2015, 6, 173-187. https://doi.org/10.3390/challe6010173
Babatunde B, Anabuike F. In Vivo Cytogenotoxicity of Electronic Waste Leachate from Iloabuchi Electronic Market, Diobu, Rivers State, Nigeria on Allium Cepa. Challenges. 2015; 6(1):173-187. https://doi.org/10.3390/challe6010173
Chicago/Turabian StyleBabatunde, Bolaji, and Felicity Anabuike. 2015. "In Vivo Cytogenotoxicity of Electronic Waste Leachate from Iloabuchi Electronic Market, Diobu, Rivers State, Nigeria on Allium Cepa" Challenges 6, no. 1: 173-187. https://doi.org/10.3390/challe6010173






