Human Genotoxic Study Carried Out Two Years after Oil Exposure during the Clean-up Activities Using Two Different Biomarkers
Abstract
:1. Introduction
2. Material and Methods
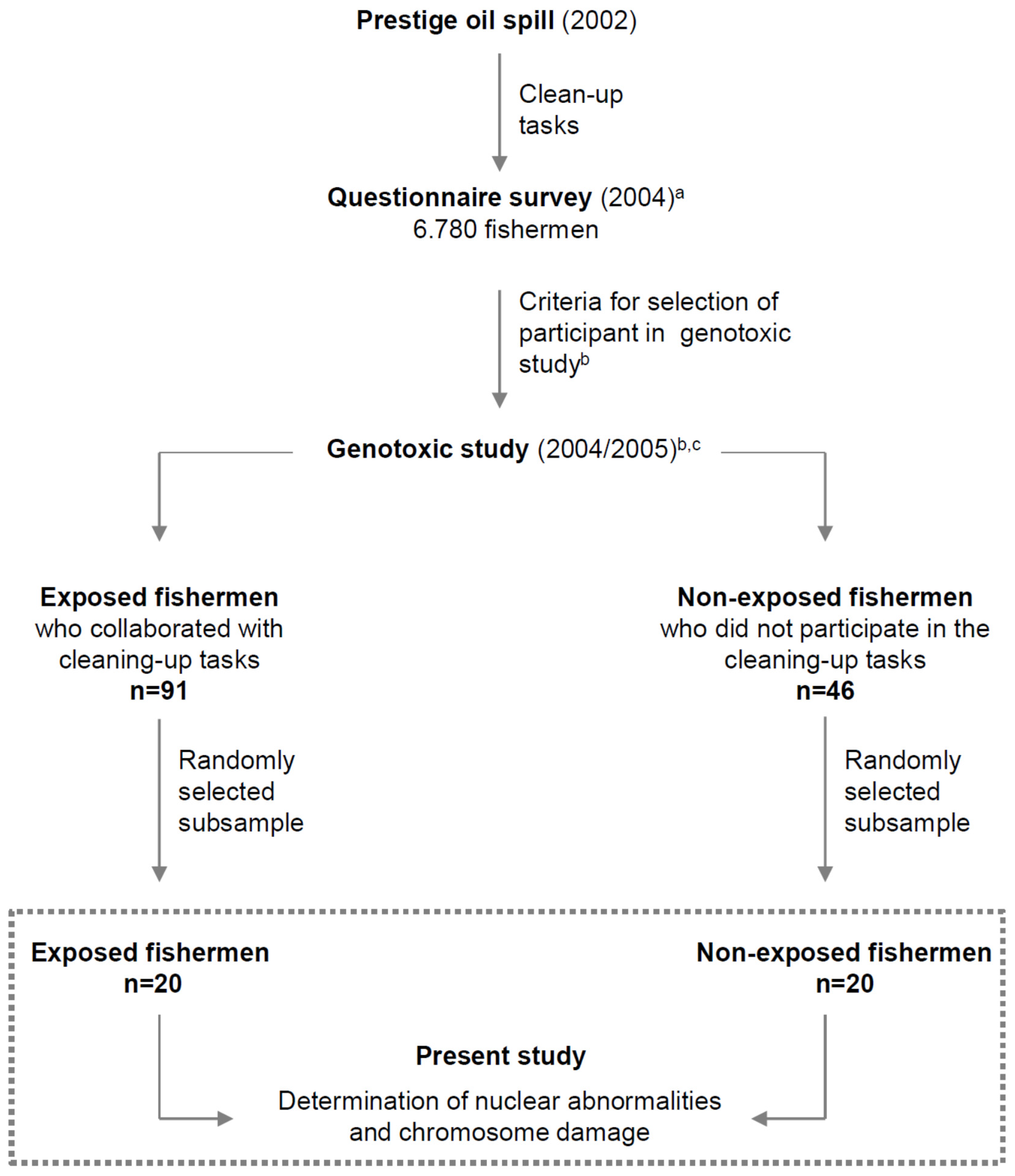
2.1. Study Population
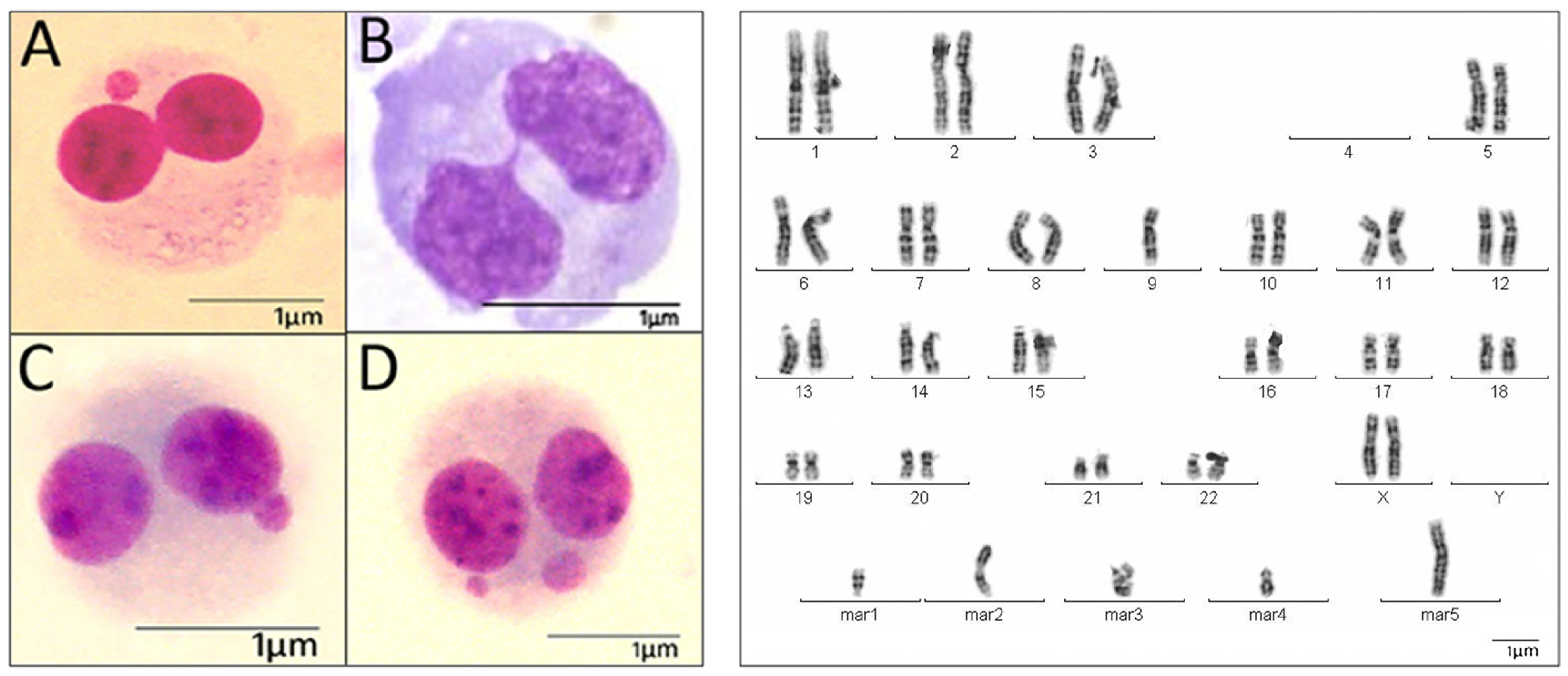
2.2. Cytogenetic Analysis
2.3. Statistical Analysis
3. Results
| Exposed | Non-Exposed | p-Value | |
|---|---|---|---|
| Total Individuals, No. | 20 | 20 | |
| Total Binucleate Cells, No. | 20.000 | 20.000 | |
| Binucleated cells with micronucleus, No. (%) | 457 (2.3) | 514 (2.6) | 0.4774 |
| 1 micronucleus/cell, No. | 399 | 450 | |
| 2 micronucleus/cell, No. | 49 | 53 | |
| 3 micronucleus/cell, No. | 9 | 11 | |
| Nucleplasmic bridges, No. (%) | 131 (0.65) | 98 (0.49) | 0.08 |
| Nuclear buds, No. (%) | 106 (0.53) | 68 (0.34) | 0.2356 |
| Total Metaphases Analyzed (Uniform Stain), No. | 2112 | 2.148 | |
| Chromosome lesion, No. (%) | 28 (1.3) | 7 (0.3) | 0.0231 |
| Total Metaphases Karyotyped (G-Banded), No. | 537 | 563 | |
| Structural chromosome alterations, No. (%) | 36 (6.7) | 16 (2.8) | 0.0972 |
| Balanced, No. | 1 | 3 | |
| Unbalanced, No. | 35 | 13 |
| Type of individuals | Binucleate cells | Chromosomal Lesions | Structural Chromosomal Alteration | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Cells | MN | NBUP | NPB | Cells | Lesions | Karyotypes | Structural Alterations | Balanced Structural Alterations | Unbalanced Structural Alterations | Type of Structural Alteration | |||||
| MN | 0 | 1 | 2 | ≥3 | |||||||||||||
| Exposed | |||||||||||||||||
| E1 | woman | 49.02 | 1000 | 29 | 971 | 27 | 2 | 0 | 4 | 7 | 100 | 1 | 27 | 5 | 0 | 5 | t(13;20)(q32;q12); t(9;?), t(10;18),mar; del(2)(q12) |
| E2 | man | 54,01 | 1000 | 23 | 977 | 20 | 3 | 0 | 2 | 4 | 116 | 3 | 28 | 0 | 0 | 0 | |
| E3 | man | 44,02 | 1000 | 21 | 979 | 19 | 2 | 0 | 5 | 3 | 105 | 6 | 29 | 1 | 0 | 1 | ace |
| E4 | man | 31,26 | 1000 | 17 | 983 | 16 | 1 | 0 | 7 | 1 | 105 | 2 | 25 | 2 | 0 | 2 | ace,ace |
| E5 | woman | 51,90 | 1000 | 25 | 975 | 22 | 3 | 0 | 11 | 8 | 103 | 0 | 26 | 0 | 0 | 0 | |
| E6 | man | 35,92 | 1000 | 17 | 983 | 16 | 1 | 0 | 3 | 8 | 108 | 1 | 30 | 2 | 0 | 2 | ace; add(12)(qter) |
| E7 | woman | 49,62 | 1000 | 43 | 957 | 37 | 4 | 2 | 9 | 5 | 100 | 2 | 25 | 6 | 0 | 6 | ace; t(4;16)(q13,p13.3); mar,mar,mar,mar |
| E8 | man | 56,45 | 1000 | 43 | 957 | 37 | 4 | 2 | 19 | 6 | 102 | 1 | 29 | 0 | 0 | 0 | |
| E9 | woman | 50,51 | 1000 | 11 | 989 | 9 | 2 | 0 | 16 | 14 | 100 | 6 | 26 | 1 | 0 | 1 | ace |
| E10 | man | 52,50 | 1000 | 9 | 991 | 8 | 1 | 0 | 2 | 17 | 102 | 1 | 26 | 0 | 0 | 0 | |
| E11 | woman | 54,86 | 1000 | 32 | 968 | 24 | 8 | 0 | 4 | 5 | 110 | 0 | 25 | 0 | 0 | 0 | |
| E12 | woman | 44,89 | 1000 | 37 | 963 | 30 | 5 | 2 | 12 | 2 | 112 | 0 | 26 | 1 | 0 | 1 | mar |
| E13 | man | 57,02 | 1000 | 18 | 982 | 18 | 0 | 0 | 9 | 3 | 100 | 1 | 31 | 1 | 1 | t(13;11)(p25;q23) | |
| E14 | woman | 46,13 | 1000 | 18 | 982 | 16 | 1 | 1 | 7 | 3 | 104 | 1 | 26 | 1 | 0 | 1 | mar |
| E15 | woman | 62,17 | 1000 | 14 | 986 | 11 | 3 | 0 | 4 | 3 | 103 | 0 | 25 | 3 | 0 | 3 | t(7;10); r, r |
| E16 | woman | 48,99 | 1000 | 10 | 990 | 8 | 2 | 0 | 1 | 1 | 112 | 0 | 25 | 1 | 0 | 1 | t(X;4)(q21;p16) |
| E17 | woman | 58,37 | 1000 | 8 | 992 | 8 | 0 | 0 | 1 | 3 | 105 | 0 | 27 | 1 | 0 | 1 | del(7)(q33) |
| E18 | woman | 36,90 | 1000 | 31 | 969 | 26 | 3 | 2 | 4 | 4 | 112 | 0 | 30 | 1 | 0 | 1 | mar |
| E19 | man | 52,98 | 1000 | 38 | 962 | 34 | 4 | 0 | 5 | 6 | 106 | 2 | 26 | 1 | 0 | 1 | del(7)(p15) |
| E20 | man | 54,94 | 1000 | 13 | 987 | 13 | 0 | 0 | 6 | 3 | 107 | 1 | 25 | 9 | 0 | 9 | mar,mar; ace, ace; mar, mar,mar,mar,mar |
| 20000 | 457 | 19543 | 399 | 49 | 9 | 131 | 106 | 2112 | 28 | 537 | 36 | 1 | 35 | ||||
| Non-Exposed | |||||||||||||||||
| NE1 | woman | 54,33 | 1000 | 22 | 978 | 19 | 3 | 0 | 3 | 0 | 103 | 0 | 25 | 0 | 0 | 0 | |
| NE2 | woman | 50,60 | 1000 | 22 | 978 | 17 | 4 | 1 | 0 | 0 | 100 | 3 | 34 | 0 | 0 | 0 | |
| NE3 | woman | 57,52 | 1000 | 13 | 987 | 10 | 2 | 1 | 4 | 2 | 105 | 0 | 25 | 4 | 0 | 4 | mar,mar,mar,mar |
| NE4 | woman | 57,19 | 1000 | 14 | 986 | 13 | 1 | 0 | 16 | 0 | 101 | 1 | 25 | 1 | 0 | 1 | del(1)(q21) |
| NE5 | man | 58,78 | 1000 | 22 | 978 | 19 | 2 | 1 | 1 | 8 | 108 | 0 | 26 | 3 | 0 | 3 | del(2)(q21); del(1)(q23), mar |
| NE6 | woman | 57,61 | 1000 | 59 | 941 | 52 | 6 | 1 | 2 | 6 | 100 | 0 | 25 | 1 | 1 | 0 | t(13;14)(q14,q32) |
| NE7 | woman | 36,56 | 1000 | 5 | 995 | 3 | 2 | 0 | 5 | 7 | 107 | 0 | 25 | 1 | 0 | 1 | del(1)(q32) |
| NE8 | woman | 53,22 | 1000 | 30 | 970 | 26 | 3 | 1 | 1 | 7 | 110 | 0 | 33 | 1 | 0 | 1 | mar |
| NE9 | woman | 58,58 | 1000 | 50 | 950 | 43 | 6 | 1 | 5 | 1 | 113 | 0 | 43 | 0 | 0 | 0 | |
| NE10 | woman | 56,00 | 1000 | 26 | 974 | 22 | 1 | 3 | 9 | 1 | 107 | 0 | 26 | 0 | 0 | 0 | |
| NE11 | woman | 56,85 | 1000 | 10 | 990 | 10 | 0 | 0 | 3 | 4 | 120 | 1 | 25 | 1 | 0 | 1 | mar |
| NE12 | woman | 46,36 | 1000 | 38 | 962 | 32 | 5 | 1 | 9 | 5 | 130 | 0 | 26 | 0 | 0 | 0 | |
| NE13 | woman | 55,82 | 1000 | 12 | 988 | 10 | 2 | 0 | 2 | 6 | 101 | 0 | 25 | 0 | 0 | 0 | |
| NE14 | woman | 45,84 | 1000 | 26 | 974 | 24 | 2 | 0 | 8 | 4 | 108 | 2 | 25 | 0 | 0 | 0 | |
| NE15 | man | 48,56 | 1000 | 41 | 959 | 33 | 7 | 1 | 8 | 3 | 107 | 0 | 25 | 0 | 0 | 0 | |
| NE16 | woman | 55,54 | 1000 | 23 | 977 | 23 | 0 | 0 | 5 | 2 | 102 | 0 | 26 | 0 | 0 | 0 | |
| NE17 | woman | 58,62 | 1000 | 36 | 964 | 32 | 4 | 0 | 8 | 4 | 112 | 0 | 26 | 1 | 0 | 1 | del(9)(q21) |
| NE18 | man | 57,15 | 1000 | 38 | 962 | 37 | 1 | 0 | 6 | 3 | 105 | 0 | 43 | 0 | 0 | 0 | |
| NE19 | woman | 49,98 | 1000 | 18 | 982 | 16 | 2 | 0 | 3 | 1 | 108 | 0 | 26 | 2 | 1 | 1 | t(8;13)((q24.1;q31); t(2;5) |
| NE20 | woman | 46,31 | 1000 | 9 | 991 | 9 | 0 | 0 | 0 | 4 | 101 | 0 | 29 | 1 | 1 | 0 | t(3;8)(q27;q13) |
| 20000 | 514 | 19486 | 450 | 53 | 11 | 98 | 68 | 2148 | 7 | 563 | 16 | 3 | 13 | ||||
4. Discussion
5. Conclusions
- nuclear abnormalies (micronucleus, nucleoplasmic bridges and nuclear buds) in binucleated cells may not detect genotoxic effects more than two years after acute oil exposure when the toxic agent is no longer present;
- chromosome damage (chromosome lesion and structural chromosome alterations) in metaphases cells is a useful biomarker for assessing genotoxic effect two years after acute oil exposure using the same peripheral blood extraction in which nuclear abnormalies were analyzed; and
- comparative study using nuclear abnormalies and chromosome damage analyses emphasizes the need to use appropriate biomarker for detection of genotoxic effect in individuals involved in toxic accidents.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aguilera, F.; Méndez, J.; Pásaro, E.; Laffon, B. Review of the effects of exposure to spilled oils on human health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.D.; Osofsky, H.J.; Lichtveld, M.Y. The Gulf oil spill. N. Engl. J. Med. 2011, 364, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- IARC. Occupational Exposures in Petroleum Refining: Crude Oil and Major Petroleum Fuels IARC. Monographs on the Evaluations of Carcinogenic Risk to Humans; International Agency for Research on Cancer: Lyon, France, 1989; Volume 45. [Google Scholar]
- Clare, M.G.; Yardley-Jones, A.; Maclean, A.C.; Dean, B.J. Chromosome analysis from peripheral blood lymphocytes of workers after an acute exposure to benzene. Br. J. Ind. Med. 1984, 41, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Beare, D.M.; Waugh, A.P.; Capulas, E.; Aldridge, K.E.; Arlett, C.F.; Green, M.H.; Crum, J.E.; Cox, D.; Garner, R.C.; et al. Biomonitoring of possible human exposure to environmental genotoxic chemicals: Lessons from a study following the wreck of the oil tanker Braer. Environ. Mol. Mutagen. 1997, 30, 97–111. [Google Scholar] [CrossRef]
- Laffon, B.; Fraga-Iriso, R.; Perez-Cadahia, B.; Méndez, J. Genotoxicity associated to exposure to Prestige oil during autopsies and cleaning of oil-contaminated birds. Food Chem. Toxicol. 2006, 44, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cadahia, B.; Laffon, B.; Pasaro, E.; Méndez, J. Genetic damage induced by accidental environmental pollutants. Sci. World J. 2006, 6, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadahía, B.; Lafuente, A.; Cabaleiro, T.; Pasaro, E.; Méndez, J.; Laffon, B. Initial study on the effects of Prestige oil on human health. Environ. Int. 2007, 33, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadahía, B.; Laffon, B.; Porta, M. Relationship between blood concentrations of heavy metals and cytogenetic and endocrine parameters among subjects involved in cleaning coastal areas affected by the Prestige tanker oil spill. Chemosphere 2008, 7, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadahía, B.; Laffon, B.; Valdiglesias, V.; Pasaro, E.; Mendez, J. Cytogenetic effects induced by Prestige oil on human populations: The role of polymorphisms in genes involved in metabolism and DNA repair. Mutat. Res. 2008, 653, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cadahia, B.; Mendez, J.; Pasaro, E.; Lafuente, A.; Cabaleiro, T.; Laffon, B. Biomonitoring of human exposure to Prestige oil: Effects on DNA and endocrine parameters. Environ. Health Insights 2008, 2, 83–92. [Google Scholar] [PubMed]
- Rodríguez-Trigo, G.; Zock, J.P.; Pozo-Rodríguez, F.; Gómez, F.P.; Monyarch, G.; Bouso, L.; Coll, M.D.; Verea, H.; Antó, J.M.; Fuster, C.; et al. SEPAR-Prestige Study Group. Health changes in fishermen 2 years after clean-up of the Prestige oil spill. Ann. Intern. Med. 2010, 153, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Monyarch, G.; de Castro-Reis, F.; Zock, J.P.; Giraldo, J.; Pozo-Rodríguez, F.; Espinosa, A.; Rodríguez-Trigo, G.; Verea, H.; Castaño-Vinyals, G.; Gómez, F.P.; et al. Chromosomal bands affected by acute oil exposure and DNA repair errors. PLoS ONE 2013, 8, e81276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildur, K.; Templado, C.; ZocK, J.P.; Giraldo, J.; Pozo-Rodríguez, F.; Frances, A.; Monyarch, G.; Rodríguez-Trigo, G.; Rodriguez-Rodriguez, E.; Souto, A.; et al. Follow-up genotoxic study: Chromosome damage two and six years after exposure to the Prestige oil spill. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Laffon, B.; Aguilera, F.; Ríos-Vázquez, J.; Valdiglesias, V.; Pásaro, E. Follow-up study of genotoxic effects in individuals exposed to oil from the tanker Prestige, seven years after the accident. Mutat. Res. 2014, 760, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mateuca, R.; Lombaert, N.; Aka, P.V.; Decordier, I.; Kirsch-Volders, M. Chromosomal changes: Induction, detection methods and applicability in human biomonitoring. Biochimie 2006, 88, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.; Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef] [PubMed]
- DeMarini, D.M. Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: A review. Mutagenesis 2013, 28, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Rossnerova, A.; Sram, R.; Romm, H.; Bolognesi, C.; Ramakumar, A.; Soussaline, F.; Schunck, C.; Elhajouji, A.; et al. HUMN project initiative and review of validation, quality control and prospects for further development of automated micronucleus assays using image cytometry systems. Int. J. Hyg. Environ. Health 2013, 216, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Norppa, H.; Bonassi, S.; Hansteen, I.L.; Hagmar, L.; Strömberg, U.; Rössner, P.; Boffetta, P.; Lindholm, C.; Gundy, S.; Lazutka, J.; et al. Chromosomal aberrations and SCEs as biomarkers of cancer risk. Mutat. Res. 2006, 600, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Norppa, H.; Ceppi, M.; Strömberg, U.; Vermeulen, R.; Znaor, A.; Cebulska-Wasilewska, A.; Fabianova, E.; Fucic, A.; Gundy, S.; et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: Results from a pooled cohort study of 22,358 subjects in 11 countries. Carcinogenesis 2008, 29, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kirsch-Volders, M.; Bonassi, S.; Knasmueller, S.; Holland, N.; Bolognesi, C.; Fenech, M.F. Commentary: Critical questions, misconceptions and a road map for improving the use of the lymphocyte cytokinesis-block micronucleus assay for in vivo biomonitoring of human exposure to genotoxic chemicals-A HUMN project perspective. Mutat. Res. 2014, 759, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bugarín, O.; Zavala-Cerna, M.G.; Nava, A.; Flores-García, A.; Ramos-Ibarra, M.L. Potential Uses, Limitations, and Basic Procedures of Micronuclei and Nuclear Abnormalities in Buccal Cells. Dis. Markers 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Lorenzo, Y.; Haug, K.; Nicolaissen, B.; Valverde, M. Epithelial cells as alternative human biomatrices for comet assay. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.; Tough, D.G. Turnover of native and memry phenotype T cells. J. Exp. Med. 1994, 179, 1127–1135. [Google Scholar]
- Dusinska, M.; Collins, A.R. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Dhawan, A.; Laubenthal, J. The comet assay in human biomonitoring. Methods Mol. Biol. 2013, 1044, 347–362. [Google Scholar] [PubMed]
- Zock, J.P.; Rodriguez-Trigo, G.; Pozo-Rodriguez, F.; Barberà, J.A.; Bouso, L.; Torralba, Y.; Antó, J.M.; Gómez, F.P.; Fuster, C.; Verea, H.; et al. Prolonged respiratory symptoms in clean-up workers of the Prestige oil spill. Am. J. Respir. Crit. Care Med. 2007, 176, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- ISCN. An International System for Human Cytogenetic Nomenclature; Shafer, L.G., McGowan-Jordan, J., Schmid, M., Eds.; S. Karger: Basel, Switzerland, 2013. [Google Scholar]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- De la Chica, R.A.; Ribas, I.; Giraldo, J.; Egozcue, J.; Fuster, C. Chromosomal instability in amniocytes from fetuses of mothers who smoke. JAMA 2005, 293, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Chromosomes and cancer cells. Chromosome Res. 2011, 19, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, N.; Umeki, S.; Hirai, Y.; Akiyama, M.; Kodama, T.; Ohama, K.; Kyoizumi, S. Stimulated rapid expression in vitro for early detection of in vivo T-cell receptor mutations induced by radiation exposure. Mutat. Res. 1997, 390, 269–282. [Google Scholar] [CrossRef]
- Littlefield, L.G.; Joiner, E.E. Analysis of chromosome aberrations in lymphocytes of long-term heavy smokers. Mutat. Res. 1986, 170, 145–150. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biern, G.; Giraldo, J.; Zock, J.-P.; Monyarch, G.; Espinosa, A.; Rodríguez-Trigo, G.; Gómez, F.; Pozo-Rodríguez, F.; Barberà, J.-A.; Fuster, C. Human Genotoxic Study Carried Out Two Years after Oil Exposure during the Clean-up Activities Using Two Different Biomarkers. J. Mar. Sci. Eng. 2015, 3, 1334-1348. https://doi.org/10.3390/jmse3041334
Biern G, Giraldo J, Zock J-P, Monyarch G, Espinosa A, Rodríguez-Trigo G, Gómez F, Pozo-Rodríguez F, Barberà J-A, Fuster C. Human Genotoxic Study Carried Out Two Years after Oil Exposure during the Clean-up Activities Using Two Different Biomarkers. Journal of Marine Science and Engineering. 2015; 3(4):1334-1348. https://doi.org/10.3390/jmse3041334
Chicago/Turabian StyleBiern, Gloria, Jesús Giraldo, Jan-Paul Zock, Gemma Monyarch, Ana Espinosa, Gema Rodríguez-Trigo, Federico Gómez, Francisco Pozo-Rodríguez, Joan-Albert Barberà, and Carme Fuster. 2015. "Human Genotoxic Study Carried Out Two Years after Oil Exposure during the Clean-up Activities Using Two Different Biomarkers" Journal of Marine Science and Engineering 3, no. 4: 1334-1348. https://doi.org/10.3390/jmse3041334