Assessment of Mesotrione Leaching Applied Alone and Mixed in Seven Tropical Soils Columns under Laboratory Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Experimental Design
2.3. Preparation of Glass Columns Packed with Soil
2.4. Chemicals and Application
2.5. Leaching Experiments
2.6. Statistical Analysis
3. Results and Discussion
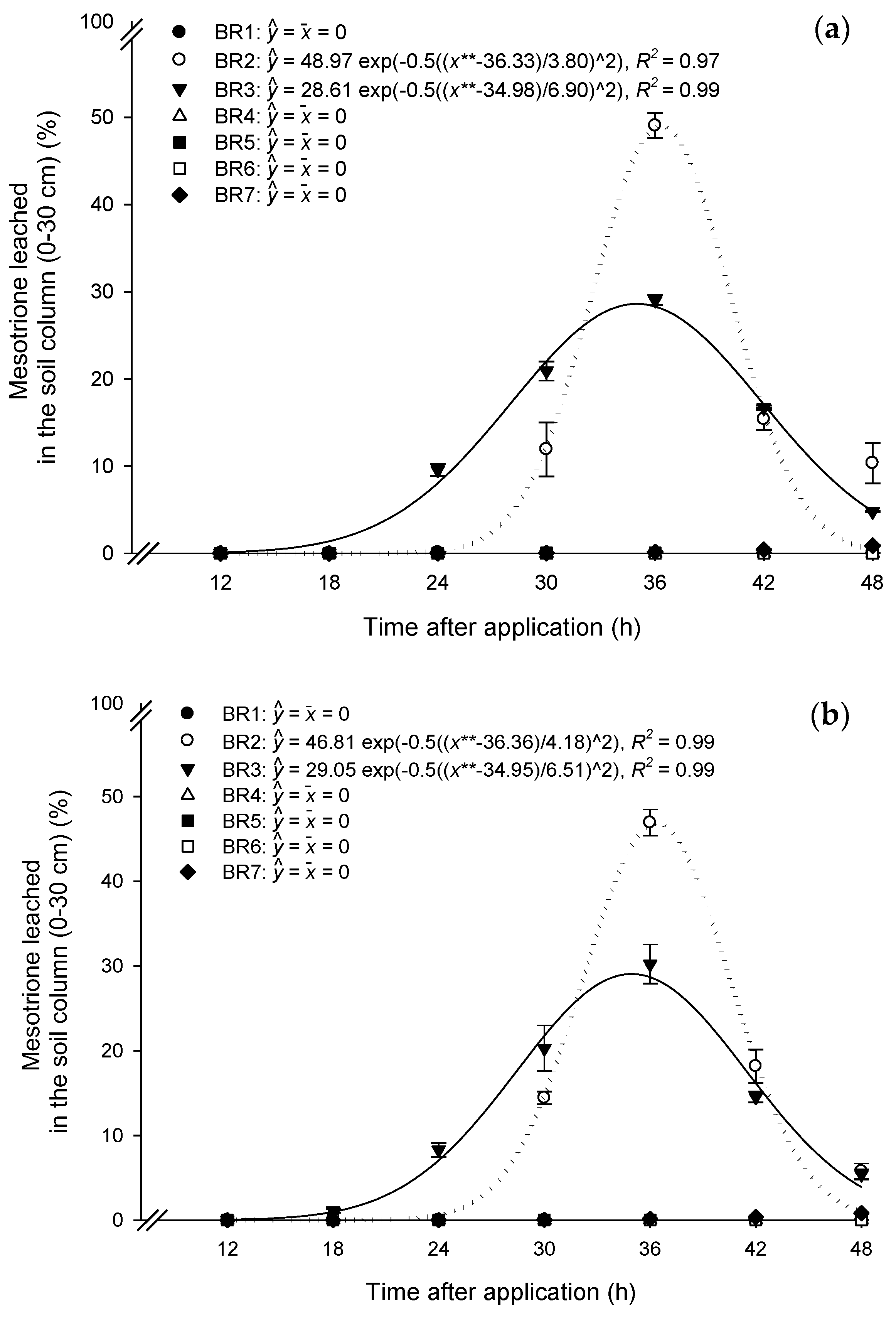
3.1. Mass Balance
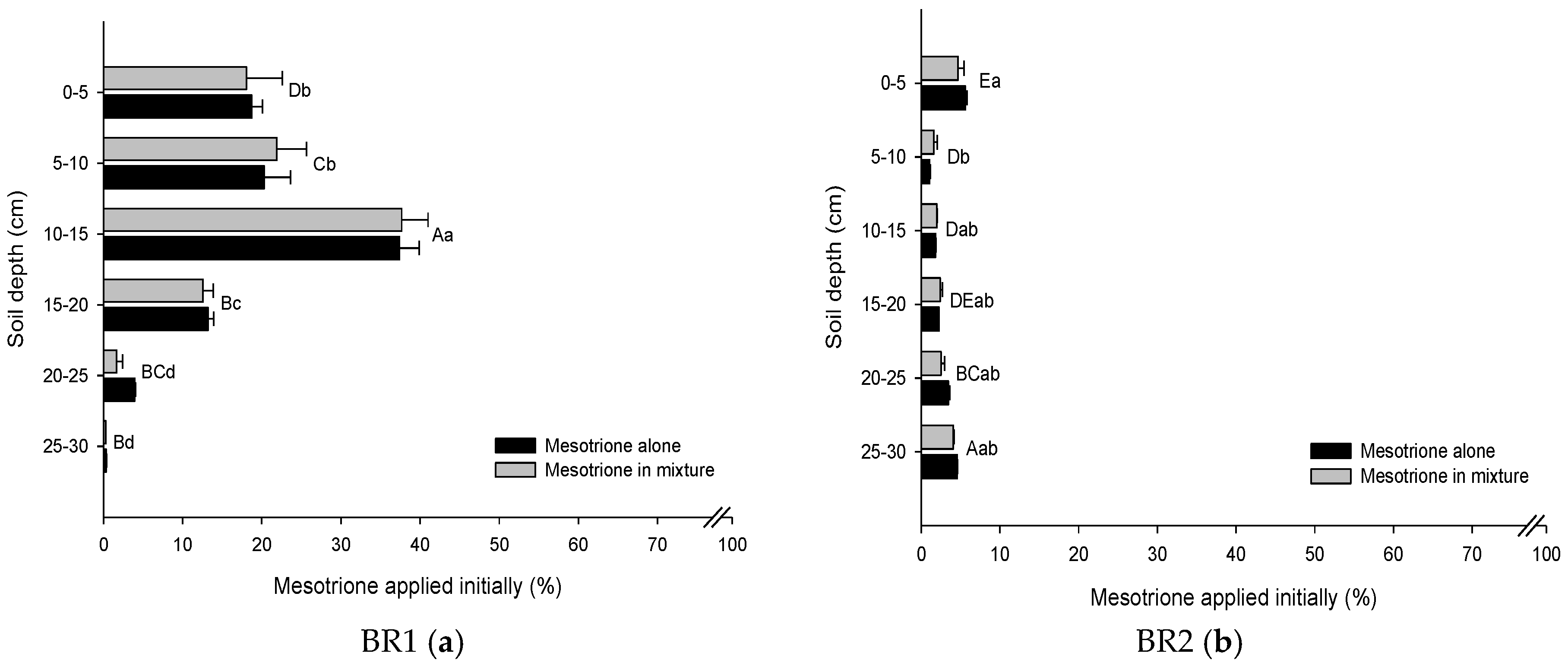
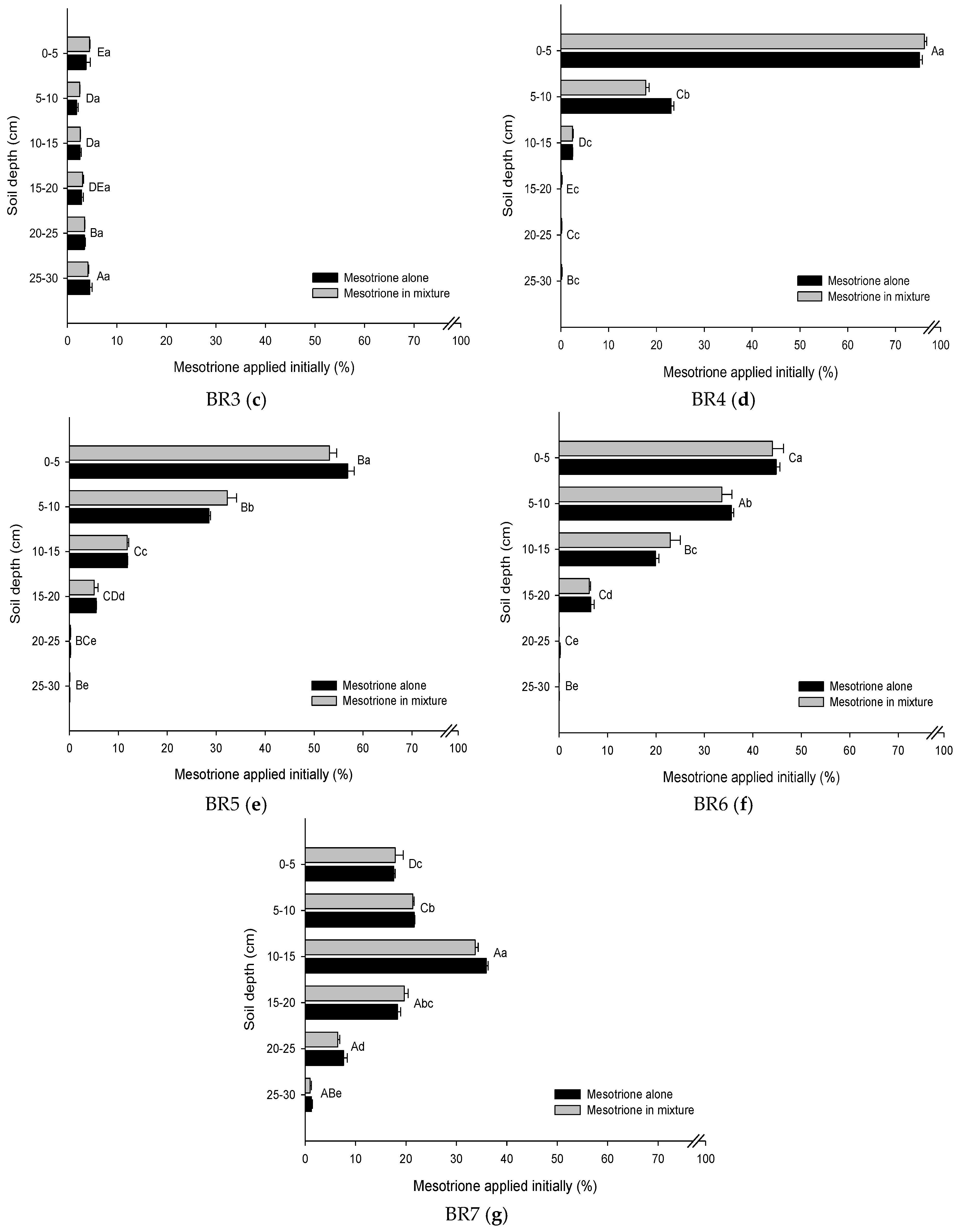
3.2. Leaching Mesotrione Alone and in a Mixture
3.3. Leachate Mesotrione Alone and in a Mixture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, H.H. Pesticide in Soil Environment: Processes, Impacts and Modeling; Soil Science Society of America: Madison, WI, USA, 1990; p. 511. [Google Scholar]
- Brady, N.C.; Weil, R.R. Soil and Chemical Pollution. In The Nature and Properties of Soils; Weil, R., Brady, N., Eds.; Pearson Education: Berkeley, CA, USA, 2016; pp. 879–935. [Google Scholar]
- Nurse, R.E.; Hamill, A.S.; Swanton, C.J.; Tardif, F.J.; Sikkema, P.H. Weed control and yield response to mesotrione in maize (Zea mays). Crop Prot. 2010, 29, 652–657. [Google Scholar] [CrossRef]
- Pinna, M.V.; Roggero, P.P.; Seddaiu, G.; Pusino, A. Soil sorption and leaching of active ingredients of Lumax® under mineral or organic fertilization. Chemosphere 2014, 111, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Milan, M.; Ferrero, A.; Fogliatto, S.; Piano, S.; Vidotto, F. Leaching of S-metolachlor, terbuthylazine, desethyl-terbuthylazine, mesotrione, flufenacet, isoxaflutole, and diketonitrile in field lysimeters as affected by the time elapsed between spraying and first leaching event. J. Environ. Sci. Health Part B 2015, 50, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Rouchaud, J.; Neus, O.; Eelen, H.; Bulcke, R. Mobility and adsorption of the triketone herbicide mesotrione in the soil of corn crops. Toxicol. Environ. Chem. 2001, 79, 211–222. [Google Scholar] [CrossRef]
- Alferness, P.; Wiebe, L. Determination of mesotrione residues and metabolites in crops, soil, and water by liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2002, 50, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Mastichiadis, C.; Christofidis, I.; Koupparis, M.A.; Willetts, C.; Kakabakos, S.E. Solid-phase fluoroimmunoassay for the determination of mesotrione—A novel triketone herbicide—In water with direct measurement of the fluorescence onto the solid support. Analyst 2003, 128, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.G.; Götz, C.W.; Ruff, M.; Singer, H.P.; Müller, S.R. Quantification of the new triketone herbicides, sulcotrione and mesotrione, and other important herbicides and metabolites, at the ng/l level in surface waters using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2004, 1028, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Barchanska, H.; Rusek, M.; Szatkowska, A. New procedures for simultaneous determination of mesotrione and atrazine in water and soil. Comparison of the degradation processes of mesotrione and atrazine. Environ. Monit. Assess. 2012, 184, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.S.; Beulke, S.; Brown, C.D.; Lane, M.C.G. Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. J. Environ. Qual. 2002, 31, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.; Brunk, G.; Nissen, S.; Westra, P.; Chen, W. Role of soil sorption and microbial degradation on dissipation of mesotrione in plant-available soil water. J. Environ. Qual. 2012, 41, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, T.; Kolyagin, Y.; Sancelme, M.; Besse-Hoggan, P. Effect of soil properties on pure and formulated mesotrione adsorption onto vertisol (Limagne plane, Puy-de-Dôme, France). Chemosphere 2014, 111, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A.; Wright, D.J. Degradation of chlorpyrifos, fenamiphos, and chlorothalonil alone and in combination and their effects on soil microbial activity. Environ. Toxicol. Chem. 2002, 21, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Swarcewicz, M.K.; Gregorczyk, A. The effects of pesticide mixtures on degradation of pendimethalin in soils. Environ. Monit. Assess. 2012, 184, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa Solos: Brasília, Brazil, 2013. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). OECD Guidelines for Testing of Chemicals; Test No. 312: Leaching in Soil Columns; Organization for Economic Co-operation and Development (OECD): Paris, France, 2004. [Google Scholar]
- Mendes, K.F.; Reis, M.R.; Inoue, M.H.; Pimpinato, R.F.; Tornisielo, V.L. Sorption and desorption of mesotrione alone and mixed with S-metolachlor + terbuthylazine in Brazilian soils. Geoderma 2016, 280, 22–28. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Pot, V.; Alletto, L.; Mamy, L.; Bedos, C.; Barriuso, E.; Benoit, P. Comparison of three pesticide fate models with respect to the leaching of two herbicides under field conditions in an irrigated maize cropping system. Sci. Total Environ. 2014, 499, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, A.M.A.; Tiktak, A.; Boesten, J.J.T.I.; Leijnse, A. Influence of pH-dependent sorption and transformation on simulated pesticide leaching. Sci. Total Environ. 2009, 407, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.B.R.J.; Freitas, M.A.M.; Gonçalves, V.A.; Silva, G.S.; da Silva, A.A.; Queiroz, M.E.L.R.; Lima, C.F.; Silva, D.V. Leaching of sulfentrazone in soils of reforestation in Brazil. Environ. Earth Sci. 2015, 74, 1211–1215. [Google Scholar] [CrossRef]
| Soil | Origin (City, State, Geographic Coordinates) | Soil Classification—Symbology a | K | Ca2+ | Mg2+ | BS | H + Al | CEC | |||||||
| (mmolc kg−1) | |||||||||||||||
| BR1 | Rio Paranaíba, MG (19°12′29″ S; 46°07′57″ W) | Oxisol—Rhodic Hapludox (Latossolo Vermelho distroférrico—LVdf) | 11 | 70 | 16 | 97 | 57 | 154 | |||||||
| BR2 | Barra do Bugres, MT (15°07′25″ S; 57°17′21″ W) | Entisol—Typic Quartzipsamments (Neossolo Quartzarênico órtico—RQo) | 1 | 11 | 3 | 15 | 29 | 44 | |||||||
| BR3 | Barra do Bugres, MT (15°04′39″ S; 57°10′51″ W) | Entisol—Typic Quartzipsamments (Neossolo Quartzarênico órtico—RQo) | 2 | 47 | 6 | 55 | 29 | 84 | |||||||
| BR4 | Tangará da Serra, MT (14°39′01″ S; 57°25′54″ W) | Oxisol—Typic Hapludox (Latossolo Vermelho distrófico—LVd) | 4 | 25 | 11 | 40 | 67 | 107 | |||||||
| BR5 | Tangará da Serra, MT (14°39′55″ S; 57°28′05″ W) | Oxisol—Typic Hapludox (Latossolo Vermelho distrófico—LVd) | 14 | 39 | 23 | 76 | 40 | 116 | |||||||
| BR6 | Piracicaba, SP (22°42′34″ S; 47°37′18″ W) | Alfisol—Paleudult (Nitossolo Vermelho eutroférrico—NVef) | 11 | 51 | 26 | 88 | 41 | 129 | |||||||
| BR7 | Piracicaba, SP (22°42′52″ S; 47°37′10″ W) | Ultisol—Typic Hapludalf (Argissolo Vermelho-Amarelo distrófico—PVAd) | 1 | 18 | 7 | 26 | 29 | 55 | |||||||
| Soil | pH (H2O) | P (mg kg−1) | V (%) | OC (g kg−1) | Sand | CM | Silt | Texture Class | |||||||
| (g kg−1) | |||||||||||||||
| BR1 | 6.4 | 67 | 63 | 27.32 | 294 | 509 | 196 | clay | |||||||
| BR2 | 7.7 | 9 | 34 | 0.58 | 932 | 50 | 18 | sand | |||||||
| BR3 | 7.3 | 19 | 65 | 4.07 | 853 | 124 | 23 | loamy sand | |||||||
| BR4 | 6.0 | 4 | 37 | 22.09 | 282 | 605 | 113 | clay | |||||||
| BR5 | 6.7 | 55 | 66 | 12.21 | 617 | 324 | 59 | sandy clay loam | |||||||
| BR6 | 6.4 | 18 | 68 | 18.02 | 466 | 376 | 158 | sandy clay | |||||||
| BR7 | 6.9 | 15 | 47 | 5.23 | 816 | 151 | 33 | sandy loam | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, K.F.; Dos Reis, M.R.; Spokas, K.A.; Tornisielo, V.L. Assessment of Mesotrione Leaching Applied Alone and Mixed in Seven Tropical Soils Columns under Laboratory Conditions. Agriculture 2018, 8, 1. https://doi.org/10.3390/agriculture8010001
Mendes KF, Dos Reis MR, Spokas KA, Tornisielo VL. Assessment of Mesotrione Leaching Applied Alone and Mixed in Seven Tropical Soils Columns under Laboratory Conditions. Agriculture. 2018; 8(1):1. https://doi.org/10.3390/agriculture8010001
Chicago/Turabian StyleMendes, Kassio F., Marcelo R. Dos Reis, Kurt A. Spokas, and Valdemar L. Tornisielo. 2018. "Assessment of Mesotrione Leaching Applied Alone and Mixed in Seven Tropical Soils Columns under Laboratory Conditions" Agriculture 8, no. 1: 1. https://doi.org/10.3390/agriculture8010001






