Limited Seed and Seed Yield Response of Calendula to Applied Nitrogen Does Not Justify Risk of Environmental Damage from High Urea Application Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Agronomic Practices and Fertilizer Treatments
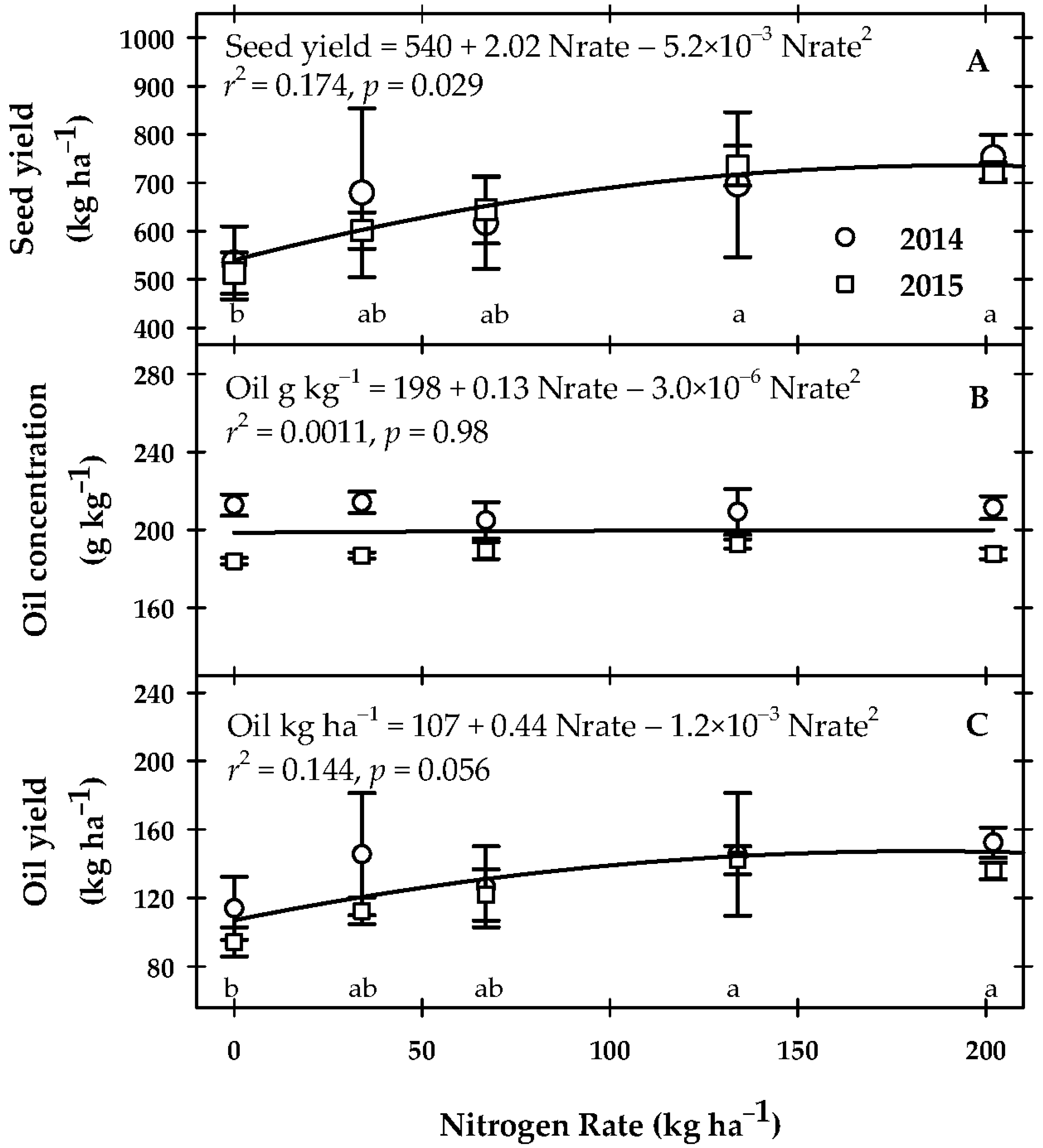
2.3. Seed and Seed Oil Yield
2.4. Plant and Soil Sample Measurements and Analysis
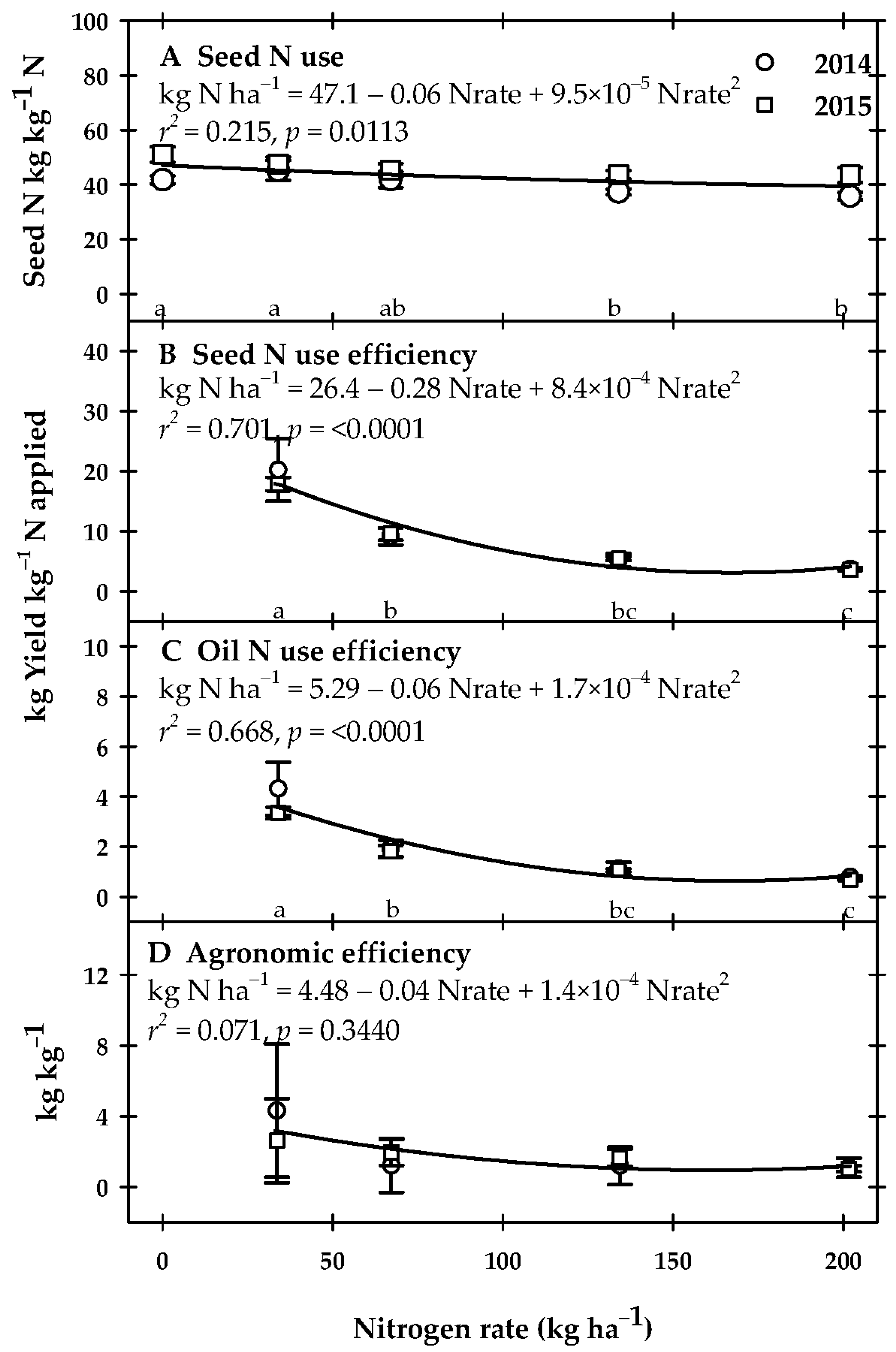
2.5. N Use Efficiency and Statistical Analysis
3. Results
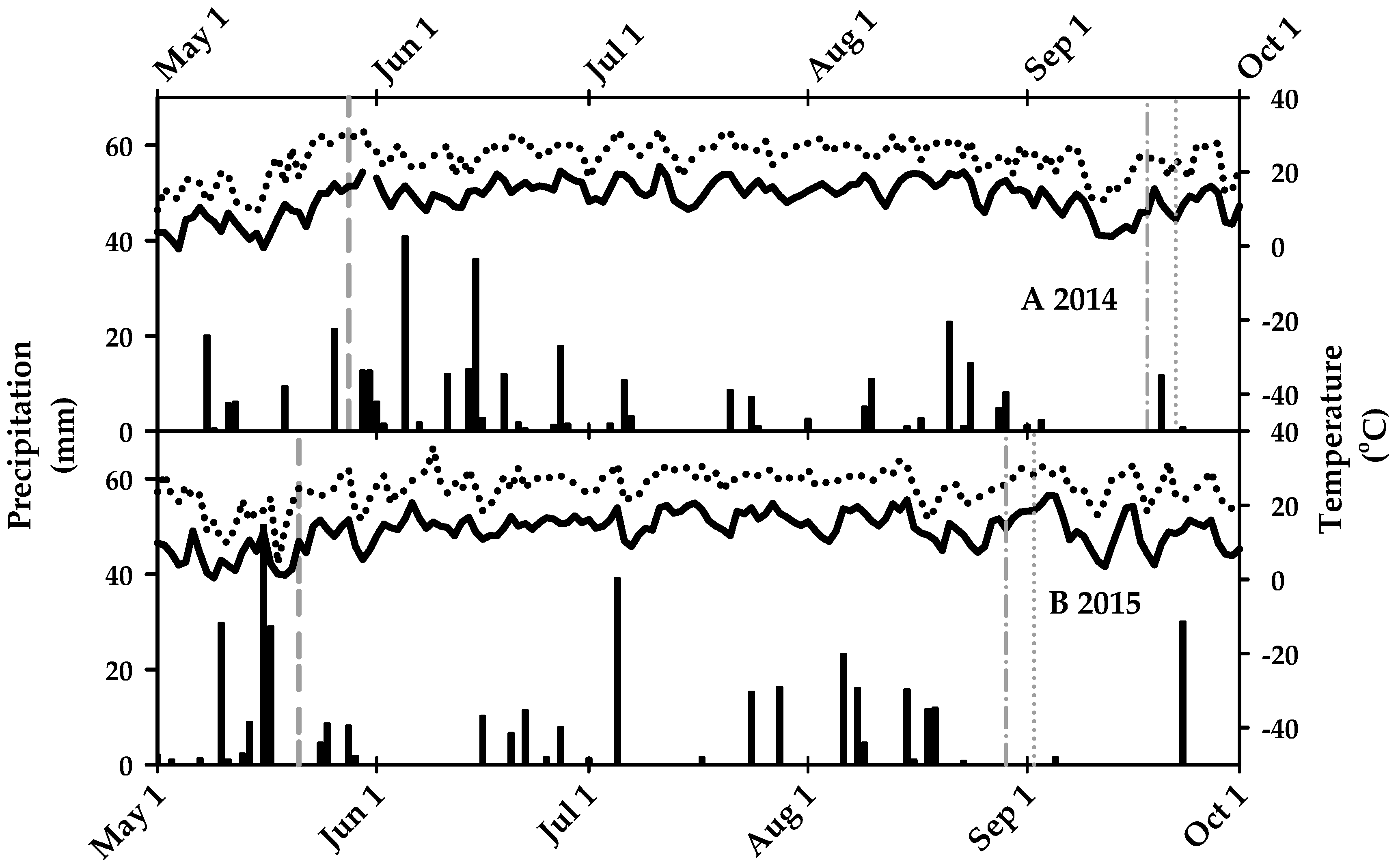
3.1. Growing Season Conditions
3.2. Yield and N-Use Efficiency
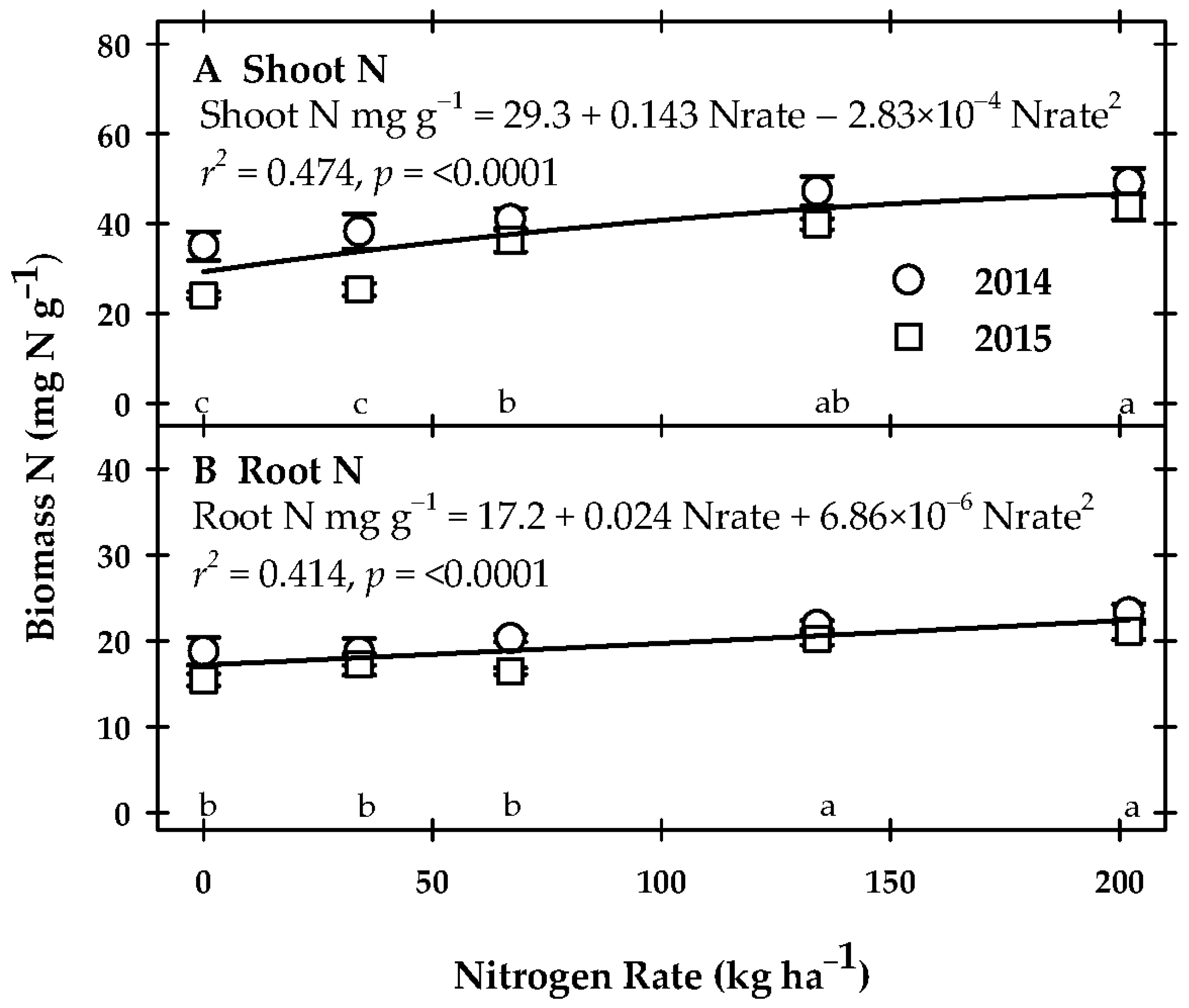
3.3. Vegetative Parameter Response
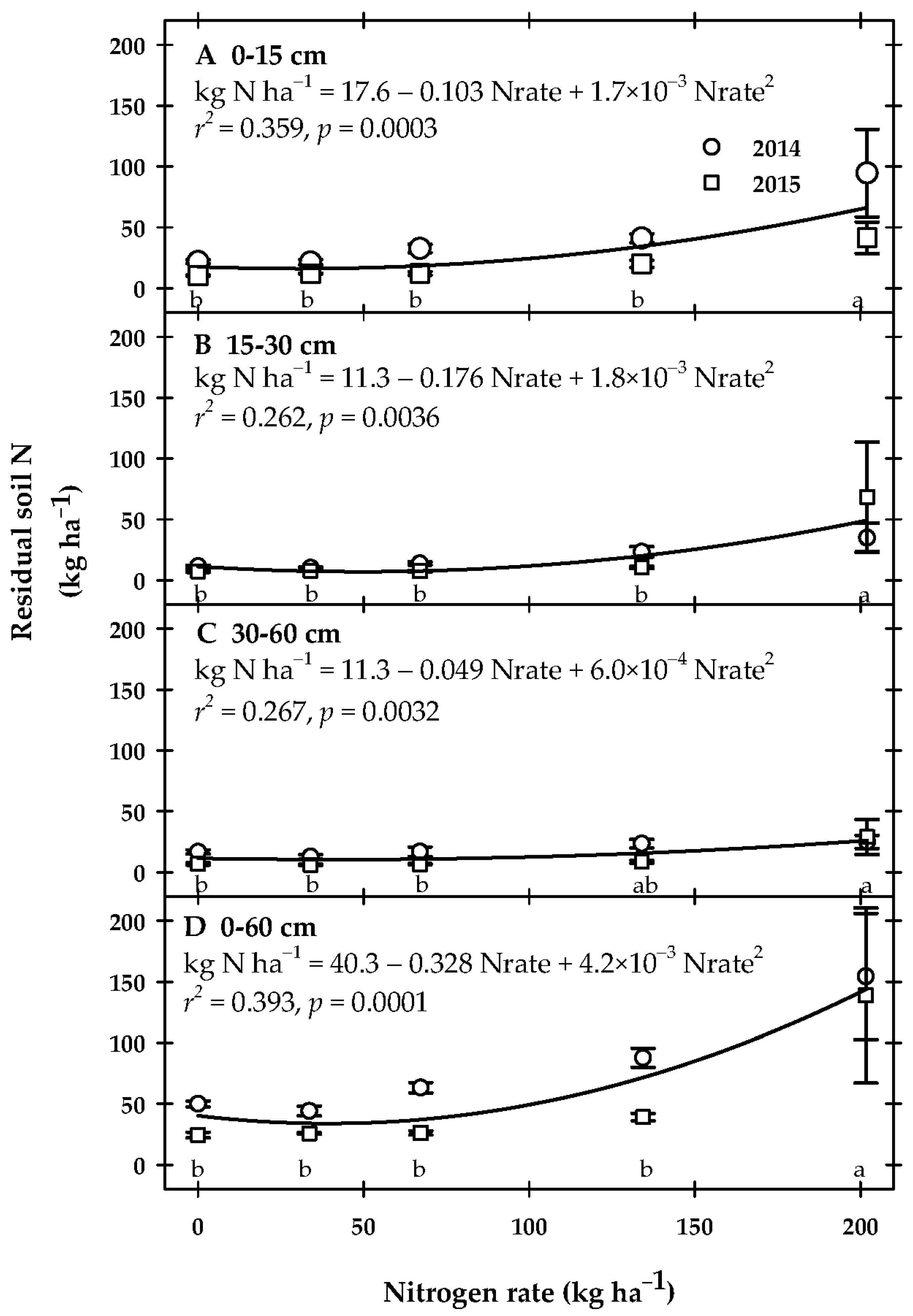
3.4. Post-Harvest Residual Soil N
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical Constituents and Pharmacological Activities of Calendula officinalis Linn (Asteraceae): A Review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Meir zu Beerentrup, H.; Röbbelen, G. Calendula and Coriandrum—New Potential Oilcrops for Industrial Uses. Fett Wiss. Technol. 1987, 89, 227–230. [Google Scholar] [CrossRef]
- Gesch, R.W. Growth and Yield Response of Calendula (Calendula officinalis) to Sowing Date in the Northern US. Ind. Crops Prod. 2013, 45, 248–252. [Google Scholar] [CrossRef]
- Muuse, B.G.; Cuperus, F.P.; Derksen, J.T.P. Composition and Physical Properties of Oils from New Oilseed Crops. Ind. Crops Prod. 1992, 1, 57–65. [Google Scholar] [CrossRef]
- Breemhaar, H.G.; Bouman, A. Harvesting and Cleaning Calendula officinalis, a New Arable Oilseed Crop for Industrial Application. Ind. Crops Prod. 1995, 4, 255–260. [Google Scholar] [CrossRef]
- Biermann, U.; Butte, W.; Holtgrefe, R.; Feder, W.; Metzger, J.O. Esters of Calendula Oil and Tung Oil as Reactive Diluents for Alkyd Resins. Eur. J. Lipid Sci. Technol. 2010, 112, 103–109. [Google Scholar] [CrossRef]
- Earle, F.R.; Miklojajczak, K.L.; Wolf, I.A. Search for New Industrial Oils: X Seed-Oils of Calendulae. J. Am. Oil Chem. Soc. 1964, 41, 345–347. [Google Scholar] [CrossRef]
- Joly, R.; Forcella, F.; Peterson, D.; Eklund, J. Planting Depth for Oilseed Calendula. Ind. Crops Prod. 2013, 42, 133–136. [Google Scholar] [CrossRef]
- Martin, R.J.; Deo, B. Effect of Plant Population on Calendula (Calendula officinalis L.). N. Z. J. Crop Hortic. Sci. 2000, 28, 37–44. [Google Scholar] [CrossRef]
- Cromack, H.T.H.; Smith, J.M. Calendula officinalis—Production Potential and Crop Agronomy in Southern England. Ind. Crops Prod. 1998, 7, 223–229. [Google Scholar] [CrossRef]
- Van Loo, E.N. (Ed.) Biotechnology and Conventional Breeding of Oil Crops for Industrial Use. In Proceedings of the Ctvo-Net: Chemical-Technical Utilization of Vegetable Oils, Bon, Germany, 20–21 June 2000. [Google Scholar]
- Zanetti, F.; Monti, A.; Berti, M.T. Challenges and Opportunities for New Industrial Oilseed Crops in Eu-27: A Review. Ind. Crops Prod. 2013, 50, 580–595. [Google Scholar] [CrossRef]
- Froment, M.; Mastebroek, D.; van Gorp, K. A Growers Manual for Calendula officinalis L.; Royal CEBECO Group: Rotterdam, The Netherlands; ADAS Bridgets Research Centre: Winchester, UK; Plant Research International: Wageningen, The Netherlands, 2003.
- Król, B. Yield and the Chemical Composition of Flower Heads of Pot Marigold (Calendula officinalis L. Cv. Orange King) Depending on Nitrogen Fertilization. Acta Sci. Pol. Hortorum Cultus 2011, 10, 235–243. [Google Scholar]
- Rahmani, N.; Daneshian, J.; Farahani, H.A.; Taherkhani, T. Evaluation of Nitrogenous Fertilizer Influence on Oil Variations of Calendula (Calendula officinalis L.) under Drought Stress Conditions. J. Med. Plants Res. 2011, 5, 696–701. [Google Scholar]
- Shakib, A.K.; Nejad, A.R.; Khalighi, A. Changes in Seed and Oil Yield of Calendula officinalis L. As Affected by Different Levels of Nitrogen and Plant Density. Res. Crops 2010, 11, 728–732. [Google Scholar]
- Moosavi, S.G.; Seghatoleslami, M.; Fazeli, M.; Jouyban, Z.; Ansarinia, E. Effect of Water Deficit Stress and Nitrogen Fertilizer on Flower Yield and Yield Components of Marigold (Calendula officinalis L.). Int. J. Biosci. 2014, 4, 42–49. [Google Scholar]
- Robertson, R.G.; Vitousek, P.M. Nitrogen in Agriculture: Balancing the Cost of an Essential Resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- McCrackin, M.L.; Harrison, J.A.; Cooter, E.J.; Dennis, R.L.; Compton, J.E. Alternative Futures of Dissolved Inorganic Nitrogen Export from the Mississippi River Basin: Influence of Crop Management, Atmospheric Deposition, and Population Growth. Biogeochemistry 2017, 133, 263–277. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Mississippi River/Gulf of Mexico Hypoxia Task Force. Available online: https://www.epa.gov/ms-htf (accessed on 2 March 2018).
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Vyn, T.J. A Comprehensive Study of Plant Density Consequences on Nitrogen Uptake Dynamics of Maize Plants from Vegetative to Reproductive Stages. Field Crops Res. 2011, 121, 2–18. [Google Scholar] [CrossRef]
- Woli, K.P.; Boyer, M.J.; Elmore, R.W.; Sawyer, J.E.; Abendroth, L.J.; Barker, D.W. Corn Era Hybrid Response to Nitrogen Fertilization. Agron. J. 2016, 108, 495–508. [Google Scholar] [CrossRef]
- USDA-Natural Resources Conservation Service. Soil Organic Carbon Map. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/use/?cid=nrcs142p2_054018 (accessed on 30 November 2017).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorologische Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Strock, J.S.; Tallaksen, J.E.; Reese, M. Corn Stover Harvest Changes Soil Hydrology and Soil Aggregation. Soil Tillage Res. 2016, 161, 106–115. [Google Scholar] [CrossRef]
- United States Department of Agriculture Agricultural Research Service. Weather Data from Swan Lake Research Farm. Available online: https://www.ars.usda.gov/midwest-area/morris-mn/soil-management-research/docs/weather/ (accessed 10 March 2018).
- Arguez, A.; Durre, I.; Applequist, S.; Squires, M.; Vose, R.; Yin, X.; Bilotta, R. Noaa‘s U.S. Climate Normals (1981–2010). [Morris West Central Research and Outreach Center MN US, Station Ghcnd:Usc00215638]. NOAA National Centers for Environmental Information, doi:10.7289/V5PN93JP. Available online: https://data.nodc.noaa.gov/cgi-bin/iso?id=gov.noaa.ncdc:C00824 (accessed on 10 March 2018).
- Gesch, R.W.; Cermak, S.C.; Isbell, T.A.; Forcella, F. Seed Yield and Oil Content of Cuphea as Affected by Harvest Date. Agron. J. 2005, 97, 817–822. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Gesch, R.W. Calendula and Camelina Response to Nitrogen Fertility. Ind. Crops Prod. 2013, 43, 684–691. [Google Scholar] [CrossRef]
- Smucker, A.J.M.; McBurney, S.L.; Srivastava, A.K. Quantitative Separation of Roots from Compacted Soil Profiles by Hydropneumatic Elutriation Systems. Agron. J. 1982, 74, 500–503. [Google Scholar] [CrossRef]
- Wagner, S.W.; Hanson, J.D.; Olness, A.; Voorhees, W.B. A Volumetric Inorganic Carbon Analysis System. Soil Sci. Soc. Am. J. 1998, 62, 690–693. [Google Scholar] [CrossRef]
- Mulvaney, R. Nitrogen-Inorganic Forms. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Bartels, J.M., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Bartels, J.M., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Helmke, P.A.; Sparks, D.L. Lithium, Sodium, Potassium, Rubidium, and Cesium. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series No. 5; Bartels, J.M., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 551–574. [Google Scholar]
- SAS Institute. SAS System for Windows, Release 9.4; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Samoon, S.A.; Kirad, K.S. Effect of Nitrogen and Phosphorus on Seed Yield Parameters of Calendula (Calendula officinalis L.) Var. Touch of Red Mixture. Prog. Hortic. 2013, 45, 149–151. [Google Scholar]
- Carpenter-Boggs, L.; Pikul, J.L., Jr.; Vigil, M.F.; Riedell, W.E. Soil Nitrogen Mineralization Influenced by Crop Rotation and Nitrogen Fertilization. Soil Sci. Soc. Am. J. 2000, 64, 2038–2045. [Google Scholar] [CrossRef]
- Wysocki, D.J.; Chastain, T.G.; Schillinger, W.F.; Guy, S.O.; Karow, R.S. Camelina: Seed Yield Response to Applied Nitrogen and Sulfur. Field Crops Res. 2013, 145, 60–66. [Google Scholar] [CrossRef] [Green Version]
- McSwiney, C.P.; Robertson, G.P. Nonlinear Response of N2o Flux to Incremental Fertilizer Addition in a Continuous Maize (Zea mays L.) Cropping System. Glob. Chang. Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change Wgi, Fourth Assessment Report, Climate Change 2007: The Physical Science Basis, Summary for Policymakers. Available online: http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_spm.pdf (accessed on 18 December 2017).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, J.M.F.; Gesch, R.W.; Barbour, N.W. Limited Seed and Seed Yield Response of Calendula to Applied Nitrogen Does Not Justify Risk of Environmental Damage from High Urea Application Rates. Agriculture 2018, 8, 40. https://doi.org/10.3390/agriculture8030040
Johnson JMF, Gesch RW, Barbour NW. Limited Seed and Seed Yield Response of Calendula to Applied Nitrogen Does Not Justify Risk of Environmental Damage from High Urea Application Rates. Agriculture. 2018; 8(3):40. https://doi.org/10.3390/agriculture8030040
Chicago/Turabian StyleJohnson, Jane M. F., Russ W. Gesch, and Nancy W. Barbour. 2018. "Limited Seed and Seed Yield Response of Calendula to Applied Nitrogen Does Not Justify Risk of Environmental Damage from High Urea Application Rates" Agriculture 8, no. 3: 40. https://doi.org/10.3390/agriculture8030040




