Relationship between Mineral Soil Surface Area and the Biological Degradation of Biosolids Added to Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application of DRM to Determine Degradation Rate
- = the carbon concentration present in the biosolids (Mg/Ha)
- = the first-order rate (year−1)
- = biosolids decomposition time (year).
- = initial concentration of C in biosolids (Mg/Ha)
- = the concentration at time t (Mg/Ha).
- = the fraction left after 1 year. decay, or , with .
2.2. Soil Particulate Size Distribution
2.2.1. Particle Mass Size Distribution Analysis
2.2.2. Particulate Surface Area Analysis
- N = the number of soil particles
- r = soil particle radius (cm)
- M = the mass of the soil particle (g)
- ρ = soil density [17] (g/cm3)
- N = the number of soil particles
- r = soil particle radius (m)
- S = particle surface area (m2).
2.3. Reference Soils from Terry
3. Results and Discussion
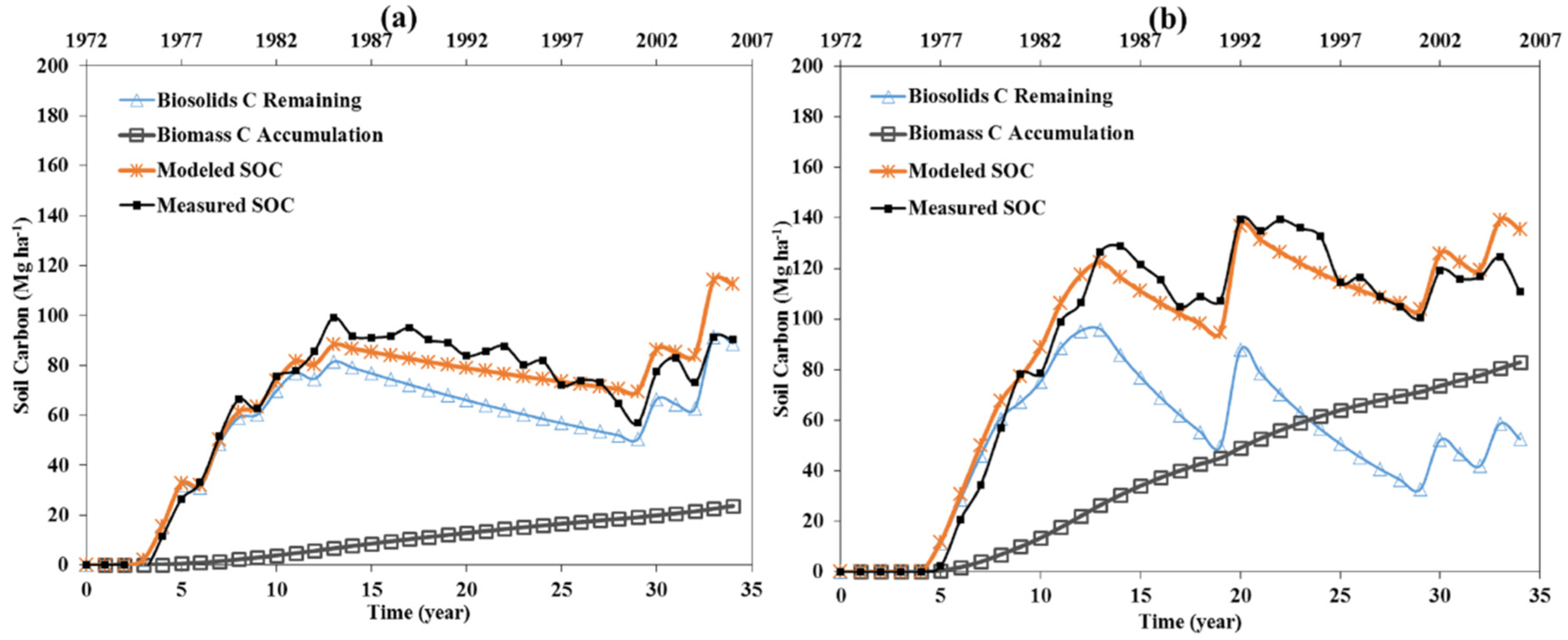
3.1. Biosolids Degradation Rate
3.1.1. DRM Estimated Rate of Biosolids Degradation
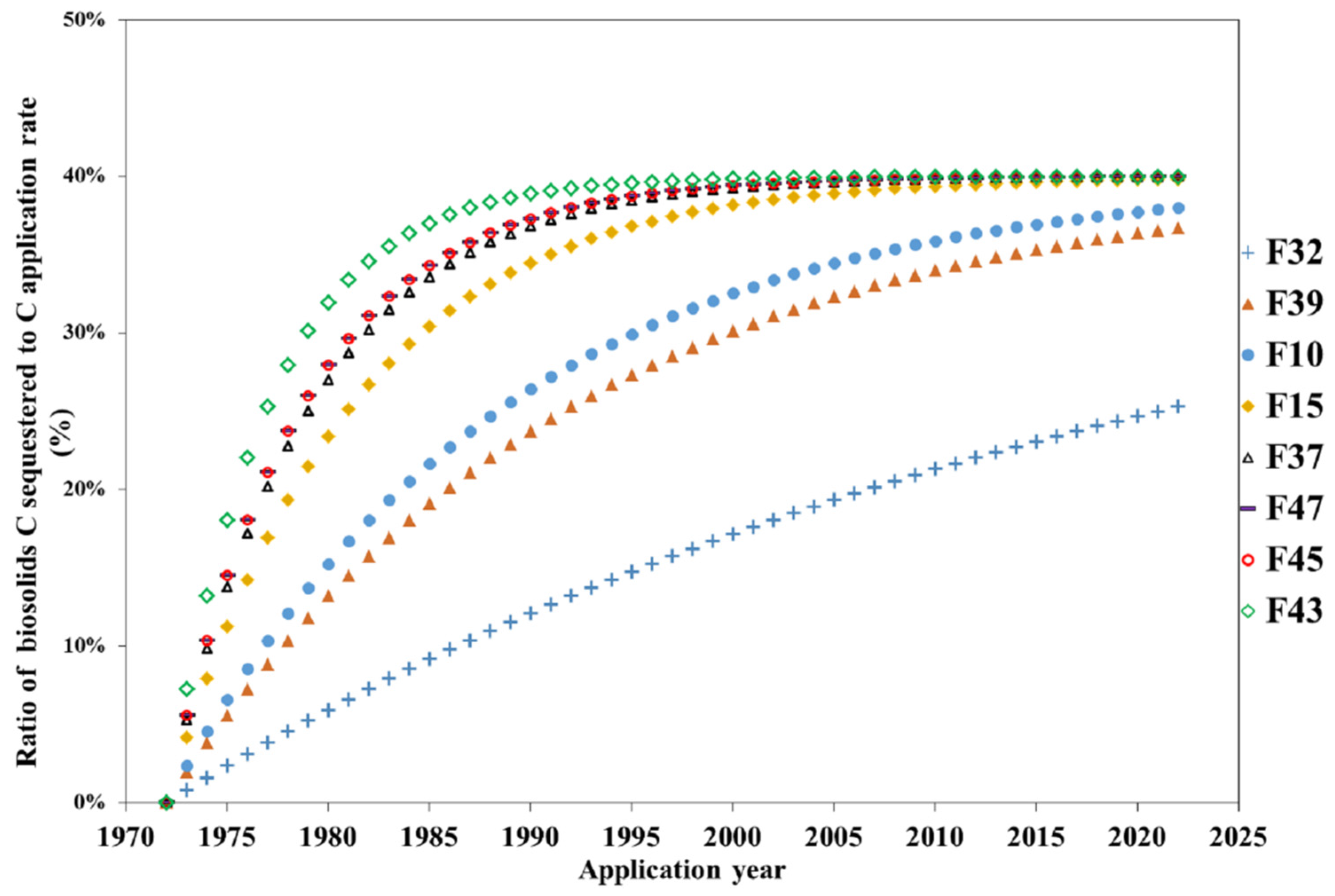
| Soil Type | Fields No. | Group No. | Biosolids Degradation Rate (year−1) | Annual Application Rate (Mg/ha) | Soil Density [17] (g/cm3) | Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| Coarse | F32 | I | 0.02 | 8.93 | 2.6 | 1.29 |
| F39 | I | 0.05 | 11.95 | 2.6 | 1.73 | |
| F10 | III | 0.06 | 12.85 | 2.6 | 2.29 | |
| F15 | I | 0.11 | 15.44 | 2.6 | 2.42 | |
| Fine | F37 | III | 0.14 | 11.75 | 2.6 | 6.55 |
| F47 | II | 0.15 | 18.02 | 2.6 | 8.54 | |
| F45 | II | 0.15 | 12.95 | 2.6 | 7.63 | |
| F43 | II | 0.20 | 15.63 | 2.6 | 7.65 | |
| Other soil samples | Fincastle | N/A | 0.13 | 5.15 | 2.6 | 4.81 |
| Chalmers | N/A | 0.10 | 5.15 | 2.6 | 4.66 | |
| [6] | Tracy | N/A | 0.07 | 5.15 | 2.6 | 2.13 |
| * | dC/dt = −kC | |||||
3.1.2. Rate of Degradation Related to Coarse and Fine Soil Type

3.1.3. Degradation Rate of Synthetic Biosolids
3.2. Particulate Mass and Surface Area Distribution Analysis

| Field | Clay | Silt | Sand | Soil Texture | |||
|---|---|---|---|---|---|---|---|
| Mass (%) | Surface Area (%) | Mass (%) | Surface Area (%) | Aass (%) | Surface Area (%) | ||
| F32 | 10.00 | 23.00 | 21.10 | 70.00 | 68.90 | 7.00 | Sandy loam |
| F39 | 10.00 | 50.50 | 23.01 | 46.30 | 67.00 | 3.21 | Sandy loam |
| F10 | 14.80 | 39.10 | 23.20 | 56.10 | 62.00 | 4.79 | Sandy loam |
| F15 | 10.00 | 54.20 | 24.10 | 43.30 | 65.90 | 3.42 | Sandy loam |
| F37 | 28.70 | 66.10 | 40.10 | 33.10 | 31.20 | 0.72 | Clay loam |
| F47 | 31.02 | 69.30 | 49.10 | 31.40 | 19.90 | 0.31 | Clay loam |
| F45 | 30.20 | 65.50 | 47.90 | 34.10 | 21.90 | 0.36 | Clay loam |
| F43 | 30.10 | 66.50 | 45.05 | 33.20 | 25.00 | 0.35 | Clay loam |
| Fincastle | 16.80 | 55.80 | 69.70 | 43.60 | 13.50 | 0.30 | Silt loam |
| Chalmers | 24.80 | 61.40 | 60.90 | 37.90 | 14.30 | 0.36 | Silt loam |
| Tracy | 8.90 | 53.80 | 37.20 | 43.00 | 53.90 | 3.10 | Sandy Loam |
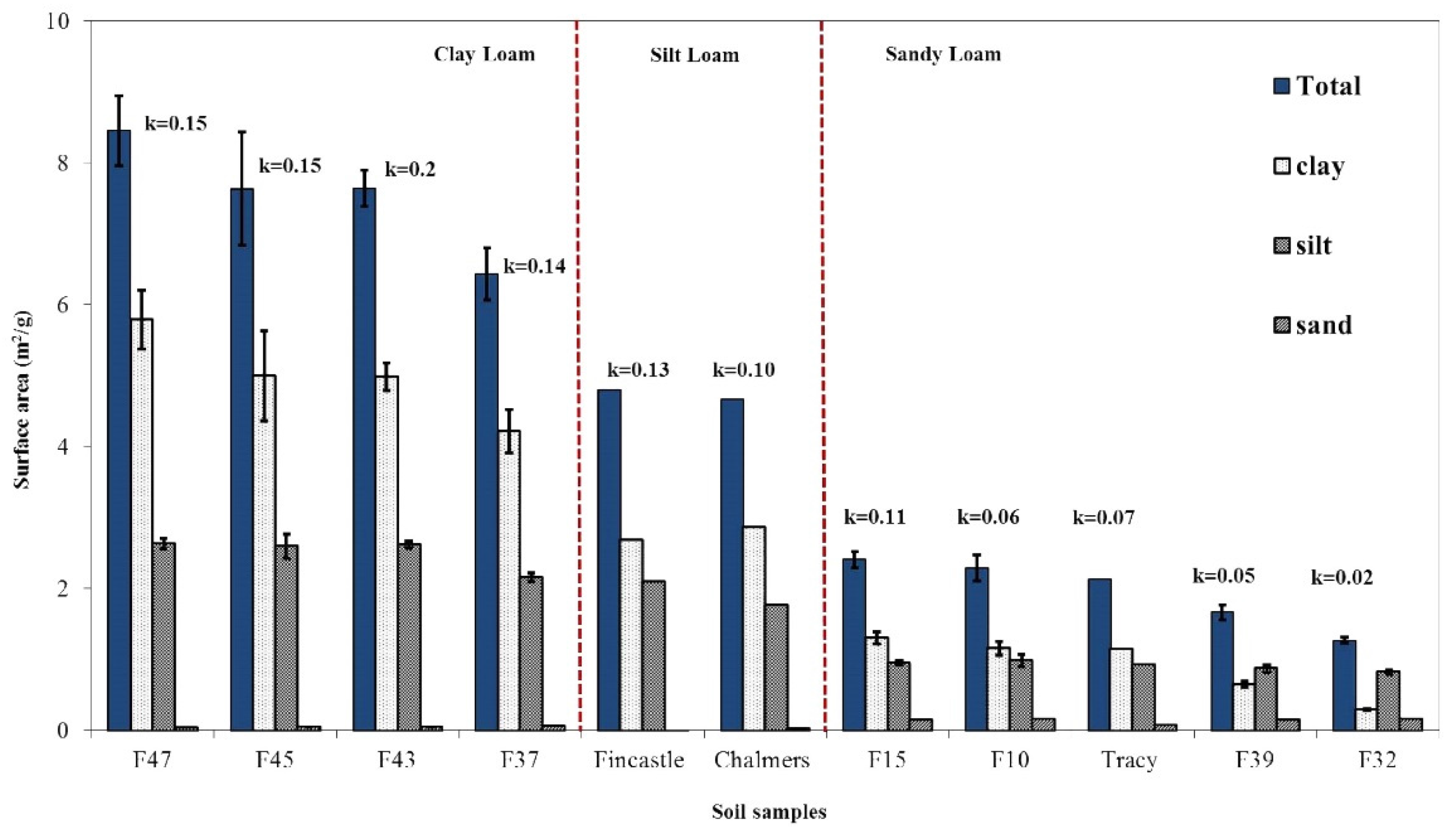
3.3. Relationship between Mineral Soil Surface Area and Biosolids Degradation
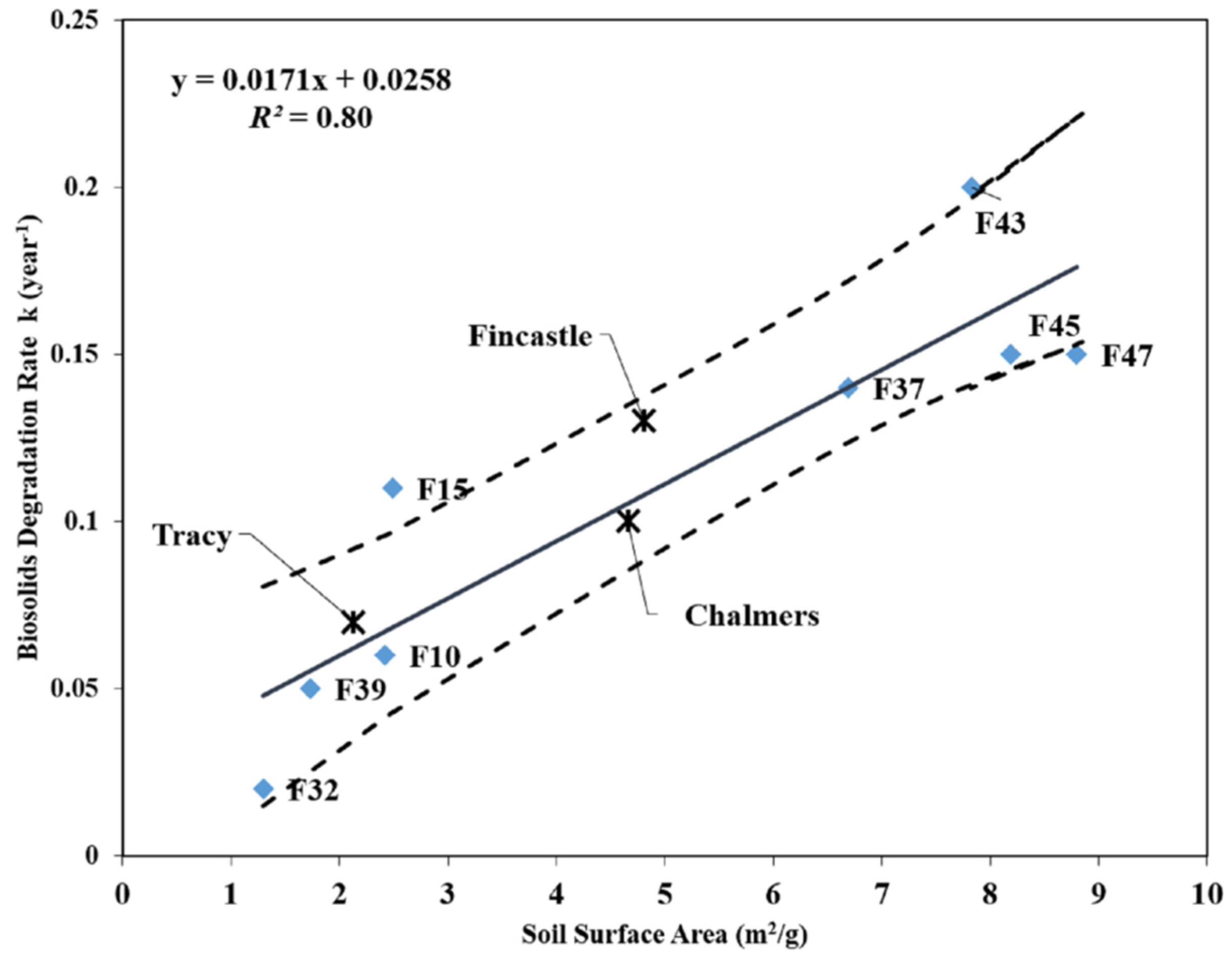
| Paired Samples | Average Biosolids Application Rate (Mg ha−1⋅year−1) | Difference of k (∆k) (year−1) | Difference of Surface Area (∆SF) (m2/g) | ∆k/∆SF (g year−1⋅m−2) |
|---|---|---|---|---|
| F39 vs. F37 | 11.85 | 0.09 | 4.82 | 0.019 |
| F10 vs. F45 | 12.90 | 0.09 | 5.34 | 0.017 |
| F15 vs. F43 | 15.54 | 0.09 | 5.23 | 0.017 |
| Average | N/A | 0.09 | 5.13 | 0.018 |
- y = the degradation rate (year−1)
- x = the total mineral soil surface area (m2/g)
3.4. C Sequestration

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Post, W.M.; Peng, T.H.; Emauel, W.R.; King, A.W.; Dale, V.H.; de Angelis, D.L. The global carbon cycle. Am. Sci. 1990, 78, 310–326. [Google Scholar]
- Brown, S.; Leonard, P. Building carbon credits with biosolids recycling. BioCycle 2004, 45, 25–29. [Google Scholar]
- Jarecki, M.K.; Lal, R. Crop management for soil carbon sequestration. Crit. Rev. Plant. Sci. 2003, 22, 471–502. [Google Scholar] [CrossRef]
- Tian, G.; Granato, T.C.; Cox, A.E.; Pietz, R.I.; Carlson, C.R.; Abedin, Z. Soil carbon sequestration resulting from long-term application of biosolids for land reclamation. J. Environ. Qual. 2009, 38, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Conant, R.T.; Paustian, K. Potential soil carbon sequestration in overgrazed grassland ecosystems. Glob. Biogeochem. Cycles 2002, 16, 901–909. [Google Scholar] [CrossRef]
- West, T.Q.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation: A global data analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Miller, A., III; Morgan, M.E.; Libbey, L.M. Lactobacillus maltaromicus, a new species producing a malty aroma. Int. J. Syst. Bacterial. 1974, 24, 346–354. [Google Scholar] [CrossRef]
- Terry, R.E.; Nelson, D.W.; Sommers, L.E. Decomposition of anaerobically digested sewage sludge as affected by soil environmental conditions. J. Environ. Qual. 1979, 8, 342–347. [Google Scholar] [CrossRef]
- Gilmour, J.T.; Gilmour, C.M. A simulation model for sludge decomposition in soil. J. Environ. Qual. 1980, 9, 194–199. [Google Scholar] [CrossRef]
- Parton, W.J.; Schimel, D.S.; Cole, C.V.; Ojima, D.S. Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci. Soc. of Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Brown, S.; Beecher, N.; Carpenter, A. Calculator tool for determining greenhouse gas emissions for biosolids processing and end use. Environ. Sci. Technol. 2010, 44, 9509–9515. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Kunhikrishnan, A.; Naidu, R. Carbon storage in a heavy clay soil landfill site after biosolids application. Sci. Total Environ. 2013, 465, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Moschandreas, D.J.; Tian, G.; Venkatesan, D.; Noll, K.E. Degradation rate model formulation to estimate soil carbon sequestration from biosolids application. Soil Sci. Soc. Am. J. 2013, 78, 238–247. [Google Scholar] [CrossRef]
- Sorenson, L.H. Carbon-nitrogen relationships during the humiliation of cellulose in soils containing different amounts of clay. Soil Biol. Bio chem. 1981, 13, 313–321. [Google Scholar] [CrossRef]
- USDA. Soil Survey of Fulton County, Illinois; U.S. Department of Agriculture: Washington, DC, USA, 1997.
- Hillel, D. Fundamentals of Soil Physics, 2nd ed.; Academic Press Inc.: Orlando, FL, USA, 1980; pp. 123–124. [Google Scholar]
- Liu, C.; Evett, J. Grain-size analysis of soil (including both mechanical and hydrometer analyses). In Soil Properties: Testing, Measurement, and Evaluation, 6th ed.; Prentice Gall: Upper Saddle River, NJ, USA, 2008; pp. 117–128. [Google Scholar]
- Hamaker, J. Mathematical prediction of cumulative levels of pesticides in soil. In Organic Pesticides in the Environment, 1st ed.; American Chemical Society: Washington, DC, USA, 1966; pp. 124–131. [Google Scholar]
- Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Zehnder, A.J. Influence of interfaces on microbial activity. Microbiol. Mol. Biol. Rev. 1990, 54, 75–87. [Google Scholar]
- Parton, W.J.; Ojima, D.S.; Cole, C.V.; Schimel, D.S. A general model for soil organic matter dynamics: Sensitivity to litter chemistry, texture and management. Soil Sci. Am. J. 1994, 39, 147–167. [Google Scholar]
- USDA. Textural Soil Classification; U.S. Department of Agriculture: Washington, DC, USA, 1987.
- Borkovec, M.; Wu, Q.; Degovics, G.; Laggner, P.; Sticher, H. Surface area and size distributions of soil particles. Colloid Surface A 1993, 73, 65–76. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Parton, W.J.; Flach, K.; Burke, I.C.; Cole, C.V.; Yonker, C.M. Texture, climate and cultivation effects on soil organic matter content in US grassland soils. Soil Sci. Am. J. 1989, 53, 800–805. [Google Scholar]
- Gilmour, J.T.; Clark, M.D. Predicting long-term decomposition of biosolids with a seven-day test. J. Environ. Qual. 1996, 25, 766–770. [Google Scholar] [CrossRef]
- Paul, E.A. Dynamics of organic matter soils. Plant Soil. 1984, 76, 275–285. [Google Scholar] [CrossRef]
- Schimel, D.S.; Coleman, D.C.; Horton, K.C. Soil Organic matter dynamics in paired rangeland and cropland topo sequences in North Dakota. Geoderma 1985, 36, 201–214. [Google Scholar] [CrossRef]
- Schimel, D.S.; Parton, W.J. Microclimatic controls of nitrogen mineralization and nitrification in shortgrass steppe soils. Plant Soil. 1986, 93, 347–357. [Google Scholar] [CrossRef]
- Allison, F.E.; Sherman, M.S.; Pinck, L.A. Maintenance of soil organic matter. Soil Sci. 1949, 68, 463–478. [Google Scholar] [CrossRef]
- Manlay, R.J.; Feller, C.; Swift, M.J. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric. Ecosyst. Environ. 2007, 119, 217–233. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Haberhauer, G.; Kirchmann, H. Soil organic matter pools and carbon-13 natural abundances in particle-size fraction of a long-term agricultural field experiment receiving organic amendments. Soil Sci. Soc. Am. J. 2001, 64, 352–358. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, D.; Zhai, W.; Moschandreas, D.; Tian, G.; Noll, K.E. Relationship between Mineral Soil Surface Area and the Biological Degradation of Biosolids Added to Soil. Agriculture 2016, 6, 1. https://doi.org/10.3390/agriculture6010001
Wen D, Zhai W, Moschandreas D, Tian G, Noll KE. Relationship between Mineral Soil Surface Area and the Biological Degradation of Biosolids Added to Soil. Agriculture. 2016; 6(1):1. https://doi.org/10.3390/agriculture6010001
Chicago/Turabian StyleWen, Dongqi, Wenjuan Zhai, Demetrios Moschandreas, Guanglong Tian, and Kenneth E. Noll. 2016. "Relationship between Mineral Soil Surface Area and the Biological Degradation of Biosolids Added to Soil" Agriculture 6, no. 1: 1. https://doi.org/10.3390/agriculture6010001






