Domestication of Marine Fish Species: Update and Perspectives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Evolution of Global Marine Fish Aquaculture Production
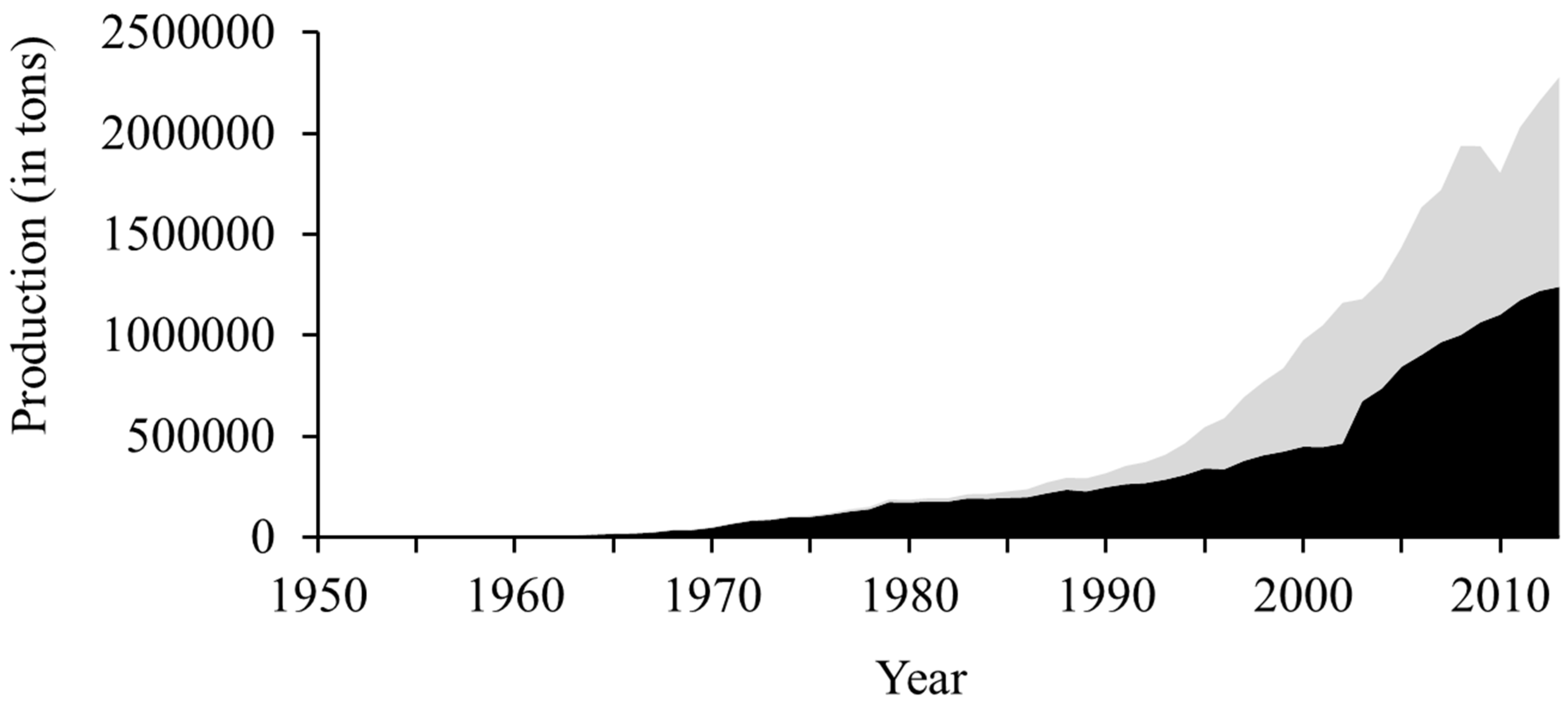
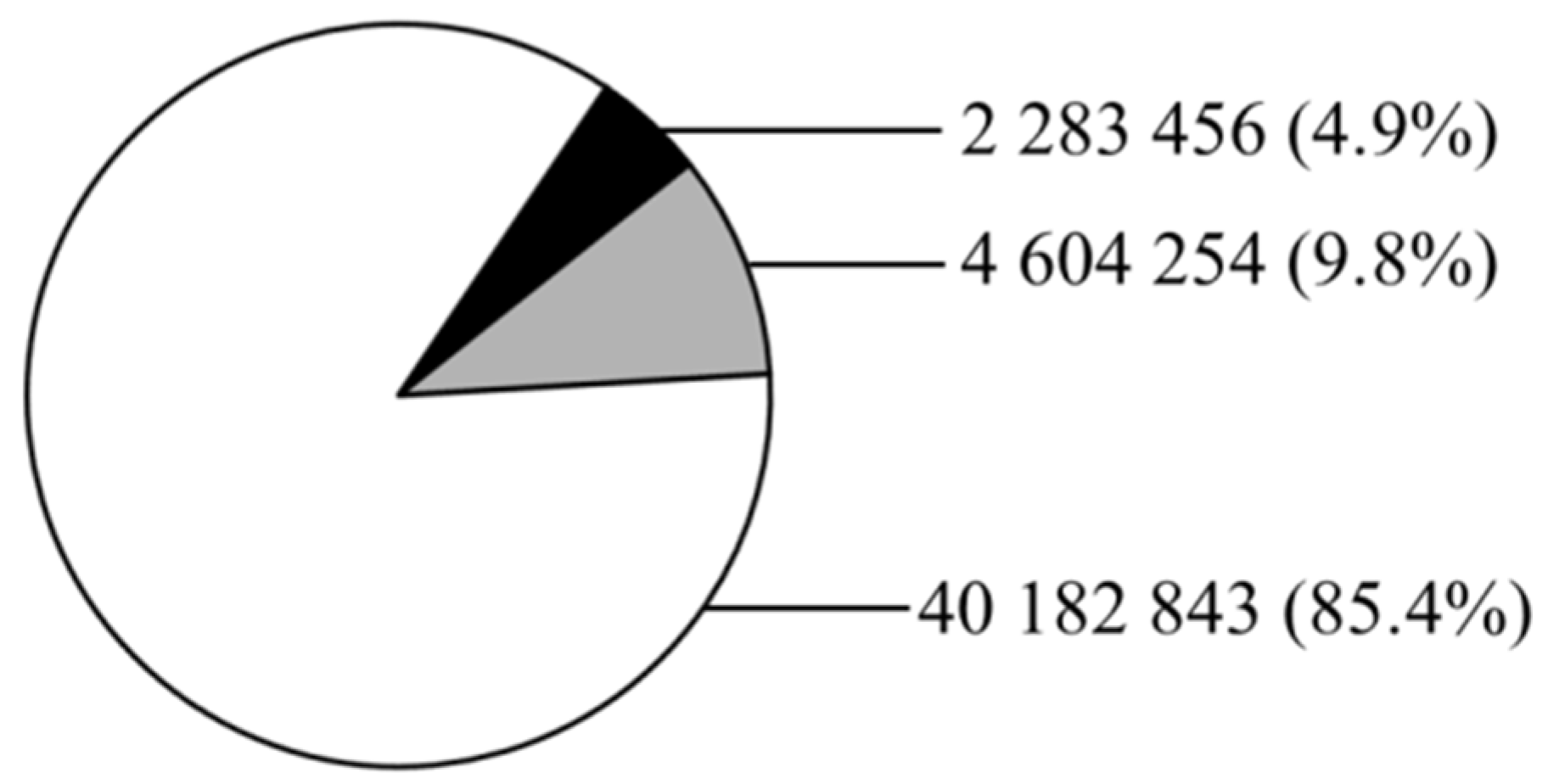

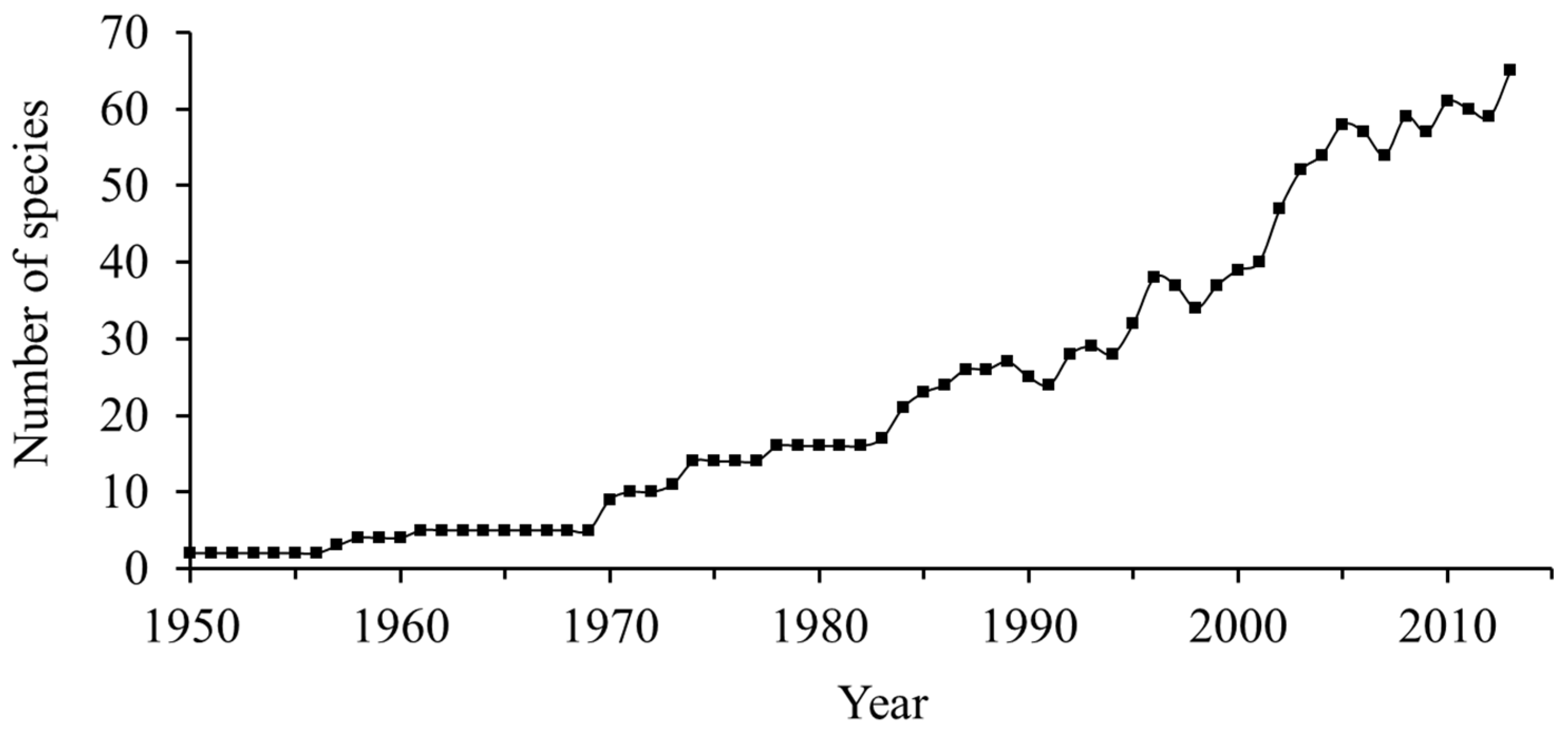
3.2. Evolution of the Number of Farmed and Domesticated Fish Species


4. Discussion
4.1. Evolution of Marine Fish Aquaculture Production
4.2. Evolution of the Number of Farmed and Domesticated Marine Species

5. Conclusions
Acknowledgments
Conflicts of Interest
Appendix
| Scientific Name | Common Name | Production in 2013 | Domestication Level | Main Reference |
|---|---|---|---|---|
| Anarhichas lupus | Atlantic wolffish | 0 | 1 | Gunnarsson et al., 2009, [33] |
| Atherina boyeri | Big-scale sand smelt | 0 | 1 | Dulcic et al., 2008, [34] |
| Bolbometopon muricatum | Green humphead parrotfish | 1 | 1 | |
| Carangoides malabaricus | Malabar trevally | 387 | 1 | |
| Caranx hippos | Crevalle jack | 0 | 1 | |
| Caranx sexfasciatus | Bigeye trevally | 1 | 1 | |
| Centropristis striata | Black seabass | 0 | 1 | Rezek et al., 2010, [35] |
| Chaetodipterus faber | Atlantic spadefish | 0 | 1 | Gaspar 2005, [36] |
| Dentex tumifrons | Yellowback seabream | 0 | 1 | |
| Dicentrarchus punctatus | Spotted seabass | 2 | 1 | Ly et al., 2012, [37] |
| Labrus bergylta | Ballan wrasse | 25 | 1 | Muncaster et al., 2010, [38] |
| Lethrinus miniatus | Trumpet emperor | 45 | 1 | |
| Lutjanus bohar | Two-spotted red snapper | 0 | 1 | |
| Megalops atlanticus | Tarpon | 0 | 1 | |
| Micropogonias furnieri | Whitemouth croaker | 5 | 1 | Velloso and Pereira Jr. 2010, [39] |
| Mugil liza | Lebranche mullet | 7 | 1 | |
| Muraenesox cinereus | Daggertooth pike conger | 0 | 1 | |
| Mycteroperca bonaci | Black grouper | 2 | 1 | |
| Pagrus major | Japanese seabream | 0 | 1 | |
| Platichthys flesus | European flounder | 0 | 1 | Engel-Sørensen et al., 2004, [40] |
| Pleurogrammus azonus | Okhotsk atka mackerel | 0 | 1 | |
| Pomatomus saltatrix | Bluefish | 0 | 1 | |
| Scophthalmus rhombus | Brill | 0 | 1 | Cruzado et al., 2004, [41] |
| Siganus canaliculatus | White-spotted spinefoot | 1 | 1 | Xu et al., 2011, [42] |
| Siganus javus | Streaked spinefoot | 0 | 1 | |
| Siganus rivulatus | Marbled spinefoot | 0 | 1 | El Dakar et al., 2011, [43] |
| Valamugil seheli | Bluespot mullet | 0 | 1 | Belal 2004, [44] |
| Acanthopagrus berda | Goldsilk seabream | 0 | 2 | Liao et al., 2001, [45] |
| Argyrosomus japonicus | Japanese meagre | 130 | 2 | Mirimin and Roodt-Wilding, 2015, [46] |
| Boleophthalmus pectinirostris | Great blue spotted mudskipper | 0 | 2 | Zhang et al., 1989, [47] |
| Centropomus undecimalis | Common snook | 0 | 2 | Carter et al., 2010a, [48] |
| Eleutheronema tetradactylum | Fourfinger threadfin | 4173 | 2 | Liao et al., 2001, [45] |
| Epinephelus areolatus | Areolate grouper | 47 | 2 | Ottolenghi et al., 2004, [49] |
| Epinephelus coioides | Orange-spotted grouper | 492 | 2 | Ottolenghi et al. 2004, [49] |
| Epinephelus fuscoguttatus | Brown-marbled grouper | 86 | 2 | Ottolenghi et al., 2004, [49] |
| Epinephelus lanceolatus | Giant grouper | 36 | 2 | Peng et al., 2015, [50] |
| Epinephelus malabaricus | Malabar grouper | 68 | 2 | Ottolenghi et al., 2004, [49] |
| Gnathanodon speciosus | Golden trevally | 58 | 2 | Liao et al., 2001, [45] |
| Liza ramada | Thinlip grey mullet | 0 | 2 | Marino et al., 1999, [51] |
| Lutjanus goldiei | Papuan black snapper | 0 | 2 | |
| Lutjanus guttatus | Spotted rose snapper | 2 | 2 | García-Ortega 2009, [52] |
| Lutjanus johnii | John's snapper | 278 | 2 | Liao et al., 2001, [45] |
| Miichthys miiuy | Mi-iuy (brown) croaker | 0 | 2 | An et al., 2012, [53] |
| Mugil soiuy | So-iuy mullet | 905 | 2 | |
| Plectropomus maculatus | Spotted coralgrouper | 7 | 2 | |
| Polydactylus sexfilis | Sixfinger threadfin | 0 | 2 | Deng et al., 2011, [54] |
| Psammoperca waigiensis | Waigieu seaperch | 5,704 | 2 | Pham et al., 2010, [55] |
| Seriola quinqueradiata | Japanese amberjack | 149,766 | 2 | Bilio 2007b, [56] |
| Seriola rivoliana | Longfin yellowtail | 400 | 2 | Roo et al., 2014, [57] |
| Thunnus albacares | Yellowfin tuna | 171 | 2 | Wexler et al., 2011, [58] |
| Thunnus maccoyii | Southern bluefin tuna | 3,482 | 2 | Carter et al., 2010b, [59] |
| Thunnus thynnus | Atlantic bluefin tuna | 3,445 | 2 | Carter et al., 2010b, [59] |
| Trachinotus blochii | Snubnose pompano | 112,499 | 2 | Liao et al., 2001, [45] |
| Trachinotus carolinus | Florida pompano | 350 | 2 | Pfeiffer and Riche 2001, [60] |
| Cromileptes altivelis | Humpback grouper | 2 | 3 | Hong and Zhang 2003, [61] |
| Dentex dentex | Common dentex | 54 | 3 | Suquet et al., 2009, [62] |
| Diplodus sargus | White seabream | 24 | 3 | Suquet et al., 2009, [62] |
| Diplodus vulgaris | Common two-banded seabream | 0 | 3 | Suquet et al., 2009, [62] |
| Epinephelus akaara | Hong Kong grouper | 0 | 3 | Hong and Zhang 2003, [61] |
| Epinephelus tauvina | Greasy grouper | 5,354 | 3 | Hong and Zhang 2003, [61] |
| Evynnis japonica | Crimson seabream | 0 | 3 | |
| Liza vaigiensis | Squaretail mullet | 0 | 3 | |
| Lutjanus argentimaculatus | Mangrove red snapper | 5,357 | 3 | Hong and Zhang 2003, [61] |
| Lutjanus russelli | Russell’s snapper | 13 | 3 | Hong and Zhang 2003, [61] |
| Melanogrammus aeglefinus | Haddock | 0 | 3 | Roselund and Skretting 2006, [63] |
| Pagellus bogaraveo | Blackspot seabream | 2 | 3 | Suquet et al., 2009, [62] |
| Pagellus erythrinus | Common pandora | 0 | 3 | Suquet et al., 2009, [62] |
| Platax orbicularis | Orbicular batfish | 8 | 3 | Coeurdacier and Gasset 2013, [64] |
| Pollachius pollachius | Pollack | 0 | 3 | Roselund and Skretting 2006, [63] |
| Pseudocaranx dentex | White trevally | 3,155 | 3 | |
| Rhabdosargus sarba | Goldlined seabream | 3 | 3 | Hong and Zhang 2003, [61] |
| Sciaena umbra | Brown meagre | 0 | 3 | Bilio 2007a, [65] |
| Seriola dumerili | Greater amberjack | 0 | 3 | Hong and Zhang 2003, [61] |
| Solea senegalensis | Senegalense sole | 640 | 3 | Imsland 2010, [66] |
| Solea solea | Common sole | 45 | 3 | Imsland 2010, [66] |
| Sparidentex hasta | Sobaity seabream | 551 | 3 | Teng et al., 1999, [67] |
| Takifugu obscurus | Obscure pufferfish | 4,860 | 3 | Kim et al., 2010, [68] |
| Takifugu rubripes | Tiger pufferfish | 19,359 | 3 | Wu et al., 2015, [69] |
| Thunnus orientalis | Pacific bluefin tuna | 16,624 | 3 | Carter et al., 2010b, [59] |
| Trachurus japonicas | Japanese jack mackerel | 958 | 3 | Masuda 2006, [70] |
| Umbrina cirrosa | Shi drum | 1,070 | 3 | Suquet et al. 2009, [62] |
| Acanthopagrus latus | Yellowfin seabream | 0 | 4 | Hong and Zhang 2003, [61] |
| Acanthopagrus schlegeli | Blackhead seabream | 1,161 | 4 | Hong and Zhang 2003, [61] |
| Anarhichas minor | Spotted wolfish | 0 | 4 | Le François et al. 2010, [71] |
| Argyrosomus regius | Meagre | 6,659 | 4 | Lazo et al., 2010, [72] |
| Diplodus puntazzo | Sharpsnout seabream | 250 | 4 | Suquet et al., 2009, [62] |
| Hippoglossus hippoglossus | Atlantic halibut | 1,485 | 4 | Imsland 2010, [66] |
| Larimichthys croceus | Large yellow croaker | 105,230 | 4 | Bilio 2007b, [56] |
| Lateolabrax japonicus | Japanese seabass | 129,334 | 4 | Hong and Zhang 2003, [61] |
| Mugil cephalus | Flathead grey mullet | 12,245 | 4 | Hong and Zhang 2003, [61] |
| Pagrus auratus | Silver seabream | 59,616 | 4 | Suquet et al., 2009, [62] |
| Pagrus pagrus | Red porgy | 350 | 4 | Suquet et al., 2009, [62] |
| Rachycentron canadum | Cobia | 43,395 | 4 | Bilio 2007a,b, [56,65] |
| Sciaenops ocellatus | Red drum | 62,197 | 4 | Lazo et al., 2010, [72] |
| Sebastes schlegeli | Korean rockfish | 23,757 | 4 | Bilio 2007b, [56] |
| Dicentrarchus labrax | European seabass | 161,059 | 5 | Jobling et al., 2010, [73] |
| Gadus morhua | Atlantic cod | 4,252 | 5 | Björnsson et al., 2010, [74] |
| Paralichthys olivaceus | Bastard halibut | 39,445 | 5 | Bilio 2007b, [56] |
| Psetta maxima | Turbot | 76,998 | 5 | Hulata 2001, [75] |
| Sparus aurata | Gilthead seabream | 173,062 | 5 | Jobling and Perruzi 2010, [76] |
References
- Price, E.O. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 1999, 65, 245–271. [Google Scholar] [CrossRef]
- Olesen, I.; Bentsen, H.B.; Phillips, M.; Ponzoni, R.W. Can the global adoption of genetically improved farmed fish increase beyond 10%, and how? J. Mar. Sci. Eng. 2015, 3, 240–266. [Google Scholar] [CrossRef]
- Zeder, M.A. Core questions in domestication research. Proc. Natl. Acad. Sci. USA 2015, 112, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Balon, E.K. About the oldest domesticates among fishes. J. Fish Biol. 2004, 65, 1–27. [Google Scholar] [CrossRef]
- Mirkena, T.; Duguma, G.; Haile, A.; Tibbo, M.; Okeyo, A.M.; Wurzinger, M.; Sölkner, J. Genetics of adaptation in domestic farm animals: A review. Livest. Sci. 2010, 132, 1–12. [Google Scholar] [CrossRef]
- Olsen, Y. How can mariculture better help feed humanity? Front. Mar. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Gjedrem, T. The first family-based breeding program in aquaculture. Rev. Aquac. 2010, 2, 2–15. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Morten, R. The importance of selective breeding in aquaculture to meet future demands for animal proteins: A review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Lind, C.E; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective breeding in fish and conservation of genetic resources for aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Teletchea, F. Domestication and genetics: What a comparison between land and aquatic species can bring. In Evolutionary Biology. Biodiversification from Genotype to Phenotype; Pontarotti, P., Ed.; Springer: Cham, Switzerland, 2015; pp. 389–402. [Google Scholar]
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Science 2007, 316, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Bartley, D.M.; Nguyen, T.T.T.; Halwart, M.; de Silva, S.S. Use and exchange of aquatic genetic resources in aquaculture: Information relevant to access and benefit sharing. Rev. Aquac. 2009, 1, 157–162. [Google Scholar] [CrossRef]
- Diana, J.S. Aquaculture production and biodiversity conservation. BioScience 2009, 59, 27–37. [Google Scholar] [CrossRef]
- Hedgecock, D. Aquaculture, the next wave of domestication. In Biodiversity in Agriculture, Domestication, Evolution, and Sustainability; Gepts, P., Famula, T.R., Bettinger, R.L., Brush, S.B., Damania, A.B., McGuire, P.E., Qualset, C.O., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 538–548. [Google Scholar]
- Gjedrem, T. Genetic improvement for the development of efficient global aquaculture: A personal opinion review. Aquaculture 2012, 344–349, 12–22. [Google Scholar] [CrossRef]
- Bilio, M. Plenary lecture: the future of capture and culture fisheries. In Proceedings of the 7th International Symposium, Keeping and Creating Diversity in Fish Production—Innovative Marine Life Science for Three Es, Edibles Environment and Education, in 21st century, Hokkaido, Japan, 17–19 November 2008; pp. 5–24.
- Teletchea, F. Qu’est-ce qu’un poisson domestique? Implications pour le développement futur de l’aquaculture. Ethnozootechnie 2012, 90, 7–12. [Google Scholar]
- Klinger, D.H.; Turnipseed, M.; Anderson, J.L.; Asche, F.; Crowder, L.B.; Guttormsen, A.G.; Halpern, B.S.; O’Connor, M.I.; Sagarin, R.; Selkoe, K.A.; et al. Moving beyond the fished or farmed dichotomy. Mar. Policy 2013, 68, 369–374. [Google Scholar] [CrossRef]
- Teletchea, F.; Fontaine, P. Levels of domestication in fish: Implications for the sustainable future of aquaculture. Fish Fish. 2014, 15, 181–195. [Google Scholar] [CrossRef]
- Watson, R.; Pauly, D. Systematic distortions in world fisheries catch trends. Nature 2001, 414, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Pauly, D. Mariculture: A global analysis of production trends since 1950. Mar. Pol. 2013, 39, 94–100. [Google Scholar] [CrossRef]
- Available online: http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en (accessed on 10 July 2015).
- De Silva, S.S. Aquaculture: A newly emergent food production sector and perspectives of its impacts on biodiversity and conservation. Biodivers. Conserv. 2012, 21, 3187–3220. [Google Scholar] [CrossRef]
- Migaud, H.; Bell, G; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.P.; Carillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquac. 2013, 5, S194–S223. [Google Scholar] [CrossRef]
- Natale, F.; Hofherr, J.; Fiore, G.; Virtanen, J. Interactions between aquaculture and fisheries. Mar. Policy 2013, 38, 205–113. [Google Scholar] [CrossRef]
- Sorgeloos, P. Aquaculture: The blue biotechnology of the future. World Aquac. 2013, 44, 16–25. [Google Scholar]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Holmer, M.; Olsen, Y.; Soto, D.; Marbà, N.; Guiu, J.; Black, K.; Karakassis, I. Will the oceans help feed humanity? BioScience 2009, 59, 967–976. [Google Scholar]
- Larson, G.; Fuller, D.Q. The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 2014, 66, 115–136. [Google Scholar] [CrossRef]
- Mylonas, C.C.; de la Gándara, F.; Corriero, A.; Belmonde Ríos, A. Atlantic Bluefin tuna (Thunnus thynnus) farming and fattening in the Mediterranean sea. Rev. Fish. Sci. 2010, 18, 266–280. [Google Scholar] [CrossRef]
- Trujillo, P.; Piroddi, C.; Jacquet, J. Fish farms at sea: The ground truth from google Earth. PLoS ONE 2012, 7, e30546. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, S.; Sigurdsson, S.; Thorarensen, H.; Imsland, A.K. Cryopreservation of sperm from spotted wolffish. Aquac. Int. 2009, 17, 385–389. [Google Scholar] [CrossRef]
- Dulcic, J.; Grubisic, L.; Pallaoro, A.; Glamuzina, B. Embryonic and larval development of big-scale sand smelt Atherina boyeri (Atherinidae). Cybium 2008, 32, 27–32. [Google Scholar]
- Rezek, T.C.; Watanabe, W.O.; Harel, M.; Seaton, P.J. Effects of dietary docosahexaenoic acid (22:6n-3) and arachidonic acid (20:4n-6) on the growth, survival, stress resistance and fatty acid composition in black sea bass Centropristis striata (Linnaeus 1758) larvae. Aquac. Res. 2010, 41, 1302–1314. [Google Scholar] [CrossRef]
- Gaspar, A.G. Induced spawning and rear larvae of spadefish Chaetodipterus faber in Margarita Island, Venezuala. In Proceedings of the 47th Gulf and Caribbean Fisheries Institute; Gulf and Caribbean Fisheries Institute: Margarita Island, Venezuala, 1995; Volume 48, pp. 15–24. [Google Scholar]
- Ly, C.-L.; Vergnet, A.; Molinari, N.; Fauvel, C.; Bonhomme, F. Fitness of early life stages in F1 interspecific hybrids between Dicentrarchus labrax and D. punctatus. Aquat. Living Resour. 2012, 25, 67–75. [Google Scholar]
- Muncaster, S.; Andersson, E.; Kjesbu, O.S.; Taranger, G.L.; Skiftesvik, A.B.; Norberg, B. The reproductive cycle of female Ballan wrasse Labrus bergylta in high latitude, temperate waters. J. Fish Biol. 2010, 77, 494–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velloso, A.L.; Pereira, J., Jr. Influence of ectoparasitism on the welfare of Micropogonias furnieri. Aquaculture 2010, 310, 43–46. [Google Scholar] [CrossRef]
- Engell-Sørensen, K.; Støttrup, J.G.; Holmstrup, M. Rearing of flounder (Platichthys flesus) juveniles in semiextensive systems. Aquaculture 2004, 230, 475–491. [Google Scholar] [CrossRef]
- Cruzado, I.H.; Herrera, M.; Quintana, D.; Rodiles, A.; Navas, J.I.; Lorenzo, A.; Almansa, E. Total lipid and fatty acid composition of brill eggs Scophthalmus rhombus L. relationship between lipid composition and egg quality. Aquac. Res. 2004, 42, 1011–1025. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Wu, Q.; Liu, X.; Wang, S.; You, C.; Li, Y. Evaluation of dried seaweed Gracilaria lemaneiformis as an ingredient in diets for teleost fish Siganus canaliculatus. Aquacult. Int. 2011, 19, 1007–1018. [Google Scholar] [CrossRef]
- El-Dakar, A.Y.; Shalaby, S.M.; Saoud, I.P. Dietary protein requirement of juvenile marbled spinefoot rabbitfish Siganus rivulatus. Aquac. Res. 2011, 42, 1050–1055. [Google Scholar] [CrossRef]
- Belal, I.E.H. Replacement of corn with mangrove seeds in bluespot mullet Valamugil seheli diets. Aquac. Nutr. 2004, 10, 25–30. [Google Scholar] [CrossRef]
- Liao, I.C.; Su, H.M.; Chang, E.Y. Techniques in finfish larviculture in Taiwan. Aquaculture 2001, 200, 1–31. [Google Scholar] [CrossRef]
- Mirimin, L.; Roodt-Wilding, R. Testing and validating a modified CTAB DNA extraction method to enable molecular parentage analysis of fertilized eggs and larvae of an emerging South African aquaculture species, the dusky kob Argyrosomus japonicas. J. Fish Biol. 2015, 86, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Hong, W.S.; Dai, Q.N.; Zhang, J.; Cai, Y.-Y.; Huang, J.L. Studies on induced ovulation, embryonic development and larval rearing of the mudskipper (Boleophthalmus pectinirostris). Aquaculture 1989, 83, 375–385. [Google Scholar] [CrossRef]
- Carter, C.; Glencross, B.; Katersky, R.S.; Bermudes, M. Chapter 14: The Snooks (Family: Centropomidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 323–336. [Google Scholar]
- Ottolenghi, F.; Silvestri, C.; Giodano, P.; Lovatelli, A.; New, M.B. Capture-Based Aquaculture, the Fattening of Eels, Groupers, Tuna and Yellowtails; FAO: Rome, Italy, 2004. [Google Scholar]
- Peng, C.; Ma, H.; Su, Y.; Wen, W.; Feng, J.; Guo, Z.; Qiu, L. Susceptibility of farmed juvenile giant grouper Epinephelus lanceolatus to a newly isolated grouper iridovirus (genus Ranavirus). Vet. Microbiol. 2015, 177, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Ingle, E.; Cataudella, S. Status of aquaculture in Italy. Cah. Opt. Méditerr. 1999, 43, 117–126. [Google Scholar]
- García-Ortega, A. Nutrition and feeding research in the spotted rose snapper (Lutjanus guttatus) and bullseye puffer (Sphoeroides annulatus), new species for marine aquaculture. Fish Physiol. Biochem. 2009, 35, 69–80. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Kim, E.M.; Lee, J.W.; Kim, D.J.; Kim, Y.C. New polymorphic microsatellite markers in the Korean mi-iuy croaker, Miichthys miiuy, and their application to the genetic characterization of wild and farmed populations. Anim. Cells Syst. 2012, 16, 41–49. [Google Scholar] [CrossRef]
- Deng, D.-F.; Ju, Z.Y.; Dominy, W.; Murashige, R.; Wilson, R.P. Optimal dietary protein levels for juvenile Pacific threadfin (Polydactylus sexfilis) fed diets with two levels of lipid. Aquaculture 2011, 316, 25–30. [Google Scholar] [CrossRef]
- Pham, H.Q.; Nguyen, A.T.; Nguyen, M.D.; Arukwe, A. Sex steroid levels, oocyte maturation and spawning performance in Waigieu seaperch (Psammoperca waigiensis) exposed to thyroxin, human chorionic gonadotropin, luteinizing hormone releasing hormone and carp pituitary extract. Comp. Biochem. Phys. A 2010, 155, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bilio, M. Controlled reproduction and domestication in aquaculture—The current state of the art, Part II. Aquac. Eur. 2007, 32, 5–23. [Google Scholar]
- Roo, J.; Fernandez-Palacios, H.; Hernandez-Cruz, C.M.; Mesa-Rodriguez, A.; Schuchardt, D.; Izquierdo, M. First results of spawning and larval rearing of longfin yellowtail Seriola rivoliana as a fast-growing candidate for European marine finfish aquaculture diversification. Aquac. Res. 2010, 45, 689–700. [Google Scholar] [CrossRef]
- Wexler, J.B.; Margulies, D.; Scholey, V.P. Temperature and dissolved oxygen requirements for survival of yellowfin tuna, Thunnus albacares, larvae. J. Exp. Mar. Biol. Ecol. 2011, 404, 63–72. [Google Scholar] [CrossRef]
- Carter, C.; Nowak, B.; Clarke, S. Chapter 20: The Tunas (Family: Scombridae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 432–449. [Google Scholar]
- Pfeiffer, T.J.; Riche, M.A. Evaluation of a low-head recirculating aquaculture system used for rearing Florida pompano to market size. J. World Aquac. Soc. 2011, 42, 198–208. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, Q. Review of captive bred species and fry production of marine fish in China. Aquaculture 2003, 227, 305–318. [Google Scholar] [CrossRef]
- Suquet, M.; Divanach, P.; Hussenot, J.; Coves, D.; Fauvel, C. Pisciculture marine de «nouvelles espèces» d’élevage pour l’Europe. Cah. Agric. 2009, 2–3, 148–156. [Google Scholar]
- Roselund, G.; Skretting, M. Worldwide status and perspective on gadoid culture. ICES J. Mar. Sci. 2006, 63, 194–197. [Google Scholar] [CrossRef]
- Coeurdacier, J.L.; Gasset, E. Dossier récapitulatif du développement de l’élevage du Platax orbicularis en Polynésie Française. Avaliable online: http://archimer.ifremer.fr/doc/00134/24490/ (accessed on 15 July 2013).
- Bilio, M. Controlled reproduction and domestication in aquaculture—The current state of the art, Part I. Aquac. Eur. 2007, 32, 5–14. [Google Scholar]
- Imsland, A.K. Chapter 21: The Flatfishes (Order: Pleuronectiformes). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 450–496. [Google Scholar]
- Teng, S.-K.; El-Zahr, C.; Al-Abdul-Elah, K.; Almatar, S. Pilot-scale spawning and fry production of blue-fin porgy, Sparidentex hasta (Valenciennes), in Kuwait. Aquaculture 1999, 178, 27–41. [Google Scholar] [CrossRef]
- Kim, J.H.; Rhee, J.-S.; Lee, J.S.; Dahms, H.U.; Lee, J.; Han, K.N.; Lee, J.S. Effect of cadmium exposure on expression of antioxidant gene transcripts in the river pufferfish, Takifugu obscurus (Tetraodontiformes). Comp. Biochem. Phys. C 2010, 152, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tang, K.; Yuan, M.; Shi, X.; Shakeela, Q.; Zhang, X.H. Studies on bacterial pathogens isolated from diseased torafugu (Takifugu rubripes) cultured in marine industrial recirculation aquaculture system in Shandong Province, China. Aquac. Res. 2015, 46, 736–744. [Google Scholar] [CrossRef]
- Masuda, R. Ontogeny of anti-predator behavior in hatchery-reared jack mackerel Trachurus japonicus larvae and juveniles: Patchiness formation, swimming capability, and interaction with jellyfish. Fish. Sci. 2006, 72, 1225–1235. [Google Scholar] [CrossRef]
- Le François, N.; Tveiten, H.; Halfyard, L.C.; Foss, A. Chapter 19. The Wolffishes (Family: Anarhichadidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 417–431. [Google Scholar]
- Lazo, J.P.; Holt, J.G.; Fauvel, C.; Suquet, M.; Quéméner, L. Chapter 18. Drum-Fish or Croakers (Family: Sciaenidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 397–416. [Google Scholar]
- Jobling, M.; Peruzzi, S.; Woods, C. Chapter 15. The Temperate Basses (Family: Moronidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 337–360. [Google Scholar]
- Björnsson, B.; Litvak, M.; Trippel, E.A.; Suquet, M. Chapter 13. The Codfishes (Family: Gadidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 290–322. [Google Scholar]
- Hulata, G. Genetic manipulations in aquaculture: A review of stock improvement by classical and modern technologies. Genetica 2001, 111, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.; Peruzzi, S. Chapter 16. Seabreams and Porgies (Family: Sparidae). In Finfish Aquaculture Diversification; Le François, N., Jobling, M., Carter, C., Blier, P., Eds.; CABI: Oxfordshire, UK, 2010; pp. 361–373. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teletchea, F. Domestication of Marine Fish Species: Update and Perspectives. J. Mar. Sci. Eng. 2015, 3, 1227-1243. https://doi.org/10.3390/jmse3041227
Teletchea F. Domestication of Marine Fish Species: Update and Perspectives. Journal of Marine Science and Engineering. 2015; 3(4):1227-1243. https://doi.org/10.3390/jmse3041227
Chicago/Turabian StyleTeletchea, Fabrice. 2015. "Domestication of Marine Fish Species: Update and Perspectives" Journal of Marine Science and Engineering 3, no. 4: 1227-1243. https://doi.org/10.3390/jmse3041227





