Effect of Elemental Sulfur as Fertilizer Ingredient on the Mobilization of Iron from the Iron Pools of a Calcareous Soil Cultivated with Durum Wheat and the Crop’s Iron and Sulfur Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Field Trial
2.2. The Nature of the FBS0 Fertilizer Granules
2.3. Determinations of Soil Parameters
2.4. Preparation of Dry Mass Digests
2.5. Rhizosoil Fractionation Scheme
2.6. Sulfate and Total Sulfur Determination in the DM of the Plant Parts
2.7. Statistical Analysis
3. Results
3.1. Dynamics of the Iron Fractions in Rhizosoil during the Crops’ Development
3.2. Dynamics of Iron and Sulfur Nutrition in the Crop’s above Ground Plant Parts during the Crops’ Development, in Relation to the Dynamics of Rhizosoil Iron Pools
3.3. FBS0 Fortified the Crop’s Iron and Sulfur Nutrition and Increased the Yield
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RS | Rhizosoil |
| OM | Organic matter |
| HS | Humic substances |
| DM | Dry mass |
| fr | Fraction |
| F | Granular conventional fertilizer |
| S0 | Elemental sulfur |
| B | Binder |
References
- Janzen, H.H.; Bettany, J.R. Release of available sulfur from fertilizers. Can. J. Soil Sci. 1986, 66, 91–103. [Google Scholar] [CrossRef]
- Germida, J.J.; Jansen, H.H. Factors affecting the oxidation of elemental sulfur in soils. Fertil. Res. 1993, 35, 101–114. [Google Scholar] [CrossRef]
- Somani, L.L.; Totawat, K.L. Mined and industrial waste products capable of generating gypsum in soil. In Handbook of Soil Conditioners; Wallace, A., Terry, R.E., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1998; pp. 257–291. [Google Scholar]
- Messick, D. Agricultural Demand for Sulphur—The Challenges, The Future, In TFI-FITR’s Outlook and Technology Conference, Georgia, USA, 19 November 2014. Available online: http://www.firt.org/sites/default/files/TFI%20FIRT%20Outlook%20-%20Agricultural%20Demand%20for%20Sulphur%20-%20TSI.pdf (accessed on 28 October 2017).
- Benardos, D. Method for Coating Fertilizer Beads with Elemental Sulfur. U.S. Patent WO2017077350A, 11 May 2017. [Google Scholar]
- Chorianopoulou, S.N.; Saridis, G.I.; Sigalas, P.P.; Margetis, M.; Benardos, D.; Mavrogiannis, H.; Bouranis, D.L. The Application of S0-coated Fertilizer to Durum Wheat Crop. In Sulfur Metabolism in Higher Plants—Fundamental, Environmental and Agricultural Aspects; Proceedings of the International Plant Sulphur Workshop; De Kok, L.J., Rennenberg, H., Hawkesford, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 115–121. [Google Scholar]
- Lindsay, W.L.; Schwab, A.P. The chemistry of iron in soils and its availability to plants. J. Plant Nutr. 1982, 5, 821–840. [Google Scholar] [CrossRef]
- Lucena, J.J. Effects of bicarbonate, nitrate and other environmental factors on iron chlorosis. A review. J. Plant Nutr. 2000, 23, 1591–1606. [Google Scholar] [CrossRef]
- Lindsay, W.L. Iron oxide solubilisation by organic matter and its effect on iron availability. In Iron Nutrition and Interaction in Plants; Chen, Y., Hadar, Y., Eds.; Kluwer: Dordrecht, The Netherlands, 1991; pp. 29–36. [Google Scholar]
- Römheld, V.; Marschner, H. Mobilization of iron in the rizosphere of different plant species. In Advances in Plant Nutrition; Tinker, B., Laüchli, A., Eds.; Praeger Scientific: New York, NY, USA, 1986; pp. 155–204. [Google Scholar]
- Rodríguez-Lucena, P.; Tomasi, N.; Pinton, R.; Hernández-Apaolaza, L.; Lucena, J.J.; Cesco, S. Evaluation of 59Fe-lignosulfonates complexes as Fe-sources for plants. Plant Soil 2009, 325, 53–63. [Google Scholar] [CrossRef]
- Lindsay, W.L. Soil and plant relationships associated with iron deficiency with emphasis on nutrient interactions. J. Plant Nutr. 1984, 7, 489–500. [Google Scholar] [CrossRef]
- Tyler, G. Mineral nutrient limitations of calcifuge plants in phosphate sufficient limestone soil. Ann. Bot. 1996, 77, 649–656. [Google Scholar] [CrossRef]
- Zohlen, Α.; Tyler, G. Immobilization of tissue iron on calcareous soil: Differences between calcicole and calcifuge plants. Oikos 2000, 89, 95–106. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 191–200. [Google Scholar]
- Jones, J.B., Jr. Soil Analysis Handbook of Reference Methods; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9780849302053. [Google Scholar]
- Chorianopoulou, S.N.; Nikologiannis, S.; Gasparatos, D.; Bouranis, D.L. Relationships between iron, sulfur, nitrogen and phosphorus in lawns grown on a calcareous soil irrigated by slightly saline water. Fresenius Environ. Bull. 2017, 26, 1240–1246. [Google Scholar]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; Lopez-Sanchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Sörbo, B. Sulfate: Turbidimetric and nephelometric methods. In Methods in Enzymology: Sulfur and Sulfur Amino Acids; Jakoby, W.B., Griffith, O.W., Eds.; Academic Press, Inc.: New York, NY, USA, 1987. [Google Scholar]
- Miller, R.O. Extractable chloride, nitrate, orthophosphate, potassium, and sulfate sulfur in plant tissue: 2% acetic acid extraction. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press LLC: Boca Raton, FL, USA, 1998; pp. 115–118. [Google Scholar]
- Astolfi, S.; Zuchi, S.; Passera, C.; Cesco, S. Does the sulfur assimilation pathway play a role in the response to Fe deficiency in maize (Zea mays L.) plants? J. Plant Nutr. 2003, 26, 2111–2121. [Google Scholar] [CrossRef]
- Chorianopoulou, S.N.; Venieraki, A.; Maniou, F.; Mavrogiannis, H.; Benardos, D.; Katinakis, P.; Bouranis, D.L. Sulfogrow®: A new type of plant biostimulant? In Proceedings of the 13th International Conference on Protection and Restoration of the Environment, Mykonos, Greece, 3–8 July 2016; pp. 733–738. [Google Scholar]
- Oburger, E.; Gruber, B.; Schindlegger, Y.; Schenkeveld, W.D.C.; Hann, S.; Kraemer, S.M.; Wenzel, W.W.; Puschenreiter, M. Root exudation of phytosiderophores from soil-grown wheat. New Phytol. 2014, 203, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Oburger, E.; Gruber, B.; Wanek, W.; Watzinger, A.; Stanetty, C.; Schindlegger, Y.; Hann, S.; Schenkeveld, W.D.C.; Kraemer, S.M.; Puschenreiter, M. Microbial decomposition of 13C- labeled phytosiderophores in the rhizosphere of wheat: Mineralization dynamics and key microbial groups involved. Soil Biol. Biochem. 2016, 98, 196–207. [Google Scholar] [CrossRef]
- Walter, M.; Oburger, E.; Schindlegger, Y.; Hann, S.; Puschenreiter, M.; Kraemer, S.M.; Schenkeveld, W.D.C. Retention of phytosiderophores by the soil solid phase—Adsorption and desorption. Plant Soil 2016, 404, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Strong, J.; Wang, H. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the “FeIII–FeII redox wheel” in dynamic redox environments? J. Soils Sediments 2012, 12, 683–693. [Google Scholar] [CrossRef]
- White, J.; Edwards, J. Wheat Growth and Development; Procrop; New South Wales, Department of Primary Industries: Orange, NSW, Australia, 2007. [Google Scholar]
- McMaster, G.S. Phytomers, phyllochrons, phenology and temperate cereal development. J. Agric. Sci. 2005, 143, 137–150. [Google Scholar] [CrossRef]
- Heyneke, E.; Watanabe, M.; Erban, A.; Duan, G.; Buchner, P.; Walther, D.; Kopka, J.; Hawkesford, M.; Hoefgen, R. Characterization of the wheat leaf metabolome during grain filling and under varied N-supply. Front. Plant Sci. 2017, 8, 2048. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nishizawa, N. Methionine as a dominant precursor of phytosiderophores in Graminea plants. Plant Cell Physiol. 1987, 28, 1081–1092. [Google Scholar]
- Ma, J.F.; Shinada, T.; Matsuda, C.; Kyosuke, N.J. Biosynthesis of phytosiderophores, mugineic acid, associated with methionine cycle. Biol. Chem. 1995, 270, 16549–16554. [Google Scholar] [CrossRef]
- Astolfi, S.; Zuchi, S.; Cesco, S.; Varanini, Z.; Pinton, R. Influence of iron nutrition on sulphur uptake and metabolism in maize (Zea mays L.) roots. Soil Sci. Plant Nutr. 2004, 50, 1079–1083. [Google Scholar] [CrossRef]
- Astolfi, S.; Zuchi, S.; Hubberten, M.-H.; Pinton, R.; Hoefgen, R. Supply of sulphur to S-deficient young barley seedlings restores their capability to cope with iron shortage. J. Exp. Bot. 2010, 61, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II; MicroMacroPublishing, Inc.: Athens, GA, USA, 1996. [Google Scholar]
- Reuter, D.J.; Robinson, J.B. Plant Analysis, an Interpretation Manual, 2nd ed.; CSIRO Publishing: Clayton, Australia, 1997; pp. 244–245. [Google Scholar]


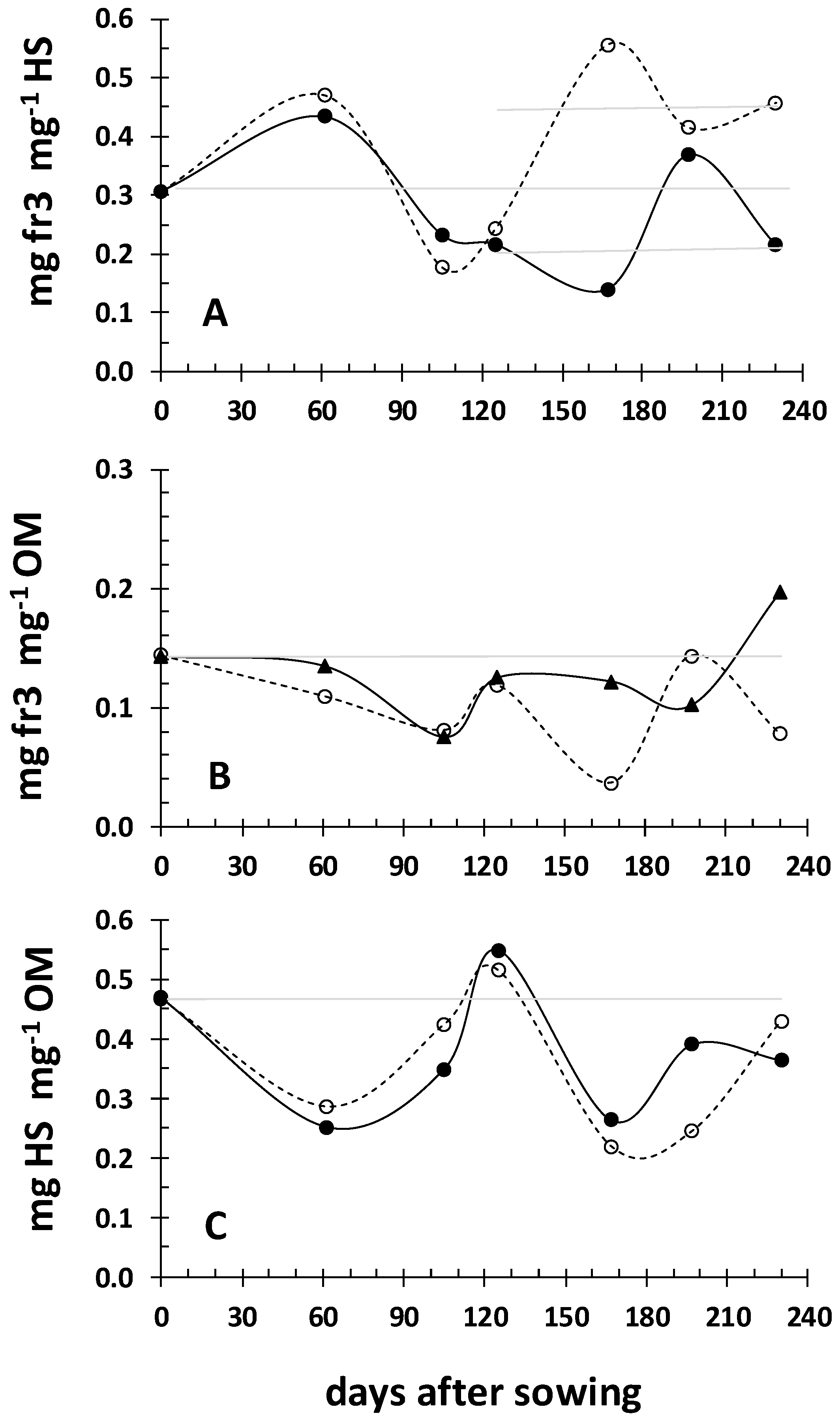
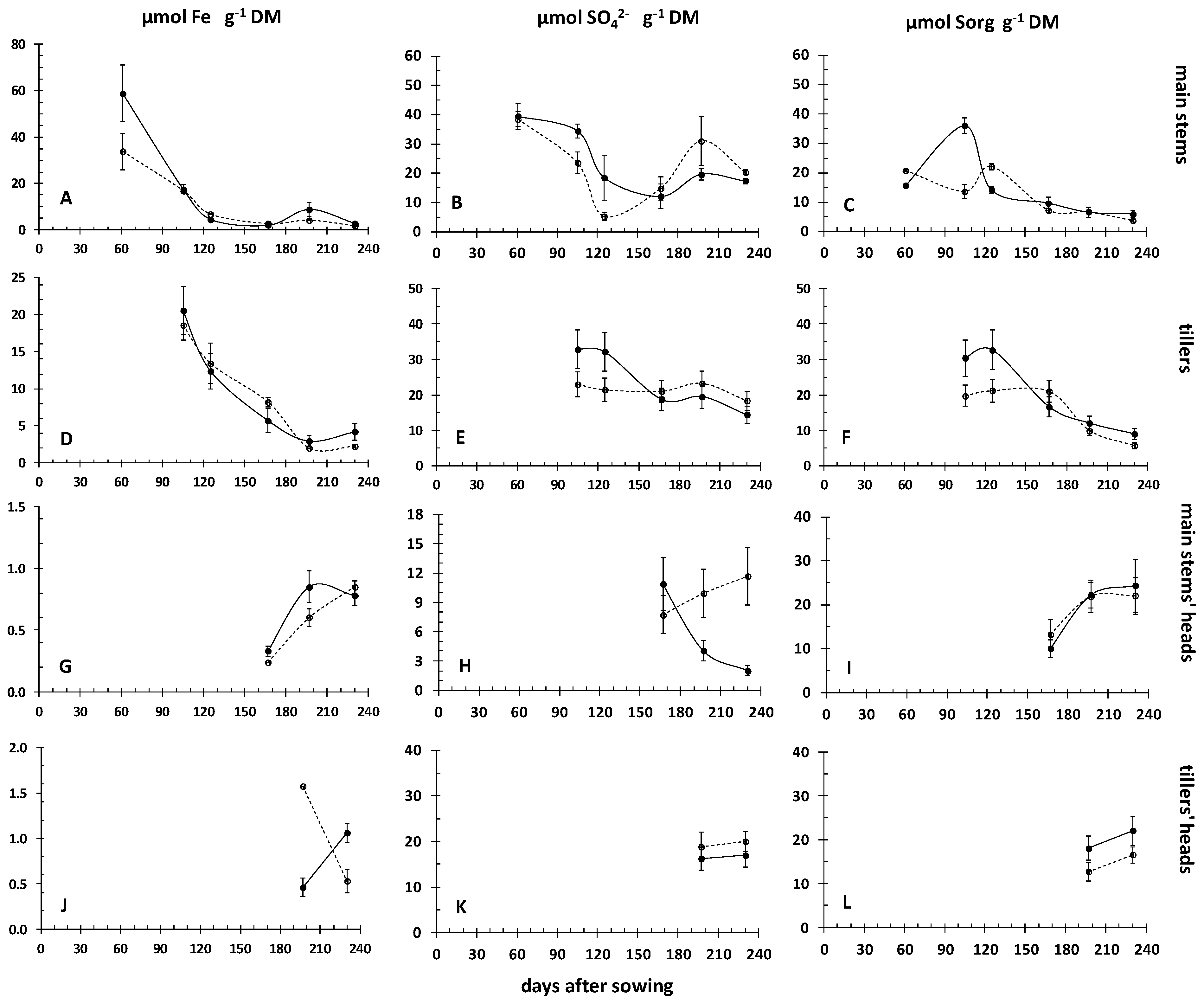
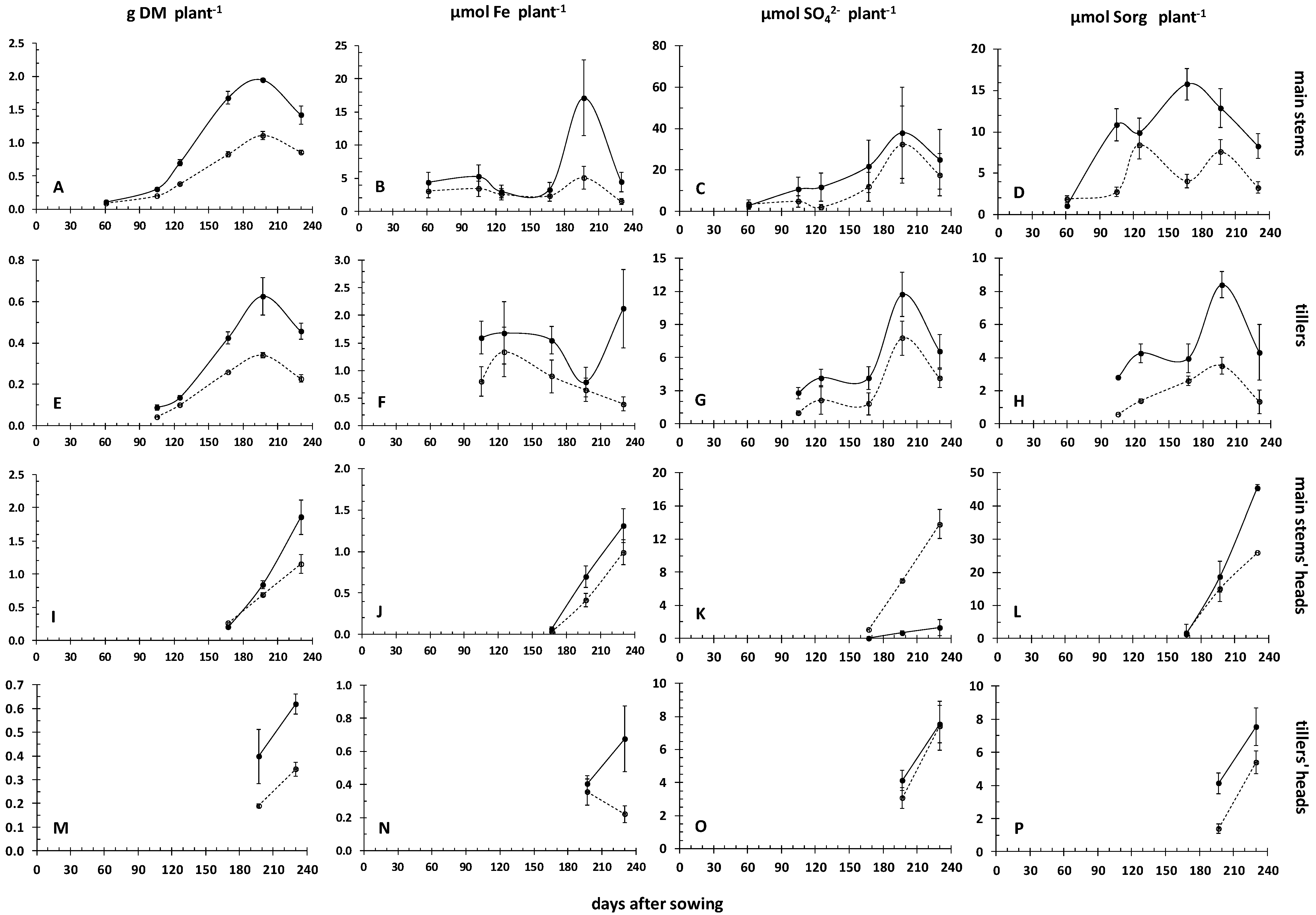
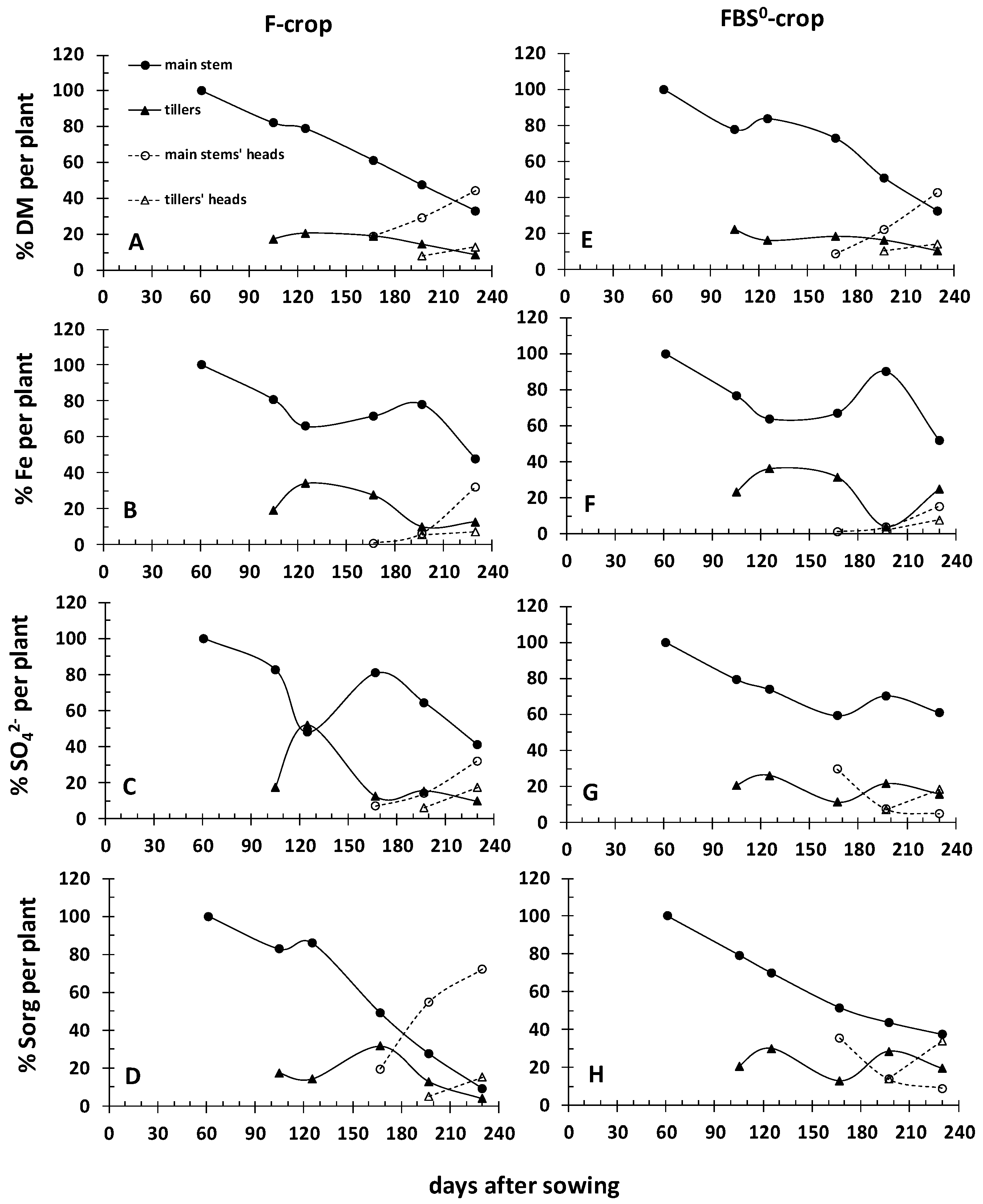
| Field | Clay | Loam | Sand | Class | CaCO3 | NO3- | P-Olsen | Kexch | Mn-DTPA | Cu-DTPA | Zn-DTPA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | ppm | ppm | ppm | ppm | ppm | ppm | ||
| F-crop | 42 | 28 | 30 | C | 13.1 | 10.51 | 18.2 | 170 | 7.51 | 1.74 | 1.1 |
| FBS0-crop | 41 | 31 | 28 | C | 16.7 | 11.68 | 19.7 | 240 | 7.84 | 1.5 | 0.92 |
| Days after Sowing | ||||
|---|---|---|---|---|
| number | F-crop | |||
| of tillers | 105 | 125 | 167 | 197 |
| 0 | 32 | 60 | 63 | 57 |
| 1 | 38 | 22 | 19 | 21 |
| 2 | 25 | 11 | 15 | 20 |
| 3+ | 5 | 7 | 3 | 2 |
| FBS0-crop | ||||
| 105 | 125 | 167 | 197 | |
| 0 | 44 | 50 | 82 | 85 |
| 1 | 15 | 20 | 14 | 13 |
| 2 | 25 | 20 | 2 | 1 |
| 3+ | 16 | 10 | 2 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouranis, D.L.; Chorianopoulou, S.N.; Margetis, M.; Saridis, G.I.; Sigalas, P.P. Effect of Elemental Sulfur as Fertilizer Ingredient on the Mobilization of Iron from the Iron Pools of a Calcareous Soil Cultivated with Durum Wheat and the Crop’s Iron and Sulfur Nutrition. Agriculture 2018, 8, 20. https://doi.org/10.3390/agriculture8020020
Bouranis DL, Chorianopoulou SN, Margetis M, Saridis GI, Sigalas PP. Effect of Elemental Sulfur as Fertilizer Ingredient on the Mobilization of Iron from the Iron Pools of a Calcareous Soil Cultivated with Durum Wheat and the Crop’s Iron and Sulfur Nutrition. Agriculture. 2018; 8(2):20. https://doi.org/10.3390/agriculture8020020
Chicago/Turabian StyleBouranis, Dimitris L., Styliani N. Chorianopoulou, Miltiadis Margetis, Georgios I. Saridis, and Petros P. Sigalas. 2018. "Effect of Elemental Sulfur as Fertilizer Ingredient on the Mobilization of Iron from the Iron Pools of a Calcareous Soil Cultivated with Durum Wheat and the Crop’s Iron and Sulfur Nutrition" Agriculture 8, no. 2: 20. https://doi.org/10.3390/agriculture8020020





