2.1. Effects of Repeated Exposure to Fine Particles on the Release of EGFR Ligands by Bronchial Epithelium
In order to test our hypothesis of a potential paracrine effect of epithelial secretome on lung fibroblasts, Wi-38 cells were exposed to basal culture media recovered when HBE cells were repeatedly exposed to fine PM as well as to basal culture media recovered in the weeks following the end of treatments.
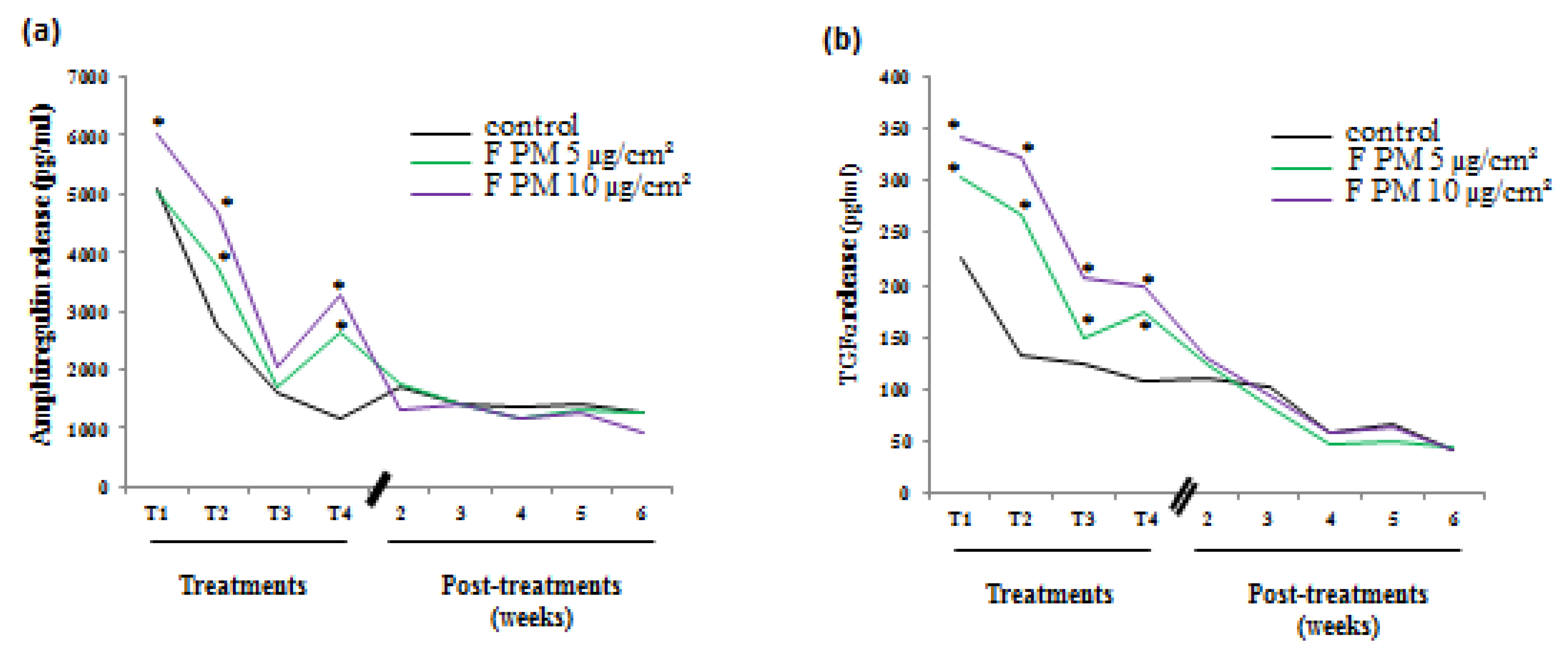
Figure 1 continues with part of the results we previously showed [
12] and, is shown to remind us of the characteristics of the conditioned culture media we used in the present study. Treatments were performed on undifferentiated HBE cells just after they started to be grown at the air-liquid interface (ALI). Four treatments, 48 hours apart, were done on the apical side of HBE cells and the release of EGFR ligands in the basal medium was measured by ELISA 48 hours after each treatment. We observed an increased and a dose-dependent release of two EGFR ligands (amphiregulin and TGF α) in PM-exposed cells compared to control cells after each treatment. The basal media collected during this treatment period were pooled for every condition in order to provide enough conditioned culture media to perform experiments with fibroblasts. At the end of the treatment period, cultures were maintained for the five following weeks and basal culture media were recovered and pooled for every week. We noticed no difference between control and PM-treated cultures considering the amphiregulin (
Figure 1a) and TGFα (
Figure 1b) release that were also less important as the culture was differentiating.
Figure 1.
Release of growth factor by human bronchial epithelial (HBE) cells induced by particulate matter (PM) exposure. Cells were exposed or not four times (48 hours between treatments) to fine PM (F PM) at 5 and 10 µg/cm². Growth factor release was measured in the basal medium 48 hours after each treatment (treatments) and during five weeks following the end of treatments (post-treatments). (a) Amphiregulin release; (b) TGF-α release. *: Different from the control (p < 0.05), n = 3.
Figure 1.
Release of growth factor by human bronchial epithelial (HBE) cells induced by particulate matter (PM) exposure. Cells were exposed or not four times (48 hours between treatments) to fine PM (F PM) at 5 and 10 µg/cm². Growth factor release was measured in the basal medium 48 hours after each treatment (treatments) and during five weeks following the end of treatments (post-treatments). (a) Amphiregulin release; (b) TGF-α release. *: Different from the control (p < 0.05), n = 3.
2.2. Effect of Conditioned Medium on Fibroblast Proliferation
The ability of the secretome produced by PM-treated HBE cells to promote lung fibroblast proliferation was investigated by counting cells after 48‑72 hours of treatment with conditioned culture media from control and PM-treated HBE cells as well as with fresh bronchial epithelial growth medium (BEGM), the culture medium used to cultivate HBE cells. In
Figure 2 we only reported results obtained with Wi-38 cells exposed to conditioned media from HBE cells recovered during the treatment period, as those recovered during the post-treatment period had no effect. Wi-38 cells exposed to conditioned medium from control HBE cells exhibited two doublings of the cell population at 48 hours but the growth rate decreased at 72 hours compared to cells exposed to fresh BEGM medium suggesting that the proliferation cannot be sustained due to nutrient depletion (
Figure 2a). Wi-38 cells exposed to conditioned medium from fine PM-treated HBE cells showed a significantly higher growth rate than Wi-38 cells exposed to conditioned medium from control HBE cells suggesting the presence of a sufficient amount of growth factors to promote cell proliferation. The growth rate was similar to the one induced by fresh BEGM medium for all used concentrations of fine PM-treatment at 48 hours. But it decreased at 72 hours with a significant effect only for the conditioned medium from HBE cells treated with fine PM at 10 µg/cm². These data suggest a growth factor/nutrient depletion over time (
Figure 2a). The decrease of growth rate for fibroblasts grown in conditioned medium recovered from HBE cells treated with fine PM at 5 µg/cm² was not associated with cell death as viability was 88% whereas for cells grown in conditioned medium recovered from control HBE cells it was lower (76%) (
Figure 2b).
Figure 2.
Effects of conditioned media on fibroblast (Wi-38) proliferation. Wi-38 cells were exposed to conditioned media recovered from normal human bronchial epithelial (NHBE) cells exposed or not (control) four times to fine (F) particles at 5 and 10 µg/cm² as well as to fresh bronchial epithelial growth medium (BEGM) medium. (a) Growth rate of Wi-38 cells at 48 hours and 72 hours exposure to conditioned medium or BEGM medium. (b) Viability of Wi-38 cells at 72 hours exposure to conditioned media. *: Different from the control (p < 0.05), n = 3.
Figure 2.
Effects of conditioned media on fibroblast (Wi-38) proliferation. Wi-38 cells were exposed to conditioned media recovered from normal human bronchial epithelial (NHBE) cells exposed or not (control) four times to fine (F) particles at 5 and 10 µg/cm² as well as to fresh bronchial epithelial growth medium (BEGM) medium. (a) Growth rate of Wi-38 cells at 48 hours and 72 hours exposure to conditioned medium or BEGM medium. (b) Viability of Wi-38 cells at 72 hours exposure to conditioned media. *: Different from the control (p < 0.05), n = 3.
Moreover immunolabeling of vimentin network was performed on fibroblasts exposed to conditioned media for 72 hours in order to appreciate fibroblast phenotype.
Figure 3 shows the characteristic vimentin network in fibroblasts when grown with fresh BEGM (
Figure 3a and b), while it was disrupted in fibroblasts grown with the conditioned medium from control HBE cells (
Figure 3c and d), likely reflecting nutrient deficiencies in this conditioned medium emphasized by the decrease of growth rate (
Figure 2a). By contrast, fibroblasts grown with conditioned medium from treated-HBE cells (
Figure 3e to 3h) exhibited an intermediate situation.
Altogether these experiments showed that conditioned media from fine PM-treated HBE cells improved the growth rate and preserved vimentin cytoskeleton of lung fibroblasts suggesting the presence of growth factors in conditioned media that could contribute to fibroblast proliferation.
Figure 3.
Wi-38 cells observation by confocal microscopy. Cells were grown during 72 hours with BEGM (a and b), conditioned medium from control HBE cells (c and d), conditioned medium from HBE cells exposed to fine (F) particles at 5 µg/cm² (e and f) or 10 µg/cm² (g and h). Cells were immunolabeled with anti-vimentin (green) antibody to label intermediate filaments, and nuclei were revealed in blue with 4',6-Diamidino-2-Phenylindole (DAPI).
Figure 3.
Wi-38 cells observation by confocal microscopy. Cells were grown during 72 hours with BEGM (a and b), conditioned medium from control HBE cells (c and d), conditioned medium from HBE cells exposed to fine (F) particles at 5 µg/cm² (e and f) or 10 µg/cm² (g and h). Cells were immunolabeled with anti-vimentin (green) antibody to label intermediate filaments, and nuclei were revealed in blue with 4',6-Diamidino-2-Phenylindole (DAPI).
2.3. Role of EGFR Ligands on Fibroblast Proliferation
Among growth factors with a mitogenic effect on fibroblasts, EGFR ligands such as amphiregulin have been shown to induce fibroblast proliferation [
9,
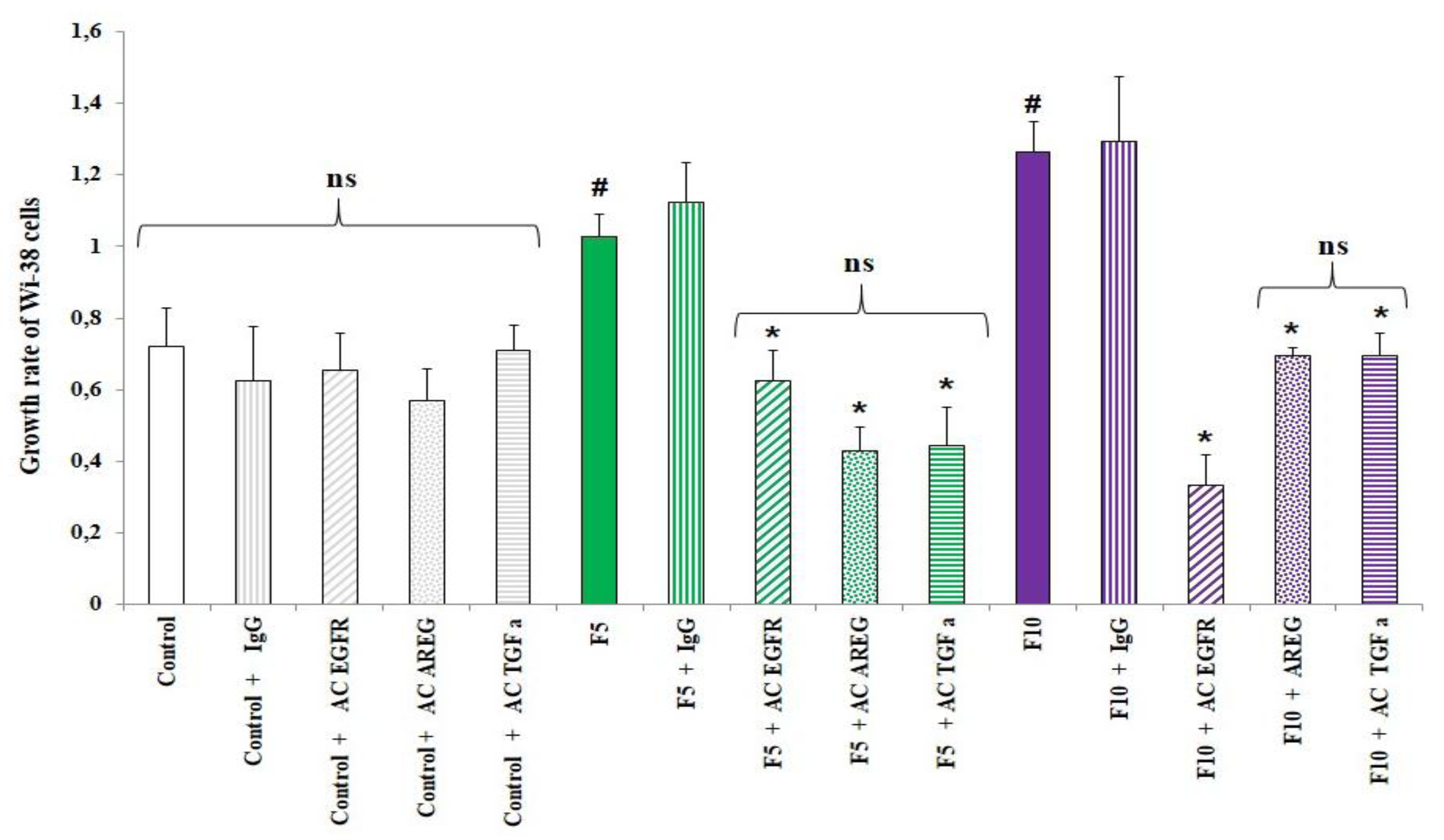
10]. To determine whether EGFR ligands, especially TGFα and amphiregulin, which we had proved to be present in conditioned media, were involved in fibroblast proliferation, we exposed WI-38 cells to conditioned media supplemented or not with anti-EGFR (preventing the binding of EGFR ligands to the receptor), anti-amphiregulin, anti-TGFα or an anti-mouse IgG as an unspecific antibody. As shown in
Figure 4, the presence of any of these antibodies modulated the growth rate of WI-38 cells treated with conditioned medium from control HBE cells. The significant increase of growth rate for Wi-38 cells exposed to conditioned media from fine PM-treated HBE cells at 5 and 10 µg/cm² was abolished when anti-EGFR was added in conditioned media but not when a control antibody (IgG) was used, underlying the involvement of EGFR and its ligands in fibroblast proliferation. In order to identify which EGFR ligand could be involved in Wi-38 proliferation, the experiment was also performed in the presence of anti-TGFα or anti-AREG in the conditioned medium. The mitogenic effect was significantly reduced whatever the antibody used and whatever the conditioned medium from fine PM treated HBE cells.
Altogether these results suggest that amphiregulin and TGFα present in the secretome of treated HBE cells could contribute to fibroblast proliferation.
Figure 4.
Involvement of anti-epidermal growth factor receptor (EGFR) ligands (amphiregulin and TGFα) in fibroblast proliferation. Wi-38 cells were treated with conditioned media from control HBE cells or HBE cells treated with fine PM (5 μg/cm², 10 µg/cm²) supplemented or not with anti-EGFR, anti-AREG, anti-TGFα or anti-mouse IgG. Wi-38 cell counting was performed 72 hours later. Cell proliferation ofWi-38 cells was expressed as growth rate. #: Different from control (p < 0.05), *: Different from the condition without antibodies, n = 3.
Figure 4.
Involvement of anti-epidermal growth factor receptor (EGFR) ligands (amphiregulin and TGFα) in fibroblast proliferation. Wi-38 cells were treated with conditioned media from control HBE cells or HBE cells treated with fine PM (5 μg/cm², 10 µg/cm²) supplemented or not with anti-EGFR, anti-AREG, anti-TGFα or anti-mouse IgG. Wi-38 cell counting was performed 72 hours later. Cell proliferation ofWi-38 cells was expressed as growth rate. #: Different from control (p < 0.05), *: Different from the condition without antibodies, n = 3.
2.4. Effect of Different Particle Size on Fibroblast Proliferation
Health effects of ambient PM are related to their size: the finest ones can go deeper in the lungs and their chemical composition is different according to the size fraction (the finest one have a larger carbonaceous content) [
13]. Our previous studies performed on bronchial epithelial cell lines demonstrated a higher pro-inflammatory effect of fine and ultrafine particles compared to coarse ones for particles coming from rural sites, traffic or background urban sites in France as well as African cities [
14,
15,
16]. This was also observed for HBE cells repeatedly exposed to fine, ultrafine and coarse PM [
12]. In order to determine whether such differences could also be observed on proliferation of fibroblasts, we compared the effects of conditioned media recovered during repeated treatments of HBE cells to either coarse, fine or ultrafine PM at 5 µg/cm². As shown in
Figure 5, a significant increase of fibroblast number was observed for Wi-38 cultures treated with conditioned media from HBE cultures exposed to either fine PM or ultrafine PM. By contrast the number of Wi-38 cells when grown with conditioned medium from HBE cultures exposed to coarse PM, was not significantly different from the one of cultures treated with conditioned media from control HBE cultures. However, the proliferative potential of these conditioned media was lower compared to fresh BEGM medium.
Figure 5.
Effect of conditioned media on fibroblast (Wi-38) proliferation. Cells were exposed for 72 hours to conditioned medium from control HBE cells or HBE cells exposed four times (48 hours between treatments) to coarse (C), fine (F) and ultrafine (UF) PM at 5 µg/cm², or to fresh BEGM medium. *: Different from the control (p < 0.05), n = 3.
Figure 5.
Effect of conditioned media on fibroblast (Wi-38) proliferation. Cells were exposed for 72 hours to conditioned medium from control HBE cells or HBE cells exposed four times (48 hours between treatments) to coarse (C), fine (F) and ultrafine (UF) PM at 5 µg/cm², or to fresh BEGM medium. *: Different from the control (p < 0.05), n = 3.
2.5. Effect of Epithelial Secretion on Fibroblast Proliferation in Co-Culture
To alternatively evaluate the paracrine effect of epithelial secretome on lung fibroblasts, co-cultures allowing the attainment of more realistic exposure conditions were carried out. Moreover it enables investigation of whether a paracrine effect can be also observed using differentiated NHBE cells. HBE cells were grown at ALI on inserts and fibroblasts were grown on the bottom of the culture plate where the inserts are placed and were submerged in BEGM medium. HBE were apically treated with fine PM at 5 µg/cm² and Wi-38 cells proliferation was measured over four days following the treatment. The experiment was firstly performed on undifferentiated HBE cells in the first week of ALI culture condition and renewed on differentiated HBE cells in the sixth week of ALI culture condition in order to assess whether fibroblast response was dependent on HBE differentiation.
Firstly we measured the amphiregulin release by HBE cells induced by fine PM exposure, whether HBE cells were grown alone or in co-culture with fibroblasts for 48 hours or 72 hours. Constitutive amphiregulin release was higher when HBE cells were cultured with Wi-38 (2,500 pg/mL at 48 hours and 3,000 pg/mL at 72 hours) than when cultured alone (1,500 pg/mL at 48 and 72 hours) (
Figure 6a). After exposure to fine PM, a significant increase of amphiregulin release (compared to control cells) was observed when HBE were grown alone but not when co-cultured with fibroblasts (
Figure 6a), confirming results shown in
Figure 1. This could be explained by the consumption of amphiregulin by Wi-38 cells. This hypothesis was strengthened by the observation of a significant increase of fibroblast growth rate at 72 hours (
Figure 6c).
For differentiated HBE cell cultures (sixth week in ALI), here again we observed that the constitutive release of amphiregulin is higher in co-culture conditions and that the PM-induced amphiregulin release at 72 hours was only significant when HBE cells were grown alone (
Figure 6b). As for undifferentiated HBE cells, the number of fibroblasts was more important when co-cultured for 96 hours with HBE cells exposed to PM compared to co-culture with control HBE cells (
Figure 6d).
Figure 6.
HBE cells-fibroblasts co-culture. (a and b) Amphiregulin release induced by exposure to fine PM of HBE cells co-cultured or not with fibroblasts. HBE cells were once exposed or not to fine PM at 5 µg/cm² in the 1st and 6th week of culture. Amphiregulin release was measured at 48 hours and 72 hours post-exposure in the HBE basal medium grown alone or co-cultured with fibroblasts. (c and d) growth rate of Wi-38 cells co-cultured with HBE cells treated or not with fine PM at 5 µg/cm². *: Different from control at the same time (p < 0.05) #: Different from control (p < 0.05), n = 3.
Figure 6.
HBE cells-fibroblasts co-culture. (a and b) Amphiregulin release induced by exposure to fine PM of HBE cells co-cultured or not with fibroblasts. HBE cells were once exposed or not to fine PM at 5 µg/cm² in the 1st and 6th week of culture. Amphiregulin release was measured at 48 hours and 72 hours post-exposure in the HBE basal medium grown alone or co-cultured with fibroblasts. (c and d) growth rate of Wi-38 cells co-cultured with HBE cells treated or not with fine PM at 5 µg/cm². *: Different from control at the same time (p < 0.05) #: Different from control (p < 0.05), n = 3.
In order to determine whether the epithelial secretome could also influence cell differentiation in addition to promoting proliferation, we studied the mRNA expression of pro-collagen I and α-smooth muscle actin and did not find significant induction using either conditioned media or co-culture (data not shown).
Altogether these results suggest that the proliferation of lung fibroblasts is associated with the mitogenic effect of the epithelial secretome. However we cannot exclude a direct effect of particles on fibroblast cells. It has been reported in
in vivo studies that particles in the nanosized range could quickly translocate through pulmonary epithelium reaching the bloodstream [
17]. For our cultures, we used inserts with membranes of a low porosity (0,4 µm) that are essential to obtain an accurate differentiation of HBE cells, however other studies from our laboratory [
18] showed it unlikely that fine PM could cross a porous membrane. However to avoid a possible direct effect of particles, conditioned media were centrifuged before treatment of Wi-38 cells in order to eliminate possibly present particles. In addition, to determine whether soluble compounds of PM could contribute to fibroblast proliferation, we investigated the effect of an aqueous extract of fine PM and found that it had no effect on Wi-38 proliferation (data not shown).
Our demonstration of the role of EGFR ligands in fibroblast proliferation is in line with
in vivo experiments performed on transgenic adult mice over-expressing TGFα that developed pulmonary fibrosis in the absence of any inflammation or activation of TGFβ, a well-known and powerful fibrotic factor [
19]. This fibrosis is characterized by an increased fibroblast proliferation and collagen deposition that was reduced when mice were treated with inhibitors of the tyrosine kinase activity of EGFR [
20].
Thanks to our
in vitro approach, we demonstrated that the epithelial secretome from particle-treated epithelium exhibited a fibrotic potential underlining that the epithelium can contribute to the fibrotic process by stimulating fibroblast hyperplasia. Fibrosis can also result from epithelial to mesenchymal transition [
21]. It was very recently demonstrated that ultrafine particles containing radicals initiate such a transition in airway epithelial cells [
22]. Our further investigations will explore such a possibility.