Selection and Breeding of Suitable Crop Genotypes for Drought and Heat Periods in a Changing Climate: Which Morphological and Physiological Properties Should Be Considered?
Abstract
:1. Introduction
2. Root Morphology and Physiology
3. Stem Properties and Solute Allocation via Xylem and Phloem
4. Leaf Morphology and Physiology
5. Reproductive Structures and Yield Formation
6. Proteomics in Search of Molecular Markers for Assisted Selection and Breeding
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| P5CS | D-1pyrroline-5-carboxylate synthetase |
| ROS | Reactive oxygen species |
References
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Schär, C.; Vidale, P.L.; Luthi, D.; Frei, C.; Haberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heat waves. Nature 2004, 427, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.M.; Knutti, R. Anthropogenic contribution to global occurrence of heavy-precipitation and high temperature extremes. Nat. Clim. Chang. 2015, 5, 560–564. [Google Scholar] [CrossRef]
- Knutti, R.; Rogelj, J.; Sedlacek, J.; Fischer, E.M. A scientific critique of the two-degree climate change target. Nat. Geosci. 2016, 9, 13–19. [Google Scholar] [CrossRef] [Green Version]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Internat. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [PubMed]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef] [PubMed]
- Krannich, C.T.; Maletzki, L.; Kurowsky, C.; Horn, R. Network candidate genes in breeding for drought tolerant crops. Int. J. Mol. Sci. 2015, 16, 16378–16400. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, V.; Signarbieux, C.; Anders, I.; Feller, U. Genotypic variation in drought stress response and subsequent recovery of wheat (Triticum aestivum L.). J. Plant Res. 2011, 124, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.H.; Yendrek, C.R.; Drag, D.; Locke, A.M.; Acosta, R.L.; Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Ort, D.R. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Chang. Biol. 2015, 21, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, M.; Picon-Cochard, C.; Morvan-Bertrand, A.; Prud’homme, M.-P.; Volaire, F. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann. Bot. 2015, 116, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Prasad, P.V.V. Variability of root traits in spring wheat germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. C R. Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Shanker, A.K.; Maheswari, M.; Yadav, S.K.; Desai, S.; Bhanu, D.; Attal, N.B.; Venkateswarlu, B. Drought stress responses in crops. Funct. Integr. Genomics 2014, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.D.; Mooney, H.A.; Sala, O.E.; Jobbagy, E.; Buchmann, N.; Bauer, G.; Canadell, J.; Jackson, R.B.; Loreti, J.; Oesterheld, M.; et al. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 1996, 108, 503–511. [Google Scholar] [CrossRef]
- Signarbieux, C.; Feller, U. Effects of an extended drought period on physiological properties of grassland species in the field. J. Plant Res. 2012, 125, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bollig, C.; Feller, U. Impacts of drought stress on water relations and carbon assimilation in grassland species at different altitudes. Agric. Ecosyst. Environ. 2014, 188, 212–220. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Vassileva, V.; Demirevska, K.; Simova-Stoilova, L.; Petrova, T.; Tsenov, N.; Feller, U. Long-term field drought affects leaf protein pattern and chloroplast ultrastructure of winter wheat in a cultivar-specific manner. J. Agric. Crop Sci. 2012, 198, 104–117. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Morito, Y.; Jitsuyama, Y.; An, P.; Inoue, T.; Inagaki, M. Effects of root water uptake efficiency on soil water utilization in wheat (Triticum aestivum L.) under severe drought environments. J. Agric. Crop Sci. 2015, 201, 161–172. [Google Scholar] [CrossRef]
- Reader, R.J.; Jalili, A.; Grime, J.P.; Spencer, R.E.; Matthews, N. A comparative-study of plasticity in seedling rooting depth in drying soil. J. Ecol. 1993, 81, 543–550. [Google Scholar] [CrossRef]
- Grieder, C.; Trachsel, S.; Hund, A. Early vertical distribution of roots and its association with drought tolerance in tropical maize. Plant Soil 2014, 377, 295–308. [Google Scholar] [CrossRef]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant. Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Von Mark, V.C.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Inukai, Y.; Kitano, H.; Yamauchi, A. Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 2011, 342, 117–128. [Google Scholar] [CrossRef]
- Babe, A.; Lavigne, T.; Severin, J.P.; Nagel, K.A.; Walter, A.; Chaumont, F.; Batoko, H.; Beeckman, T.; Draye, X. Repression of early lateral root initiation events by transient water deficit in barley and maize. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012, 367, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Lamont, K.J.; Pan, H.Y.; Barry, L.A.; Hall, A.; Rogiers, S.Y. Spring root-zone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Aust. J. Grape Wine Res. 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Nippert, J.B.; Holdo, R.M. Challenging the maximum rooting depth paradigm in grasslands and savannas. Funct. Ecol. 2015, 29, 739–745. [Google Scholar] [CrossRef]
- Zhang, H.M.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.B.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Desnos, T. Root branching responses to phosphate and nitrate. Curr. Opin. Plant Biol. 2008, 11, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [PubMed]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Rubio-Wilhelmi, M.M.; Blasco, B.; Constan-Aguilar, C.; Romero, L.; Ruiz, J.M. Variation in the use efficiency of N under moderate water deficit in tomato plants (Solanum lycopersicum) differing in their tolerance to drought. Acta Physiol. Plant. 2011, 33, 1861–1865. [Google Scholar] [CrossRef]
- Ge, T.D.; Sun, N.B.; Bai, L.P.; Tong, C.L.; Sui, F.G. Effects of drought stress on phosphorus and potassium uptake dynamics in summer maize (Zea mays) throughout the growth cycle. Acta Physiol. Plant. 2012, 34, 2179–2186. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Waraich, E.A.; Naeem, M.S.; Shabbir, R.N. Nutrient uptake, physiological responses, and yield attributes of wheat (Triticum aestivum L.) exposed to early and late drought stress. J. Plant Nutr. 2012, 35, 961–974. [Google Scholar]
- Ren, H.B.; Wei, K.F.; Jia, W.S.; Davies, W.J.; Zhang, J.H. Modulation of root signals in relation to stomatal sensitivity to root-sourced abscisic acid in drought-affected plants. J. Integrat. Plant Biol. 2007, 49, 1410–1420. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B.R. Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. J. Exp. Bot. 2011, 62, 5311–5333. [Google Scholar] [CrossRef] [PubMed]
- De Ollas, C.; Hernando, B.; Arbona, V.; Gomez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrainzar, E.; Molenaar, J.A.; Wienkoop, S.; Gil-Quintana, E.; Alibert, B.; Limami, A.M.; Arrese-Igor, C.; Gonzalez, E.M. Drought stress provokes the down-regulation of methionine and ethylene biosynthesis pathways in Medicago truncatula roots and nodules. Plant Cell Environ. 2014, 37, 2051–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, H.D.; MacAdam, J.W. Effect of drought on growth, carbohydrates, and soil water use by perennial ryegrass, tall fescue, and white clover. Crop Sci. 2001, 41, 156–166. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Sanchez-Diaz, M.; Avice, J.C.; Ourry, A. Effect of drought, elevated CO2 and temperature on accumulation of N and vegetative storage proteins (VSP) in taproot of nodulated alfalfa before and after cutting. Plant Sci. 2007, 172, 903–912. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Adaptive responses of Beta vulgaris L. and Cichorium intybus L. root and leaf forms to drought stress. J. Agric. Crop Sci. 2014, 200, 108–118. [Google Scholar]
- Kivuva, B.M.; Githiri, S.M.; Yencho, G.C.; Sibiya, J. Screening sweetpotato genotypes for tolerance to drought stress. Field Crops Res. 2015, 171, 11–22. [Google Scholar] [CrossRef]
- Ruizlozano, J.M.; Azcon, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance—Physiological and nutritional plant-responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar]
- Roberts, S.K. Regulation of K+ channels in maize roots by water stress and abscisic acid. Plant Physiol. 1998, 116, 145–153. [Google Scholar] [CrossRef]
- Pilot, G.; Gaymard, F.; Mouline, K.; Cherel, I.; Sentenac, H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; Zimmermann, U. Hydraulic conductance and K+ transport into the xylem depend on radial volume flow, rather than on xylem pressure, in roots of intact, transpiring maize seedlings. New Phytol. 2009, 181, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; Stefano, G.; Shabala, L.; Rossi, M.; Mancuso, S.; Shabala, S. Sequential depolarization of root cortical and stelar cells induced by an acute salt shock—Implications for Na+ and K+ transport into xylem vessels. Plant Cell Environ. 2011, 34, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Benefit, cost and water-use efficiency of arbuscular mycorrhizal durum wheat grown under drought stress. Mycorrhiza 1998, 8, 41–45. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.J.; Mortimer, P.E.; Lintnaar, A.; Borgo, R. Drought responses of arbuscular mycorrhizal grapevines. Symbiosis 2006, 41, 127–133. [Google Scholar]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hort. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Borowicz, V.A. The impact of arbuscular mycorrhizal fungi on strawberry tolerance to root damage and drought stress. Pedobiologia 2010, 53, 265–270. [Google Scholar] [CrossRef]
- Barzana, G.; Aroca, R.; Paz, J.A.; Chaumont, F.; Martinez-Ballesta, M.C.; Carvajal, M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann. Bot. 2012, 109, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.L.; Zhang, S.B.; Chen, B.D. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [PubMed]
- Jensen, E.S.; Nielsen, H.H. How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Crews, T.E.; Peoples, M.B. Legume versus fertilizer sources of nitrogen: Ecological tradeoffs and human needs. Agric. Ecosyst. Environ. 2004, 102, 279–297. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Voisin, A.S.; Oury, V.; Salon, C.; Crozat, Y.; Jeuffroy, M.H. Inhibition and recovery of symbiotic N-2 fixation by peas (Pisum sativum L.) in response to short-term nitrate exposure. Plant Soil 2011, 346, 275–287. [Google Scholar]
- Graham, P.H.; Vance, C.P. Legumes: importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Scott, P.T.; Gresshoff, P.M. Tree legumes as feed—Stock for sustainable biofuel production: opportunities and challenges. J. Plant Physiol. 2011, 168, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- González, E.M.; Larrainzar, E.; Marino, D.; Wienkoop, S.; Gil-Quintana, E.; Arrese-Igor, C. Physiological responses of N2-fixing legumes to water limitation. In Legume Nitrogen Fixation in a Changing Environment; Springer International Publishing: Berlin, Germany; Heidelberg, Germany, 2015; pp. 5–33. [Google Scholar]
- Vadez, V.; Sinclair, T.R.; Serraj, R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol. Plant. 2000, 110, 215–223. [Google Scholar] [CrossRef]
- Streeter, J.G. Effects of drought on nitrogen fixation in soybean root nodules. Plant Cell Environ. 2003, 26, 1199–1204. [Google Scholar] [CrossRef]
- Boonkerd, N.; Weaver, R.W. Survival of cowpea rhizobia in soil as affected by soil temperature and moisture. Appl. Environ. Microbiol. 1982, 43, 585–589. [Google Scholar] [PubMed]
- Miller, M.S.; Pepper, I.L. Survival of a fast-growing strain of lupin rhizobia in Sonoran Desert soils. Soil Biol. Biochem. 1988, 20, 323–327. [Google Scholar] [CrossRef]
- Williams, P.M.; de Mallorca, M.S. Effect of osmotically induced leaf moisture stress on nodulation and nitrogenase activity of Glycine max. Plant Soil 1984, 80, 267–283. [Google Scholar] [CrossRef]
- Morieri, G.; Martinez, E.A.; Jarynowski, A.; Driguez, H.; Morris, R.; Oldroyd, G.E.; Downie, J.A. Host-specific Nod-factors associated with Medicago truncatula nodule infection differentially induce calcium influx and calcium spiking in root hairs. New Phytol. 2013, 200, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Worrall, V.S.; Roughley, R.J. The effect of moisture stress on infection of Trifolium subterraneum L. by Rhizobium trifolii Dang. J. Exp. Bot. 1976, 27, 1233–1241. [Google Scholar] [CrossRef]
- Kirova, E.; Tzvetkova, N.; Vaseva, I.; Ignatov, G. Photosynthetic responses of nitrate-fed and nitrogen-fixing soybeans to progressive water stress. J. Plant Nutr. 2008, 31, 445–458. [Google Scholar] [CrossRef]
- Marquez-Garcia, B.; Shaw, D.; Cooper, J.W.; Karpinska, B.; Quain, M.D.; Makgopa, E.M.; Kunert, K.; Foyer, C.H. Redox markers for drought-induced nodule senescence, a process occurring after drought-induced senescence of the lowest leaves in soybean (Glycine max). Ann. Bot. 2015, 116, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Ladrera, R.; Marino, D.; Larrainzar, E.; Gonzalez, E.M.; Arrese-Igor, C. Reduced carbon availability to bacteroids and elevated ureides in nodules, but not in shoots, are involved in the nitrogen fixation response to early drought in soybean. Plant Physiol. 2007, 145, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Izaguirre-Mayoral, M.L. Early signaling, synthesis, transport and metabolism of ureides. J. Plant Physiol. 2016, 193, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.R.; Serraj, R. Legume nitrogen-fixation and drought. Nature 1995, 378, 344. [Google Scholar] [CrossRef]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Charlson, D.V.; Korth, K.L.; Purcell, L.C. Allantoate amidohydrolase transcript expression is independent of drought tolerance in soybean. J. Exp. Bot. 2009, 60, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Arrese-Igor, C.; González, E.M.; Marino, D.; Ladrera, R.; Larrainzar, E.; Gil-Quintana, E. Physiological responses of legume nodules to drought. Plant Stress 2011, 5, 24–31. [Google Scholar]
- Kirova, E.; Nedeva, D.; Nikolova, A.; Ignatov, G. Changes in the electrophoretic spectra of antioxidant enzymes in nitrate-fed and nitrogen-fixing soybean subjected to gradual water stress. Acta Agron. Hung. 2005, 52, 323–332. [Google Scholar] [CrossRef]
- Sassi, S.; Gonzalez, E.M.; Aydi, S.; Arrese-Igor, C.; Abdelly, C. Tolerance of common bean to long-term osmotic stress is related to nodule carbon flux and antioxidant defenses: Evidence from two cultivars with contrasting tolerance. Plant Soil 2008, 312, 39–48. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Kaur, N.; Sandhu, J.S.; Gupta, S.K. Antioxidative enzymes and sucrose synthase contribute to cold stress tolerance in chickpea. J. Agron. Crop Sci. 2009, 195, 393–397. [Google Scholar] [CrossRef]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.L.; Mathesius, U. Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2016, 43, 87–104. [Google Scholar]
- Kaufmann, I.; Schulze-Till, T.; Schneider, H.U.; Zimmermann, U.; Jakob, P.; Wegner, L.H. Functional repair of embolized vessels in maize roots after temporal drought stress, as demonstrated by magnetic resonance imaging. New Phytol. 2009, 184, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Trifilo, P.; Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Salleo, S. Ion-mediated compensation for drought-induced loss of xylem hydraulic conductivity in field-growing plants of Laurus nobilis. Funct. Plant Biol. 2011, 38, 606–613. [Google Scholar] [CrossRef]
- Brodersen, C.R.; McElrone, A.J.; Choat, B.; Lee, E.F.; Shackel, K.A.; Matthews, M.A. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol. 2013, 161, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S. Phloem transport and drought. J. Exp. Bot. 2014, 65, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.J.; Raymond, C.A.; Bloomfield, C.; King, G.J. Perturbation of nutrient source-sink relationships by post-anthesis stresses in differential accumulation of nutrients in wheat grain. J. Plant Nutr. 2015, 178, 89–98. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Wei, S. Effects of PEG-induced water deficit in Solanum nigrum on Zn and Ni uptake and translocation in splot root systems. Plants 2015, 4, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A.; Golan, G.; Mayer, J.; Sinmena, B. The effect of dwarfing genes on sorghum grain filling from remobilized stem reserves under stress. Field Crops Res. 1997, 52, 43–54. [Google Scholar] [CrossRef]
- Pinheiro, C.; Passarinho, J.A.; Ricardo, C.P. Effect of drought and rewatering on the metabolism of Lupinus albus organs. J. Plant Physiol. 2004, 161, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C(3) plants. Biol. Plant. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Boughalleb, F.; Hajlaoui, H.; Denden, M. Effect of salt stress on growth, water relations, solute composition and photosynthetic capacity of the xero-halophyte Nitraria retusa (L.). Environ. Res. J. 2012, 6, 1–13. [Google Scholar]
- Shabala, L.; Mackay, A.; Tian, Y.; Jacobsen, S.E.; Zhou, D.W.; Shabala, S. Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa). Physiol. Plant. 2012, 146, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Springer: Berlin, Germany; Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S.; Shimizu, H. Effects of soil drought with nocturnal warming on leaf stomatal traits and mesophyll cell ultrastructure of a perennial grass. Crop Sci. 2009, 49, 1843–1851. [Google Scholar] [CrossRef]
- Das, A.; Mukhopadhyay, M.; Sarkar, B.; Saha, D.; Mondal, T.K. Influence of drought stress on cellular ultrastructure and antioxidant system in tea cultivars with different drought sensitivities. J. Environ. Biol. 2015, 36, 875–882. [Google Scholar] [PubMed]
- Vani, B.; Saradhi, P.P.; Mohanty, P. Alteration in chloroplast structure and thylakoid membrane composition due to in vivo heat treatment of rice seedlings: Correlation with the functional changes. J. Plant Physiol. 2001, 158, 583–592. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Jubany-Marí, T.; Alegre, L. Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant, Cell Environ. 2001, 24, 1319–1327. [Google Scholar] [CrossRef]
- Vassileva, V.; Simova-Stoilova, L.; Demirevska, K.; Feller, U. Variety-specific response of wheat (Triticum aestivum L.) leaf mitochondria to drought stress. J. Plant Res. 2009, 122, 445–454. [Google Scholar] [PubMed]
- Grigorova, B.; Vassileva, V.; Klimchuk, D.; Vaseva, I.; Demirevska, K.; Feller, U. Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J. Plant Interact. 2012, 7, 204–213. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and dessication. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals thet modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor (Book Chapter). In Crop Stress and Its Management: Perspectives and Strategies; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; de Vosa, D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Burgess, P.; Huang, B. Root antioxidant mechanisms in relation to root thermotolerance in perennial grass species contrasting in heat tolerance. PLoS ONE 2015, 10, e0138268. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhoua, P.; Huang, B. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 2013, 87, 159–166. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Chauhan, S. Wheat cultivars differing in heat tolerance show a differential response to oxidative stress during monocarpic senescence under high temperature stress. Protoplasma 2015, 252, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Simova-Stoilova, L.; Demirevska, K.; Petrova, T.; Tsenov, N.; Feller, U. Antioxidative protection in wheat varieties under severe recoverable drought at seedling stage. Plant Soil Environ. 2008, 54, 529–536. [Google Scholar]
- Simova-Stoilova, L.; Demirevska, K.; Petrova, T.; Tsenov, N.; Feller, U. Antioxidative protection and proteolytic activity in tolerant and sensitive wheat (Triticum aestivum L.) varieties subjected to long-term field drought. Plant Growth Regul. 2009, 58, 107–117. [Google Scholar]
- Bazargani, M.M.; Sarhadi, E.; Bushehri, A.S.; Matros, A.; Mock, H.P.; Naghavi, M.R.; Hajihoseini, V.; Mardi, M.; Hajirezaei, M.R.; Moradi, F.; et al. A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J. Proteomics 2011, 74, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant response to drought in red and white clover. Acta Physiol. Plant. 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Wilson, R.A.; Sangha, M.K.; Banga, S.S.; Atwal, A.K.; Gupta, S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J. Environ. Biol. 2014, 35, 383–387. [Google Scholar] [PubMed]
- Hu, X.; Li, Y.; Li, C.; Yang, H.; Wang, W.; Lu, M. Characterization of small heat shock proteins associated with maize tolerance to combined drought and heat stress. J. Plant Growth Regul. 2010, 29, 455–464. [Google Scholar] [CrossRef]
- Signorelli, S.; Casaretto, E.; Sainz, M.; Díaz, P.; Monza, J.; Borsani, O. Antioxidant and photosystem II responses contribute to explain the drought—Heat contrasting tolerance of two forage legumes. Plant Physiol. Biochem. 2013, 70, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J. Dehydrins: A commonality in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Volaire, F.; Lelievre, F. Drought survival in Dactylis glomerata and Festuca arundinacea under similar rooting conditions in tubes. Plant Soil 2001, 229, 225–234. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Anders, I.; Feller, U. Identification and expression of different dehydrin subclasses involved in the drought response of Trifolium repens. J. Plant Physiol. 2014, 171, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E. Association of Rubisco activase with chaperonin-60 beta: A possible mechanism for protecting photosynthesis during heat stress. J. Exp. Bot. 2008, 58, 1923–1933. [Google Scholar]
- Cheng, Z.; Dong, K.; Ge, P.; Bian, Y.; Dong, L.; Deng, X.; Li, X.; Yan, Y. Identification of leaf proteins differentially accumulated between wheat cultivars distinct in their levels of drought tolerance. PLoS ONE 2015, 10, e0125302. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dinler, B.S.; Vignjevic, M.; Jacobsen, S.; Wollenweber, B. Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci. 2015, 230, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Kannan, M.; Reddy, A.R. A root proteomics-based insight reveals dynamic regulation of root proteins under progressive drought stress and recovery in Vigna radiata (L.) Wilczek. Planta 2011, 233, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Zhu, X.; Gong, Y.; Xiang, F.; Sun, X.; Liu, L. Proteomic analysis of heat stress response in leaves of radish (Raphanus sativus L.). Plant Mol. Biol. Rep. 2013, 31, 195–203. [Google Scholar]
- Su, M.; Li, X.F.; Ma, X.Y.; Peng, X.J.; Zhao, A.G.; Cheng, L.Q.; Chen, S.Y.; Liu, G.S. Cloning two P5CS genes from bioenergy sorghum and their expression profiles under abiotic stresses and MeJA treatment. Plant Sci. 2011, 181, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Singh, V.; van Oosterom, E.J.; Chapman, S.C.; Jordan, D.R.; Hammer, G.L. Genetic variability in high temperature effects on seed-set in sorgum. Funct. Plant Biol. 2013, 40, 439–448. [Google Scholar] [CrossRef]
- Balla, K.; Bencze, S.; Bonis, P.; Arendas, T.; Veisz, O. Changes in the photosynthetic efficiency of winter wheat in response to abiotic stress. Centr. Eur. J. Biol. 2014, 9, 519–530. [Google Scholar] [CrossRef]
- Jagadish, K.S.V.; Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V. Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. Adv. Agron. 2014, 127, 111–156. [Google Scholar]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Rushton, P.J.; Rohila, J.S. The potential of proteomics te chnologies for crop improvement under drought conditions. Crit. Rev. Plant Sci. 2011, 30, 471–490. [Google Scholar] [CrossRef]
- Abreu, I.A.; Farinha, A.P.; Negrão, S.; Gonçalves, N.; Fonseca, C.; Rodrigues, M.; Batista, R.; Saibo, N.J.M.; Oliveira, M.M. Coping with abiotic stress: Proteome changes for crop improvement. J. Proteomics 2013, 93, 145–168. [Google Scholar]
- Raorane, M.L.; Pabuayon, I.M.; Varadarajan, A.R.; Mutte, S.K.; Kumar, A.; Treumann, A.; Kohli, A. Proteomic insights into the role of the large-effect QTL qDTY12.1 for rice yield under drought. Mol. Breed. 2015, 35, 139. [Google Scholar]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Klíma, M.; Roy, A.; Prášil, I.T. Biological networks underlying abiotic stress tolerance in temperate crops—A proteomic perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar]
- Hashiguchi, A.; Ahsan, N.; Komatsu, S. Proteomics application of crops in the context of climatic changes. Food Res. Int. 2010, 43, 1803–1813. [Google Scholar] [CrossRef]
- Luo, J.; Tang, S.; Peng, X.; Yan, X.; Zeng, X.; Li, J.; Li, X.; Wu, G. Elucidation of cross-talk and specificity of early response mechanisms to salt and PEG simulated drought stresses in Brassica napus using comparative proteomic analysis. PLoS ONE 2015, 10, e0138974. [Google Scholar] [CrossRef] [PubMed]
- Timabud, T.; Yin, X.; Pongdontri, P.; Komatsu, S. Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J. Proteomics 2016, 133, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.I.; Qadir, S.; Zolla, L. Proteomics-based dissection of stress-responsive pathways in plants. J. Plant Physiol. 2007, 164, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhu, J.; Gu, A.; Lv, D.; Ge, P.; Chen, G.; Li, X.; Yan, Y. An integrative proteome analysis of different seedling organs in tolerant and sensitive wheat cultivars under drought stress and recovery. Proteomics 2015, 15, 1544–1563. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Soltani, N.; Sarhadi, E.; Pascovici, D.; Keighley, T.; Salekdeh, G.H.; Haynes, P.A.; Atwell, B.J. Shotgun proteomic analysis of long-distance drought signaling in rice roots. J. Proteome Res. 2012, 11, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Gayen, D.; Datta, S.K.; Datta, K. Dissecting root proteome of transgenic rice cultivars unravels metabolic alterations and accumulation of novel stress responsive proteins under drought stress. Plant Sci. 2015, 234, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, J.; He, X.; Sun, H.; Zhang, G.; Wu, F. Comparative proteomic analysis of drought tolerance in the two contrasting Tibetan wild genotypes and cultivated genotype. BMC Genomics 2015, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Lyon, D.; Staudinger, C.; Wienkoop, S.; González, E.M. Medicago truncatula and Glycine max: Different drought tolerance and similar local response of the root nodule proteome. J. Proteome Res. 2015, 14, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Chen, H.; Li, X.J.; Yang, M.F.; Liu, G.S.; Shen, S.H. A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochim. Biophys. Acta 2009, 1794, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.P.; Mishra, V.; Singh, N.K.; Tiwari, R.; Sharma, P.; Gupta, R.K.; Sharma, I. Deciphering the dynamics of changing proteins of tolerant and intolerant wheat seedlings subjected to heat stress. Mol. Biol. Rep. 2015, 42, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, Z.; Qiao, Z.; Wu, Z.; Cheng, L.; Wang, Y. Proteomics analysis of alfalfa response to heat stress. PLoS ONE 2013, 8, e82725. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.N.; Aman, S.; Haq, N.U.; Heckathorn, S.A.; Luthe, D. Proteomic and transcriptomic analyses of Agave americana in response to heat stress. Plant Mol. Biol. Rep. 2013, 31, 840–851. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Muthurajan, R.; Rahman, H.; Selvam, J.; Peng, S.; Zou, Y.; Jagadish, K.S.V. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Bilyeu, K.D.; Beuselinck, P.R. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Zhao, F.; Zhang, D.; Li, N.; Zhu, G.; Li, C.; Wang, W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015, 6, 298. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K.; Muthurajan, R.; Rang, Z.W.; Malo, R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22). Rice 2011, 4, 1–11. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Xiong, L.Z. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Chimungu, J.G.; Brown, K.M. Root anatomical phenes associated with water acquisition from drying soil: Targets for crop improvement. J. Exp. Bot. 2014, 65, 6155–6166. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Riesen, O.; Feller, U. Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. J. Plant Nutr. 2005, 28, 412–430. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, J.; Zhu, J.S.; Zhou, C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 75, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Reynolds-Henne, C.E.; Langenegger, A.; Mani, J.; Schenk, N.; Zumsteg, A.; Feller, U. Interactions between temperature, drought and stomatal opening in legumes. Environ. Exp. Bot. 2010, 68, 37–43. [Google Scholar] [CrossRef]
- Gallé, A.; Feller, U. Changes of photosynthetic traits in beech saplings (Fagus sylvatica) under severe drought stress and during recovery. Physiol. Plant. 2007, 131, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Stockey, R.A.; Ko, H. Cuticle micromorphology of Araucaria dejussieu. Bot. Gaz. 1986, 147, 508–548. [Google Scholar] [CrossRef]
- Lee, B.R.; Lee, D.G.; Avice, J.C.; Kim, T.H. Characterization of vegetative storage protein (VSP) and low molecular proteins induced by water deficit in stolon of white clover. Biochem. Biophys. Res. Commun. 2014, 443, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, M.C.; Eldem, V.; Hajyzadeh, M.; Unver, T. Genome-wide analysis of the bZIP transcription factors in cucumber. PLoS ONE 2014, 9, e96014. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.R.; Jia, H.H.; Chen, X.B.; Hao, L.L.; An, H.L.; Guo, X.Q. The Cotton WRKY Transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef] [PubMed]
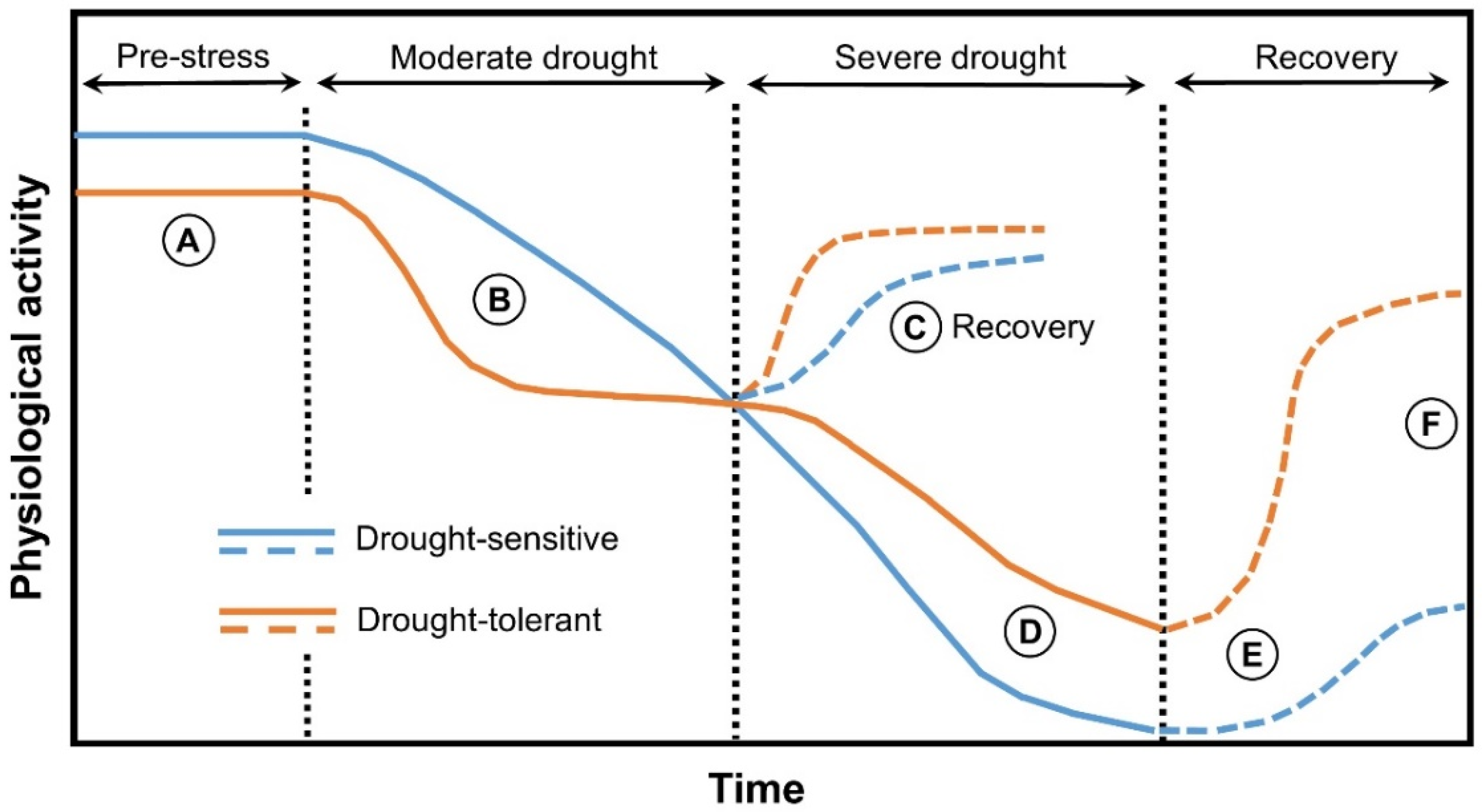
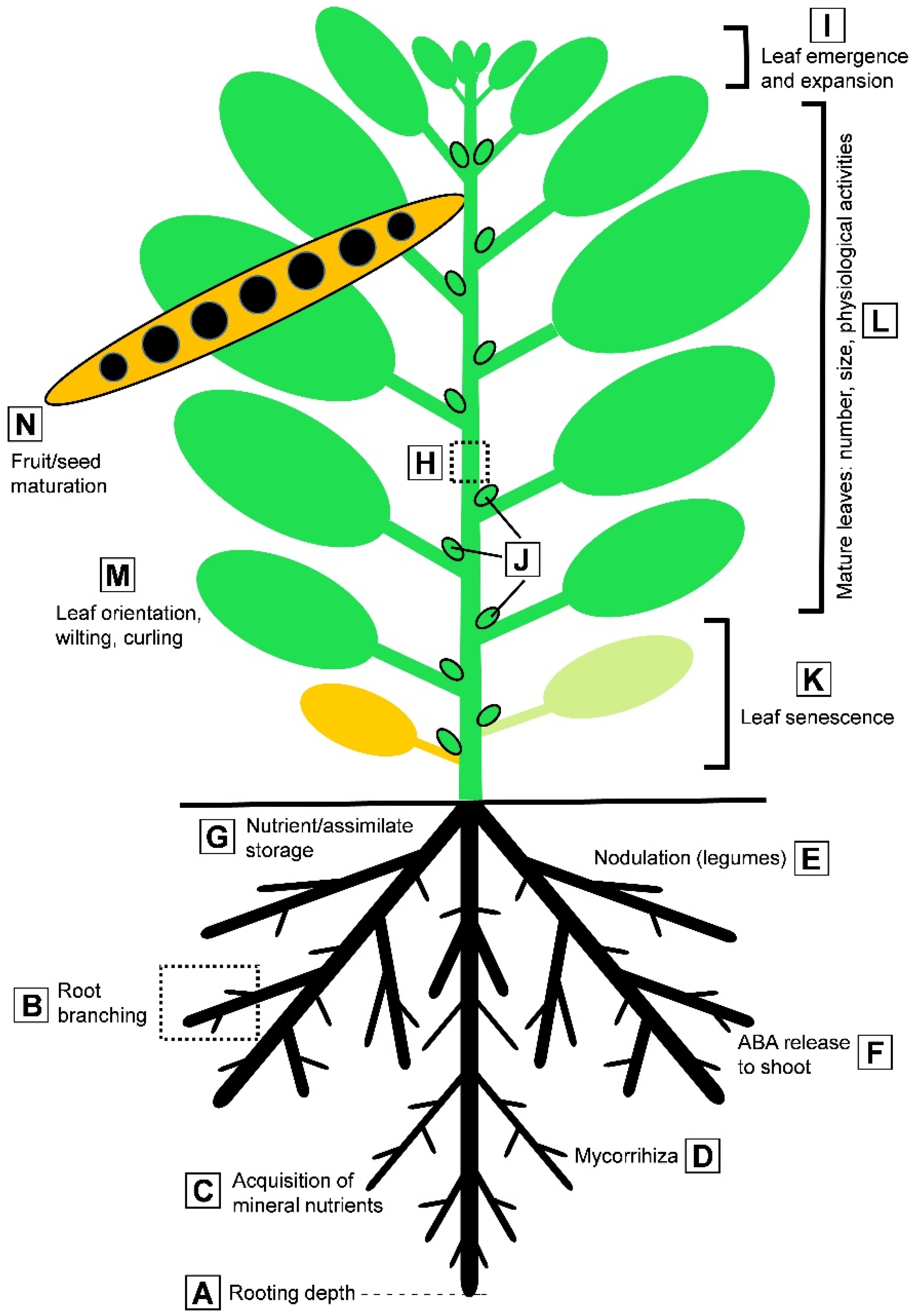

| Protein | Proposed Function | References |
|---|---|---|
| Aquaporins | H2O transport through membranes | [133] |
| Dehydrins | Stabilization of macromolecules | [134,135,136] |
| Chaperonin-60 β | Stabilization of macromolecules | [137] |
| Heat shock proteins | Stabilization of cell constituents | [138,139,140] |
| Cu/Zn Superoxide dismutase | Detoxification of reactive oxygen species | [124,141] |
| Mn Superoxide dismutase | Detoxification of reactive oxygen species | [124] |
| Fe Superoxide dismutase | Detoxification of reactive oxygen species | [124] |
| Ascorbate peroxidase | Detoxification of reactive oxygen species | [124,142] |
| Catalase | Detoxification of reactive oxygen species | [124] |
| P5CS 1 | Accumulation of proline | [143] |
| Rubisco activase | Activation of Rubisco (Calvin cycle) | [6,144] |
| Trait | Relevance for Abiotic Stress Response | References |
|---|---|---|
| Rooting depth | Access to more suitable soil regions p,c | [25,26,27,28,29] |
| Root branching | Access to more suitable soil regions p,c | [29,30,31,32,33,34,35,36,37] |
| Nutrient uptake into roots | Acquisition of mineral nutrients r,c | [44,45,46,59] |
| Xylem loading in roots | Transfer of nutrients to the shoot r,c | [57,58,59,60] |
| Nutrient assimilation in roots | Acquisition of mineral nutrients r,c | [44,45,46] |
| Mycorrhization | Acquisition of mineral nutrients/water r,c | [56,61,62,63,64,65,66,67,68,69,70] |
| Nodulation in legumes | Symbiotic nitrogen fixation p,c | [76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95] |
| Storage functions in roots | Stress survival and recovery p,c | [13,52,53] |
| Release of nutrients to shoot | Supply of aerial parts with nutrients p,c | [44,45,46] |
| Phytohormone release to shoot | Root-to-shoot signaling r | [47,48,49,50,51] |
| Root senescence | Root architecture and functions p,c | [43] |
| Xylem-to-phloem transfer | Solute channeling to leaves and fruits r | [176] |
| Xylem embolism and repair | Acropetal flux of water and solutes p | [97,98,99] |
| Storage of reserves in the stem | Accumulation of reserves for recovery r | [104,105] |
| Shoot apical meristem activity | Shoot architecture and performance p,c | [103] |
| Development of axillary buds | Shoot architecture and performance p,c | [103] |
| Leaf expansion (final size) | Shoot architecture and performance i,c | [103] |
| Leaf orientation | Light interception r | [103] |
| Leaf senescence | Loss of assimilatory capacity i,c | [103] |
| Leaf surface (wax deposition) | Reduction of non-stomatal transpiration i | [177,178] |
| Density and size of stomates | Stomatal transpiration i | [107,108,109,110] |
| Stomatal regulation | Reversible control of stomatal transpiration r | [106,179] |
| Formation of stomatal plug | Reduction of stomatal transpiration i | [180,181] |
| Mesophyll conductance | CO2 diffusion inside the leaf i | [106] |
| Vegetative storage proteins | Intermediate storage of mobilized nitrogen r | [182] |
| Intactness of organelles | Functionality of plastids and mitochondria p | [26,112,113,114,115,116,117] |
| Photosystems | Light energy conversion to ATP/NADPH r | [132] |
| Rubisco activase | Activation of Rubisco (Calvin cycle) p,c | [103,144] |
| Detoxification of ROS | Protection of cell constituents/metabolism r | [119,120,121,122,123,124,125,126,127,128,129,130,131,132] |
| Respiration in leaves | Maintenance of basic cellular functions | [9] |
| Compatible solutes | Protection of cell constituents/metabolism r | [143] |
| Transcription factors | Regulation of gene expression under stress r | [183,184,185] |
| Dehydrin pattern | Protection of cell constituents r | [134,135,136] |
| Aquaporins | Water/CO2 transport across membranes p | [133] |
| Chaperonins | Protection of enzymes r | [137,138,139,140] |
| Cytokinin levels/effects | Regulation of metabolism and senescence r | [48] |
| Proteolytic activities | Intracellular protein degradation p,c | [127] |
| Solute transport to fruits | Yield formation p,c | [145,146,147,148] |
| Seed maturation/composition | Yield quantity and quality in seed crops p,c | [5,160,168] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simova-Stoilova, L.; Vassileva, V.; Feller, U. Selection and Breeding of Suitable Crop Genotypes for Drought and Heat Periods in a Changing Climate: Which Morphological and Physiological Properties Should Be Considered? Agriculture 2016, 6, 26. https://doi.org/10.3390/agriculture6020026
Simova-Stoilova L, Vassileva V, Feller U. Selection and Breeding of Suitable Crop Genotypes for Drought and Heat Periods in a Changing Climate: Which Morphological and Physiological Properties Should Be Considered? Agriculture. 2016; 6(2):26. https://doi.org/10.3390/agriculture6020026
Chicago/Turabian StyleSimova-Stoilova, Lyudmila, Valya Vassileva, and Urs Feller. 2016. "Selection and Breeding of Suitable Crop Genotypes for Drought and Heat Periods in a Changing Climate: Which Morphological and Physiological Properties Should Be Considered?" Agriculture 6, no. 2: 26. https://doi.org/10.3390/agriculture6020026








