Combination of Ascophyllum nodosum Extract and Humic Acid Improve Early Growth and Reduces Post-Harvest Loss of Lettuce and Spinach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Material
2.2. Preparation of Humic Acid and Seaweed Extract
2.3. Early Growth Assay
2.4. Treatments
2.5. Sample Preparation and Post-Harvest Storage
2.6. Weight Loss
2.7. Determination of Lipid Peroxidation
2.8. Determination of Pigments
2.9. Determination of Total Ascorbic Acid
2.10. Determination of Total Phenolic Content
2.11. Determination of Total Antioxidants
2.12. Statistical Analysis
3. Results
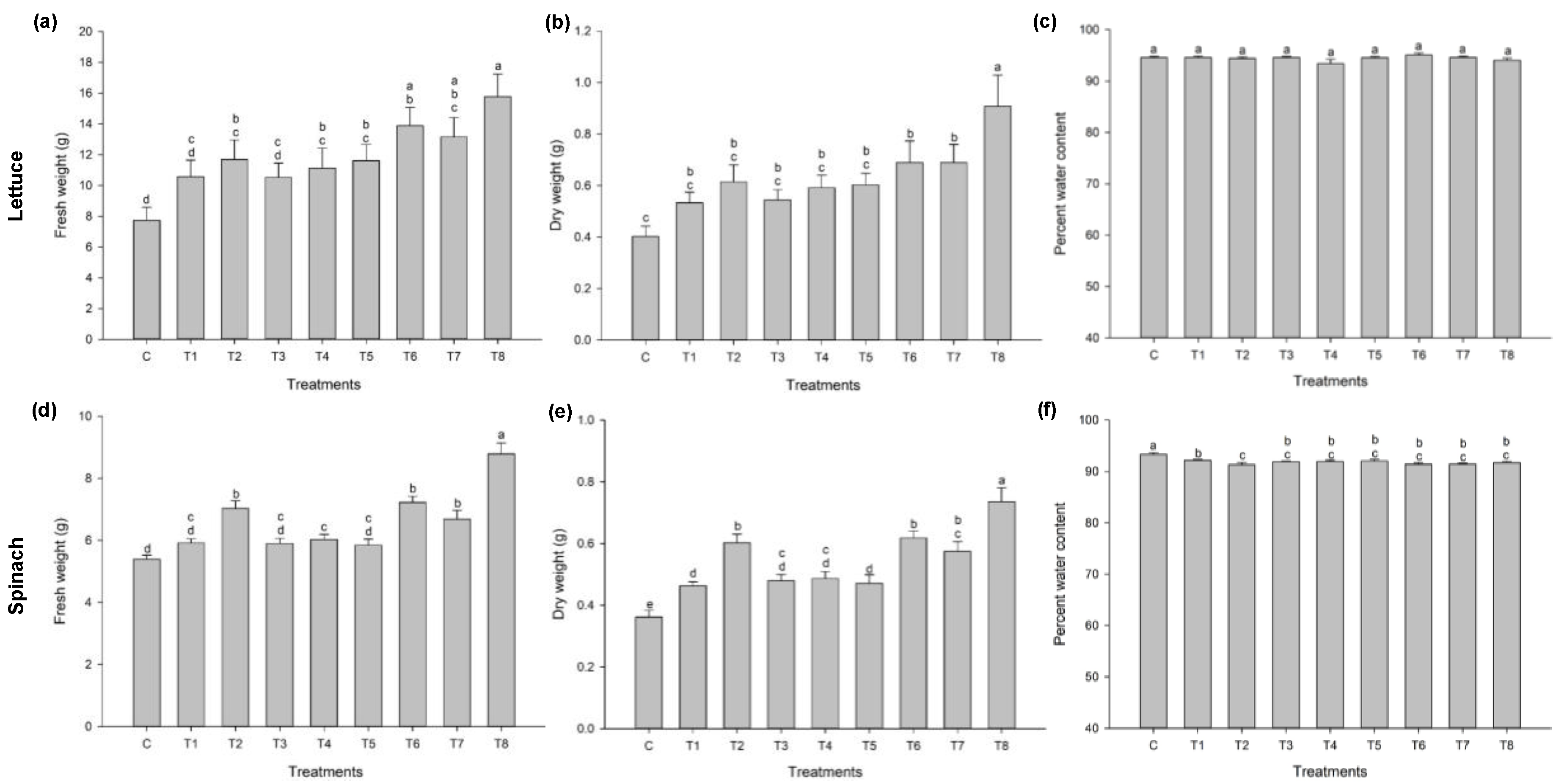
3.1. Ascophyllum nodosum Extract (ANE) and Humic Acid (HA) Improves Early Growth of Lettuce and Spinach
3.1.1. Lettuce
3.1.2. Spinach
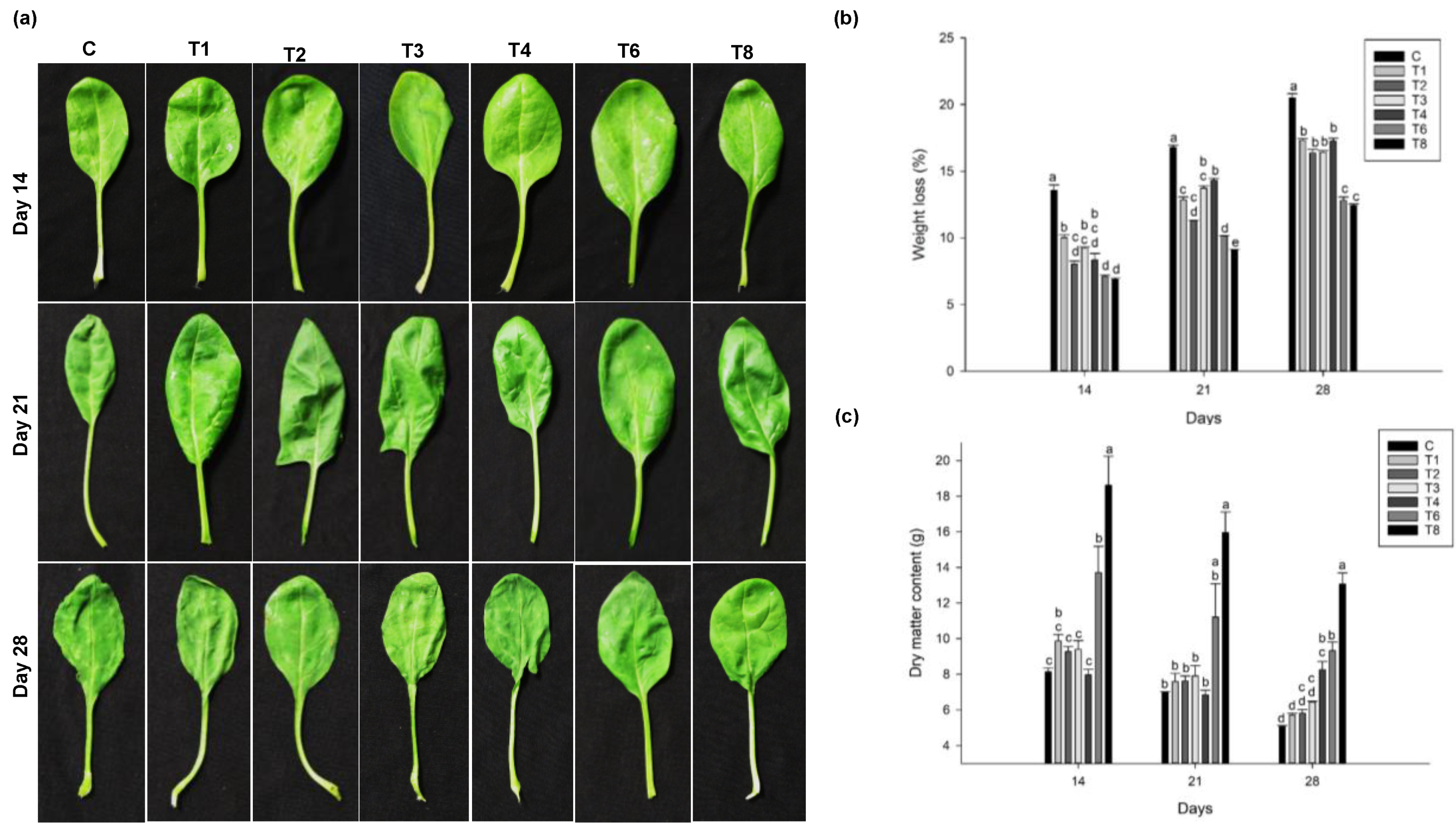
3.2. Effect of Pre-Harvest Treatment of Ascophyllum nodosum Extract (ANE) and Humic Acid (HA) on Yield and Post-Harvest Weight-Loss
3.2.1. Lettuce
3.2.2. Spinach
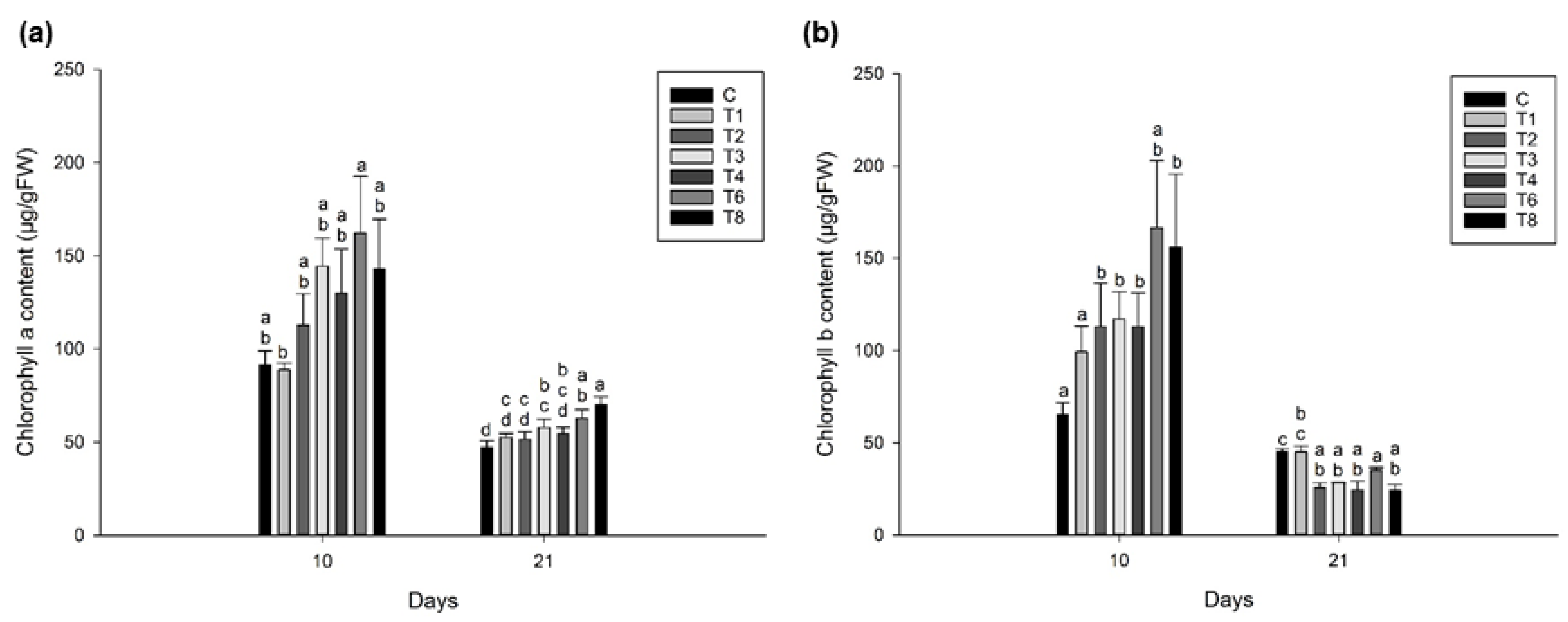
3.3. Ascophyllum nodosum Extract (ANE) and Humic Acid (HA) Retains Pigmentation during Post-Harvest Storage of Lettuce and Spinach
3.3.1. Lettuce
3.3.2. Spinach
3.4. Ascophyllum nodosum Extract (ANE) and Humic Acid (HA) Reduces Lipid Peroxidation during Post-Harvest Storage of Lettuce and Spinach
3.4.1. Lettuce
3.4.2. Spinach
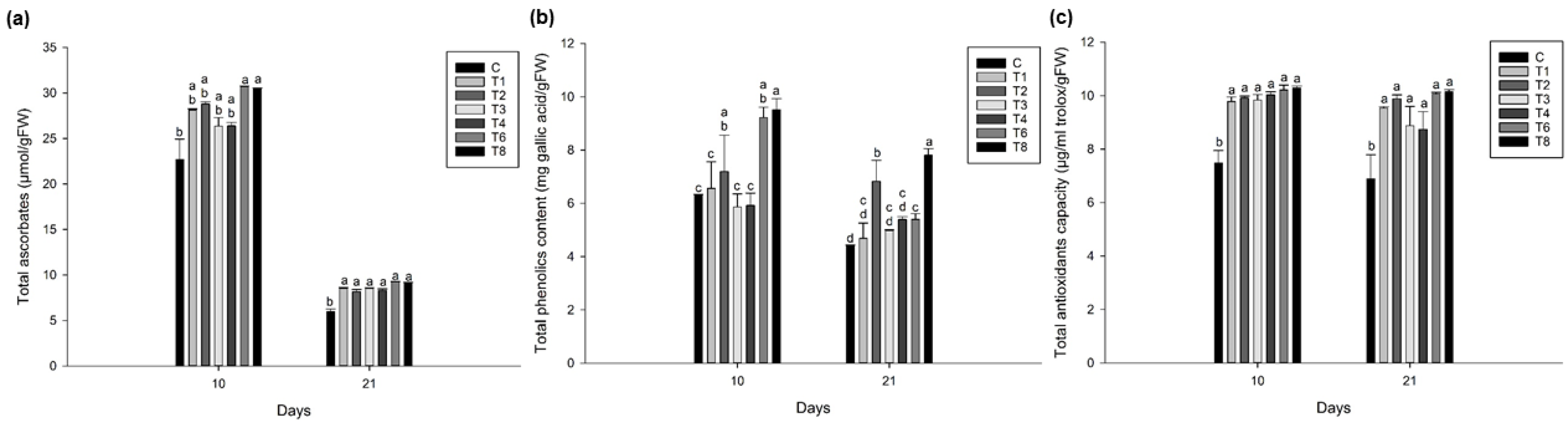
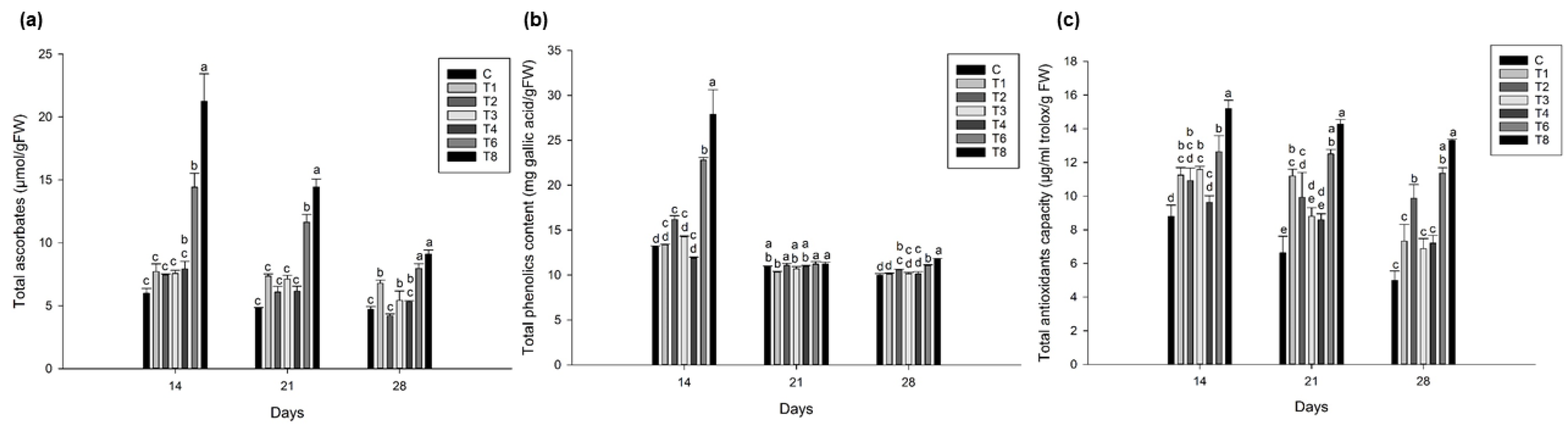
3.5. Ascophyllum nodosum Extract (ANE) and Humic Acid (HA) Showed Higher Total Ascorbic Acid, Phenolics, and Antioxidant Content in Lettuce and Spinach during Post-Harvest Storage
3.5.1. Lettuce
3.5.2. Spinach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. (Amsterdam) 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J. Hortic. Sci. Biotechnol. 2009, 84, 131–137. [Google Scholar] [CrossRef]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Rayirath, P.; Allan-Wojtas, P.; Prithiviraj, B.; Mark Hodges, D.; Critchley, A.T.; MacKinnon, S.; Benkel, B. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis Thaliana. Planta 2009, 230, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Khan, W.; Evans, F.; Critchley, A.T.; Prithiviraj, B. Tasco®: A product of Ascophyllum nodosum enhances immune response of Caenorhabditis elegans against Pseudomonas aeruginosa infection. Mar. Drugs 2012, 10, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-Derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. (Amsterdam) 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H.A. Effect of different levels of Humic Acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 212–219. [Google Scholar] [CrossRef]
- Phuong, H.K.; Tichý, V. Activity of humus acids from peat as studied by means of some growth regulator bioassays. Biol. Plant 1976, 18, 1951–1999. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Seaweed extract, humic acid, and propiconazole improve tall fescue sod heat tolerance and posttransplant quality. Hortic. Sci. 2003, 38, 4404–4443. [Google Scholar] [CrossRef]
- Pishchik, V.N.; Vorobyov, N.I.; Walsh, O.S.; Surin, V.G.; Khomyakov, Y.V. Estimation of synergistic effect of humic fertilizer and Bacillus subtilis on lettuce plants by reflectance measurements. J. Plant Nutr. 2016, 39, 1074–1086. [Google Scholar] [CrossRef]
- Billard, V.; Etienne, P.; Jannin, L.; Garnica, M.; Cruz, F.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Two biostimulants derived from algae or humic acid induce similar responses in the mineral content and gene expression of winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2014, 33, 305–316. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.; Schmidt, R. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 4924–4996. [Google Scholar]
- Ferguson, I.; Volz, R.; Woolf, A. Preharvest factors affecting physiological disorders of fruit. Postharvest Biol. Technol. 1999, 15, 2552–2562. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest technology of horticultural crops—An overview from farm to fork. J. Appl. Sci. Technol. 2013, 1, 1–8. [Google Scholar]
- Weston, L.A.; Barth, M.M. Preharvest factors affecting postharvest quality of vegetables. HortScience 1997, 32, 812–816. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Fan, D.; Kandasamy, S.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. Pre-Harvest treatment of spinach with Ascophyllum nodosum extract improves post-harvest storage and quality. Sci. Hortic. 2014, 170, 70–74. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Nikbakht, A.; Kafi, M.; Babalar, M.; Xia, Y.P.; Luo, A.; Etemadi, N.A. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Shukla, P.S.; Gupta, K.; Agarwal, P.; Jha, B.; Agarwal, P.K. Overexpression of a novel SbMYB15 from Salicornia brachiata confers salinity and dehydration tolerance by reduced oxidative damage and improved photosynthesis in transgenic tobacco. Planta 2015, 242, 1291–1308. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Handbook of Food Analytical Chemistry; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 9780471709084. [Google Scholar]
- Brand-Williams; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Bradford, K.J.; Dahal, P.; Van Asbrouck, J.; Kunusoth, K.; Bello, P.; Thompson, J.; Wu, F. The dry chain: Reducing postharvest losses and improving food safety in humid climates. Trends Food Sci. Technol. 2018, 71, 84–93. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol.-Part C: Toxicol. Pharmacol. 2007, 146, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 71–74. [Google Scholar] [CrossRef]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Prusky, D. Reduction of the incidence of postharvest quality losses, and future prospects. Food Secur. 2011, 3, 4634–4674. [Google Scholar] [CrossRef]
- Hodges, D.M.; Toivonen, P.M.A. Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biol. Technol. 2008, 48, 1551–1562. [Google Scholar] [CrossRef]
- Mancuso, S.; Azzarello, E.; Mugnai, S.; Briand, X. Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv. Hortic. Sci. 2006, 20, 1000–1006. [Google Scholar]
- Bergquist, S.A.M.; Gertsson, U.E.; Olsson, M.E. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2006, 86, 346–355. [Google Scholar] [CrossRef]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Khan, W.; Hiltz, D.; Critchley, A.T.; Prithiviraj, B. Bioassay to detect Ascophyllum nodosum extract-induced cytokinin-like activity in Arabidopsis thaliana. J. Appl. Phycol. 2011, 23, 409–414. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-Containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Palma, T.; Stanley, D.W. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–217. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. HortScience 2000, 35, 575–579. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Page, T.; Griffiths, G.; Buchanan-Wollaston, V. Molecular and biochemical characterization of postharvest senescence in broccoli. Plant. Physiol. 2001, 125, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Schmidt, R.E. Hormone-Containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 207–220. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandepogu, M.; Shukla, P.S.; Asiedu, S.; Yurgel, S.; Prithiviraj, B. Combination of Ascophyllum nodosum Extract and Humic Acid Improve Early Growth and Reduces Post-Harvest Loss of Lettuce and Spinach. Agriculture 2019, 9, 240. https://doi.org/10.3390/agriculture9110240
Sandepogu M, Shukla PS, Asiedu S, Yurgel S, Prithiviraj B. Combination of Ascophyllum nodosum Extract and Humic Acid Improve Early Growth and Reduces Post-Harvest Loss of Lettuce and Spinach. Agriculture. 2019; 9(11):240. https://doi.org/10.3390/agriculture9110240
Chicago/Turabian StyleSandepogu, Monica, Pushp Sheel Shukla, Samuel Asiedu, Svetlana Yurgel, and Balakrishnan Prithiviraj. 2019. "Combination of Ascophyllum nodosum Extract and Humic Acid Improve Early Growth and Reduces Post-Harvest Loss of Lettuce and Spinach" Agriculture 9, no. 11: 240. https://doi.org/10.3390/agriculture9110240




