Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry
Abstract
:1. Introduction
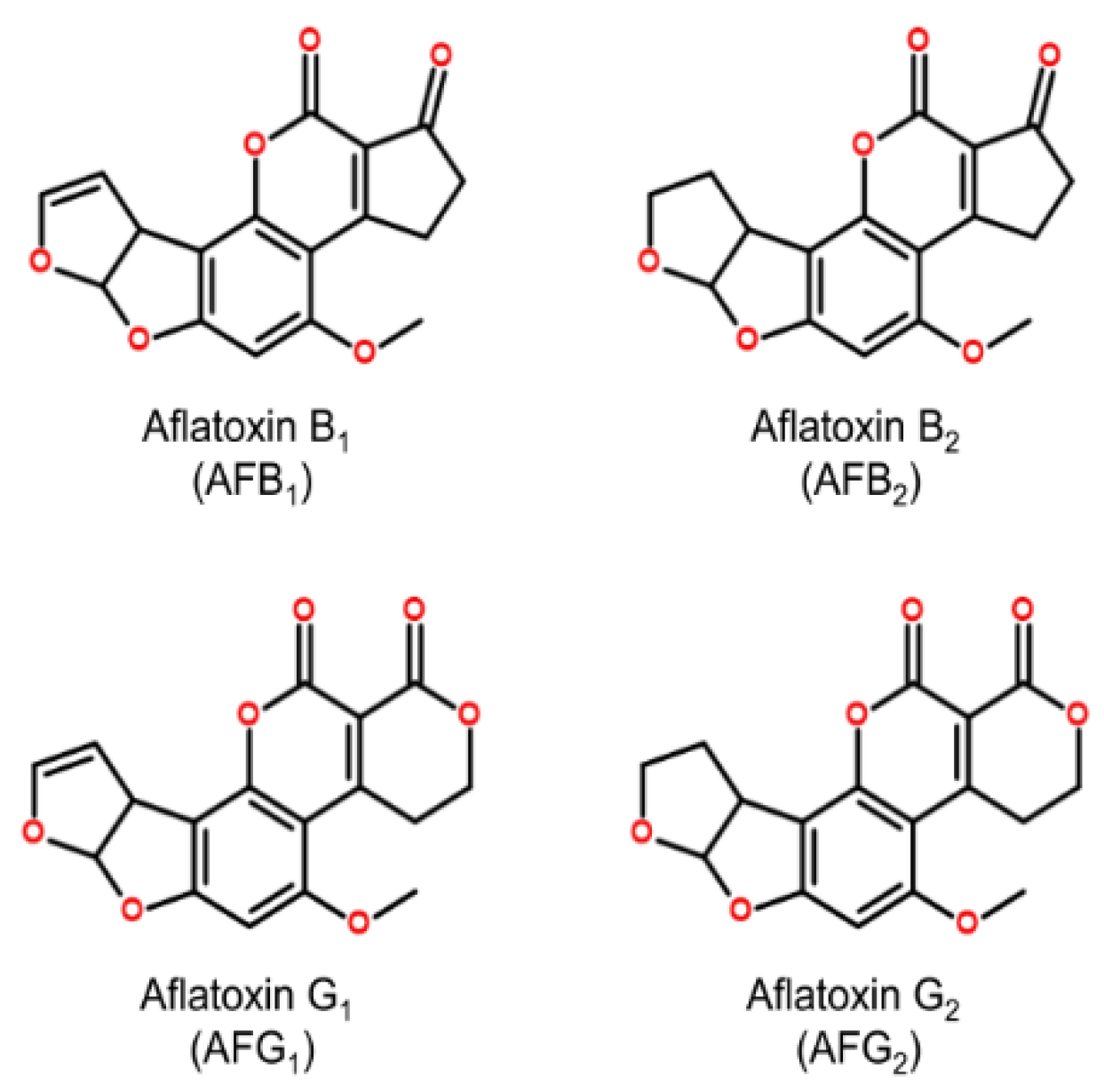
2. Etiology of Aflatoxicosis
| Species | Age | Oral LD50 (mg/kg Body weight) 1 |
|---|---|---|
| Baboon | A | 2.0–2.2 |
| Cat | A | 0.6 |
| Chicken | E | 0.3–5.0 |
| Chicken | Y | 6.5–18.0 |
| Dog | A | 0.5–1.0 |
| Duck | E | 0.5–1.0 |
| Duck | N | 0.3–0.6 |
| Guinea Pig | Y | 1.4–2.0 |
| Hamster | Y | 10.2–12.8 |
| Macaque (Cynomolgus) | A | 2.2 |
| Macaque (Rhesus) | A | 7.8–8.0 |
| Mouse | N | 1.5 |
| Mouse | Y | 7.3–9.0 |
| Rabbit | Y | 0.3–0.5 |
| Rat | N | 0.6–1.0 |
| Rat | Y | 5.5–7.4 |
| Rat | A | 6.3–18.0 |
| Sheep | A | 2.0 |
| Swine | Y | 0.6 |
| Trout | Y | 0.5 |
| Turkey | Y | 1.4–3.2 |
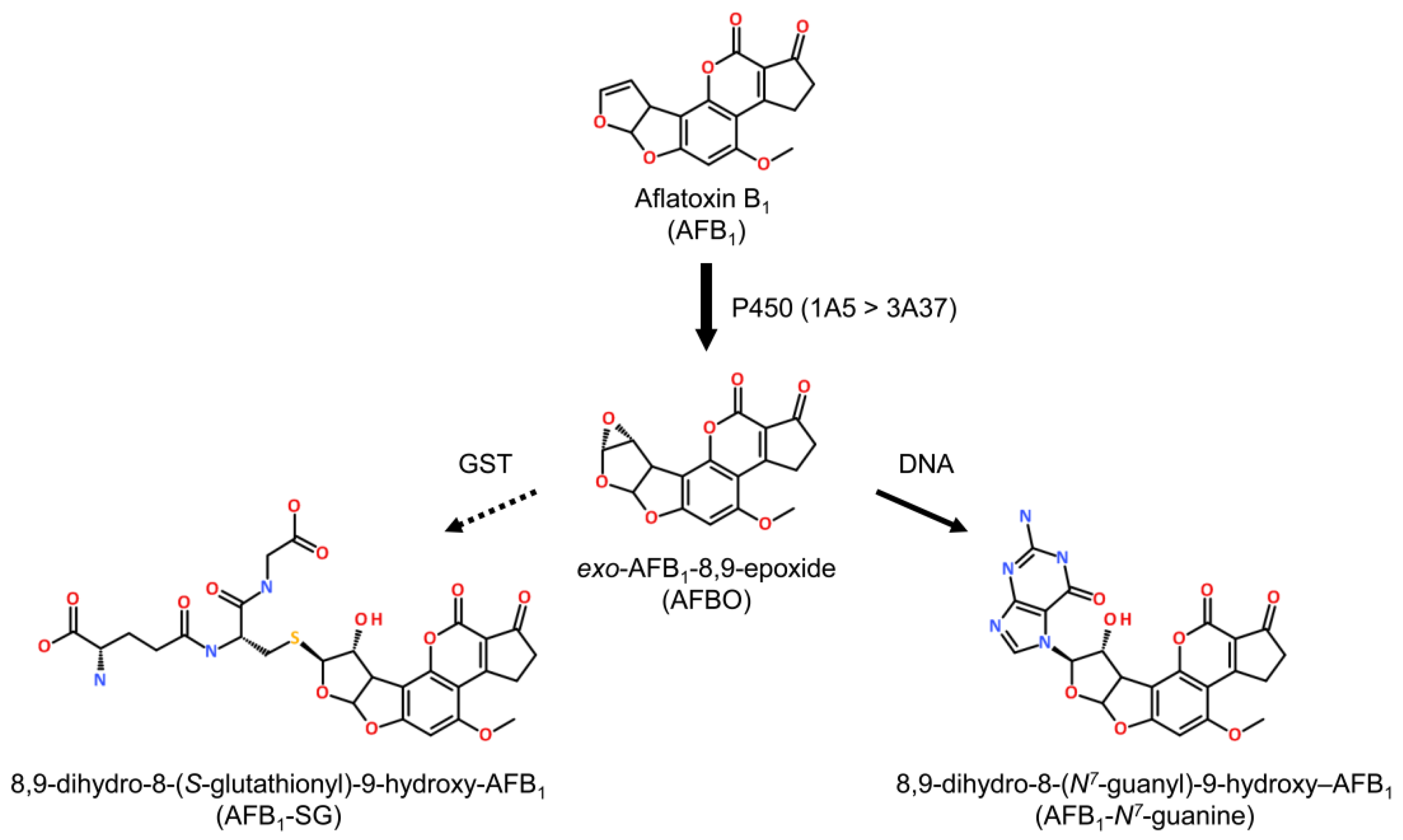
3. AFB1 Metabolism and Sensitivity
3.1. Metabolism
3.2. Sensitivity: Mice to Humans
3.3. Sensitivity: Poultry
| Species | Minimum Dietary Contamination Level (ppb) to Cause | ||
|---|---|---|---|
| 100% Lethality | Gross Hepatic Lesions | Impaired Production | |
| Chicken | NR (>4000) | 800 1 | 800 1 |
| Duck | 1000 2 | 500 2 | 500 2 |
| Goose | 4000 2 | 500 2 | 700 3 |
| Pheasant | 4000 2 | 500 2 | 1000 2 |
| Quail (Bobwhite) | ND | ND | 700 3 |
| Turkey | 800 1 | 400 1 | 400 1 |
4. Effects of AFB1 Exposure
4.1. AFB1 Adducts
4.2. Mutagenicity
4.3. Production Losses
4.4. Hepatotoxicity
4.5. Immunotoxicity
4.6. Intestinal Toxicity
4.7. Embryotoxicity
5. Gene Expression and AFB1
5.1. P450 and GST Enzymes
5.2. Cytokines and the MHC
5.3. Moving towards Transcriptomics
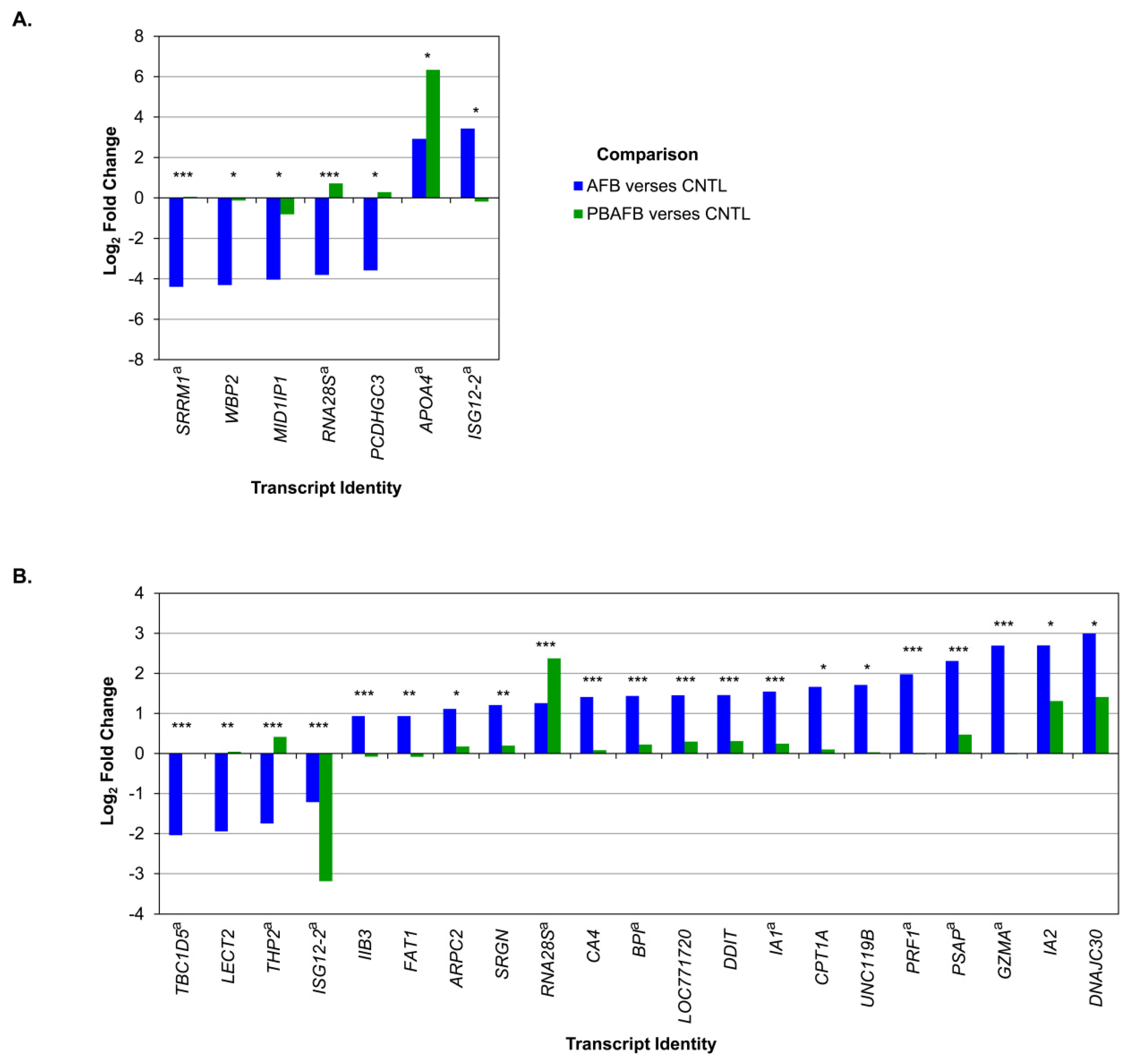
5.4. Poultry Transcriptomics and AFB1
6. Strategies to Reduce AFB1 Toxicity
6.1. Chemical Detoxification
6.2. Feed Additives
6.3. Probiotics
6.4. Selection for Resistance
7. Conclusions: Suggested Areas for Further Research
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bedard, L.L.; Massey, T.E. Aflatoxin B1-induced DNA damage and its repair. Cancer Lett. 2006, 241, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, R.A., Jr. Biological action of mycotoxins. J. Dairy Sci. 1993, 76, 880–891. [Google Scholar] [CrossRef]
- Eaton, D.L.; Gallagher, E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.D.; Kensler, T.W. Role of metabolism and viruses in aflatoxin-induced liver cancer. Toxicol. Appl. Pharmacol. 2005, 206, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-Year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Diaz, G.J.; Summers, J.D. Aflatoxins. In Poultry Metabolic Disorders and Mycotoxins; University Books: Guelph, Canada, 1995. [Google Scholar]
- Yabe, K.; Nakajima, H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004, 64, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Kensler, T.W.; Groopman, J.D. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addit. Contam. 2012, 29, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Newberne, P.M.; Butler, W.H. Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals: A review. Cancer Res. 1969, 29, 236–250. [Google Scholar] [PubMed]
- Patterson, D.S.P. Metabolism as a factor in determining the toxic action of the aflatoxins in different animal species. Food Cosmet. Toxicol. 1973, 11, 287–294. [Google Scholar] [CrossRef]
- Pier, A.C. Aflatoxicosis and Immunosuppression in Mammalian Animals. In Aflatoxin in Maize: A Proceedings of the Workshop; Zuber, M.S., Lillehoj, E.B., Renfro, B.L., Eds.; CIMMYT: El Batan, Mexico, 1986. [Google Scholar]
- Robens, J.F.; Richard, J.L. Aflatoxins in animal and human health. Rev. Environ. Contam. Toxicol. 1992, 127, 69–94. [Google Scholar] [PubMed]
- Wogan, G.N. Chemical nature and biological effects of the aflatoxins. Bacteriol. Rev. 1966, 30, 460–470. [Google Scholar] [PubMed]
- Wogan, G.N. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992, 52, 2114s–2118s. [Google Scholar] [PubMed]
- Blount, W.P. Turkey “X” disease. Turkeys 1961, 9, 77. [Google Scholar]
- Wannop, C.C. The histopathology of turkey “X” disease in Great Britain. Avian Dis. 1961, 5, 371–381. [Google Scholar] [CrossRef]
- Nesbitt, B.F.; O’Kelly, J.; Sargeant, K.; Sheridan, A. Aspergillus flavus and turkey X disease. Toxic metabolites of Aspergillus flavus. Nature 1962, 195, 1062–1063. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Klich, M.A.; Beltz, S.B. Characterization of aflatoxin-producing fungi outside of Aspergillus section Flavi. Mycologia 2005, 97, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Skouboe, P.; Samson, R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst. Appl. Microbiol. 2005, 28, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Newberne, P.M. Acute Hepatotoxicity of Aflatoxins. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: London, UK, 1993. [Google Scholar]
- CAST. Mycotoxins: Risks in Plant, Animal and Human Systems; No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Gqaleni, N.; Smith, J.E.; Lacey, J.; Gettinby, G. Effects of temperature, water activity, and incubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Appl. Environ. Microbiol. 1997, 63, 1048–1053. [Google Scholar] [PubMed]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modeling growth rate and assessing aflatoxins production by Aspergillus flavus as a function of water activity and temperature on polished and brown rice. J. Food Sci. 2013, 78, M56–M63. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.F.; Palmer, J.G.; Eisenberg, W.V. Aflatoxin production by Aspergillus flavus as related to various temperatures. Appl. Microbiol. 1967, 15, 1006–1009. [Google Scholar] [PubMed]
- Schroeder, H.W.; Hein, H. Aflatoxins: Production of the toxins in vitro in relation to temperature. Appl. Microbiol. 1967, 15, 441–445. [Google Scholar] [PubMed]
- Trenk, H.L.; Hartman, P.A. Effects of moisture content and temperature on aflatoxin production in corn. Appl. Microbiol. 1970, 19, 781–784. [Google Scholar] [PubMed]
- Aly, S.A.; Anwer, W. Effect of naturally contaminated feed with aflatoxins on performance of laying hens and the carryover of aflatoxin B1 residues in table egg. Pakistan J. Nutr. 2009, 8, 181–186. [Google Scholar]
- Chen, C.; Pearson, A.M.; Coleman, T.H.; Gray, J.I.; Pestka, J.J.; Aust, S.D. Tissue deposition and clearance of aflatoxins from broiler chickens fed a contaminated diet. Food Chem. Toxicol. 1984, 22, 447–451. [Google Scholar] [CrossRef]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M1 in milk and milk products: A worldwide review. J. Food Prot. 1996, 59, 1079–1090. [Google Scholar]
- Pandey, I.; Chauhan, S.S. Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. Br. Poult. Sci. 2007, 48, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Purchase, I.F.H. Aflatoxin residues in food of animal origin. Food Cosmet. Toxicol. 1972, 10, 531–544. [Google Scholar] [CrossRef]
- Richard, J.L.; Stubblefield, R.D.; Lyon, R.L.; Peden, W.M.; Thurston, J.R.; Rimler, R.B. Distribution and clearance of aflatoxins B1 and M1 in turkeys fed diets containing 50 or 150 ppb aflatoxin from naturally contaminated corn. Avian Dis. 1986, 30, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Wolzak, A.; Pearson, A.M.; Coleman, T.H.; Pestka, J.J.; Gray, J.I. Aflatoxin deposition and clearance in the eggs of laying hens. Food Chem. Toxicol. 1985, 23, 1057–1061. [Google Scholar] [CrossRef]
- Wolzak, A.; Pearson, A.M.; Coleman, T.H.; Pestka, J.J.; Gray, J.I.; Chen, C. Aflatoxin carryover and clearance from tissues of laying hens. Food Chem. Toxicol. 1986, 24, 37–41. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of aflatoxin on young turkeys and broiler chickens. Poult. Sci. 1985, 64, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.J.; Buckner, R.; Kelly, J.; Coulombe, R.A., Jr. Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B1. Toxicol. Appl. Pharmacol. 2000, 165, 45–52. [Google Scholar] [CrossRef] [PubMed]
- CAST. Mycotoxins: Economic and Health Risks; No. 116; Council for Agricultural Science and Technology: Ames, IA, USA, 1989. [Google Scholar]
- Gratz, S.; Mykkänen, H.; el-Nezami, H. Aflatoxin B1 binding by a mixture of Lactobacillus and Propionibacterium.: In vitro versus ex vivo. J. Food Prot. 2005, 68, 2470–2474. [Google Scholar] [PubMed]
- Guengerich, F.P.; Johnson, W.W.; Ueng, Y.F.; Yamazaki, H.; Shimada, T. Involvement of cytochrome P450, glutathione S-transferase, and epoxide hydrolase in the metabolism of aflatoxin B1 and relevance to risk of human liver cancer. Environ. Health Perspect. 1996, 104, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Godoy, H.M.; Neal, G.E. Some studies of the effects of aflatoxin B1 in vivo and in vitro on nucleic acid synthesis in rat and mouse. Chem. Biol. Interact. 1976, 13, 257–277. [Google Scholar] [CrossRef]
- Monroe, D.H.; Eaton, D.L. Comparative effects of butylated hydroxyanisole on hepatic in vivo DNA binding and in vitro biotransformation of aflatoxin B1 in the rat and mouse. Toxicol. Appl. Pharmacol. 1987, 90, 401–409. [Google Scholar] [CrossRef]
- Pelkonen, P.; Lang, M.A.; Wild, C.P.; Negishi, M.; Juvonen, R.O. Activation of aflatoxin B1 by mouse CYP2A enzymes and cytotoxicity in recombinant yeast cells. Eur. J. Pharmacol. 1994, 292, 67–73. [Google Scholar] [CrossRef]
- Pelkonen, P.; Lang, M.A.; Negishi, M.; Wild, C.P.; Juvonen, R.O. Interaction of aflatoxin B1 with cytochrome P450 2A5 and its mutants: Correlation with metabolic activation and toxicity. Chem. Res. Toxicol. 1997, 10, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, H.S.; Eaton, D.L. Species susceptibility to aflatoxin B1 carcinogenesis: Comparative kinetics of microsomal biotransformation. Cancer Res. 1990, 50, 615–620. [Google Scholar] [PubMed]
- Yanagimoto, T.; Itoh, S.; Sawada, M.; Hashimoto, H.; Kamataki, T. Molecular cloning and functional expression of a mouse cytochrome P-450 (Cyp3a-13): Examination of Cyp3a-13 enzyme to activate aflatoxin B1 (AFB1). Biochim. Biophys. Acta 1994, 1201, 405–410. [Google Scholar] [CrossRef]
- Yanagimoto, T.; Itoh, S.; Sawada, M.; Kamataki, T. Mouse cytochrome P450 (Cyp3a11): Predominant expression in liver and capacity to activate aflatoxin B1. Arch. Biochem. Biophys. 1997, 340, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Buetler, T.M.; Slone, D.; Eaton, D.L. Comparison of the aflatoxin B1–8,9-epoxide conjugating activities of two bacterially expressed alpha class glutathione S-transferase isozymes from mouse and rat. Biochem. Biophys. Res. Commun. 1992, 188, 597–603. [Google Scholar] [CrossRef]
- Hayes, J.D.; Judah, D.J.; Neal, G.E.; Nguyen, T. Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S-transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J. 1992, 285, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ilic, Z.; Crawford, D.; Vakharia, D.; Egner, P.A.; Sell, S. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1. Toxicol. Appl. Pharmacol. 2010, 242, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.H.; Eaton, D.L. Effects of modulation of hepatic glutathione on biotransformation and covalent binding of aflatoxin B1 to DNA in the mouse. Toxicol. Appl. Pharmacol. 1988, 94, 118–127. [Google Scholar] [CrossRef]
- Quinn, B.A.; Crane, T.L.; Kocal, T.E.; Best, S.J.; Cameron, R.G.; Rushmore, T.H.; Farber, E.; Hayes, M.A. Protective activity of different hepatic cytosolic glutathione S-transferases against DNA-binding metabolites of aflatoxin B1. Toxicol. Appl. Pharmacol. 1990, 105, 351–363. [Google Scholar] [CrossRef]
- Slone, D.H.; Gallagher, E.P.; Ramsdell, H.S.; Rettie, A.E.; Stapleton, P.L.; Berlad, L.G.; Eaton, D.L. Human variability in hepatic glutathione S-transferase-mediated conjugation of aflatoxin B1-epoxide and other substrates. Pharmacogenetics 1995, 5, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Buetler, T.M.; Bammler, T.K.; Hayes, J.D.; Eaton, D.L. Oltipraz-mediated changes in aflatoxin B1 biotransformation in rat liver: Implications for human chemointervention. Cancer Res. 1996, 56, 2306–2313. [Google Scholar] [PubMed]
- Imaoka, S.; Ikemoto, S.; Shimada, T.; Funae, Y. Mutagenic activation of aflatoxin B1 by pulmonary, renal, and hepatic cytochrome P450s from rats. Mutat. Res. 1992, 269, 231–236. [Google Scholar] [CrossRef]
- Johnson, W.W.; Ueng, Y.F.; Widersten, M.; Mannervik, B.; Hayes, J.D.; Sherratt, P.J.; Ketterer, B.; Guengerich, F.P. Conjugation of highly reactive aflatoxin B1 exo-8,9-epoxide catalyzed by rat and human glutathione transferases: Estimation of kinetic parameters. Biochemistry 1997, 36, 3056–3060. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Judah, D.J.; McLellan, L.I.; Kerr, L.A.; Peacock, S.D.; Neal, G.E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1–8,9-epoxide. Biochem. J. 1991, 279, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Nguyen, T.; Judah, D.J.; Petersson, D.G.; Neal, G.E. Cloning of cDNAs from fetal rat liver encoding glutathione S-transferase Yc polypeptides. The Yc2 subunit is expressed in adult rat liver resistant to the hepatocarcinogen aflatoxin B1. J. Biol. Chem. 1994, 269, 20707–20717. [Google Scholar] [PubMed]
- Hayes, J.D.; Pulford, D.J.; Ellis, E.M.; McLeod, R.; James, R.F.; Seidegård, J.; Mosialou, E.; Jernström, B.; Neal, G.E. Regulation of rat glutathione S-transferase A5 by cancer chemopreventive agents: Mechanisms of inducible resistance to aflatoxin B1. Chem. Biol. Interact. 1998, 111–112, 51–67. [Google Scholar] [CrossRef]
- Forrester, L.M.; Neal, G.E.; Judah, D.J.; Glancey, M.J.; Wolf, C.R. Evidence for involvement of multiple forms of cytochrome P-450 in aflatoxin B1 metabolism in human liver. Proc. Natl. Acad. Sci. USA 1990, 87, 8306–8310. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.P.; Wienkers, L.C.; Stapleton, P.L.; Kunze, K.L.; Eaton, D.L. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Res. 1994, 54, 101–108. [Google Scholar] [PubMed]
- Macé, K.; Aguilar, F.; Wang, J.S.; Vautravers, P.; Gómez-Lechón, M.; Gonzalez, F.J.; Groopman, J.; Harris, C.C.; Pfeifer, A.M. Aflatoxin B1-induced DNA adduct formation and p53 mutations in CYP450-expressing human liver cell lines. Carcinogenesis 1997, 18, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, H.S.; Parkinson, A.; Eddy, A.C.; Eaton, D.L. Bioactivation of aflatoxin B1 by human liver microsomes: Role of cytochrome P450 IIIA enzymes. Toxicol. Appl. Pharmacol. 1991, 108, 436–447. [Google Scholar] [CrossRef]
- Van Vleet, T.R.; Watterson, T.L.; Klein, P.J.; Coulombe, R.A., Jr. Aflatoxin B1 alters the expression of p53 in cytochrome P450-expressing human lung cells. Toxicol. Sci. 2006, 89, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Raney, K.D.; Meyer, D.J.; Ketterer, B.; Harris, T.M.; Guengerich, F.P. Glutathione conjugation of aflatoxin B1 exo- and endo-epoxides by rat and human glutathione S-transferases. Chem. Res. Toxicol. 1992, 5, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Gross-Steinmeyer, K.; Stapleton, P.L.; Tracy, J.H.; Bammler, T.K.; Strom, S.C.; Eaton, D.L. Sulforaphane- and phenethyl isothiocyanate-induced inhibition of aflatoxin B1-mediated genotoxicity in human hepatocytes: Role of GSTM1 genotype and CYP3A4 gene expression. Toxicol. Sci. 2010, 116, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Taylor, J.; Linko, P.; Lucier, G.W.; Thompson, C.L. Glutathione S-transferase mu in human lymphocyte and liver: Role in modulating formation of carcinogen-derived DNA adducts. Carcinogenesis 1991, 12, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Rosvold, E.A.; Lustbader, E.D.; Hu, Y.; Clapper, M.L.; Zhou, T.; Wild, C.P.; Xia, X.L.; Baffoe-Bonnie, A.; Ofori-Adjei, D.; et al. Susceptibility to hepatocellular carcinoma is associated with genetic variation in the enzymatic detoxification of aflatoxin B1. Proc. Natl. Acad. Sci. USA 1995, 92, 2384–2387. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.S.; Bloomer, R.J.; Wilson, H.R.; Simpson, C.F.; Harms, R.H. Susceptibility of various poultry species to dietary aflatoxin. Br. Poult. Sci. 1981, 22, 431–436. [Google Scholar]
- Gumbmann, M.R.; Williams, S.N.; Booth, A.N.; Vohra, P.; Ernst, R.A.; Bethard, M. Aflatoxin susceptibility in various breeds of poultry. Proc. Soc. Exp. Biol. Med. 1970, 134, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.C.; Diaz, G.J. Microsomal and cytosolic biotransformation of aflatoxin B1 in four poultry species. Br. Poult. Sci. 2006, 47, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.D.; Carlson, C.W.; Semeniuk, G.; Harshfield, G.S. The response of chicks, ducklings, goslings, pheasants and poults to graded levels of aflatoxins. Poult. Sci. 1970, 49, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Mendoza, K.M.; Reed, K.M.; Coulombe, R.A., Jr. Structure, genetic mapping, and function of the cytochrome P450 3A37 gene in the turkey (Meleagris gallopavo). Cytogenet. Genome Res. 2009, 125, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Yip, S.S.; Coulombe, R.A., Jr. Cloning, expression and functional characterization of cytochrome P450 3A37 from turkey liver with high aflatoxin B1 epoxidation activity. Chem. Res. Toxicol. 2010, 23, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Coulombe, R.A., Jr. Metabolism of aflatoxin B1 in turkey liver microsomes: The relative roles of cytochromes P450 1A5 and 3A37. Toxicol. Appl. Pharmacol. 2011, 254, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.; Coulombe, R.A. Molecular cloning and expression of a novel cytochrome P450 from turkey liver with aflatoxin B1 oxidizing activity. Chem. Res. Toxicol. 2006, 19, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Bioactivation of aflatoxin B1 by turkey liver microsomes: Responsible cytochrome P450 enzymes. Br. Poult. Sci. 2010, 51, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult. Sci. 2010, 89, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M.; Boermans, H.J. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010, 39, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Murcia, H.W.; Díaz, G.J.; Cepeda, S.M. Enzymatic activity in turkey, duck, quail and chicken liver microsomes against four human cytochrome P450 prototype substrates and aflatoxin B1. J. Xenobiotics 2011, 1, 17–21. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of purified aflatoxin on turkeys. Poult. Sci. 1985, 64, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.J.; van Vleet, T.R.; Hall, J.O.; Coulombe, R.A., Jr. Biochemical factors underlying the age-related sensitivity of turkeys to aflatoxin B1. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 193–201. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.M.; Washburn, K.W.; Wyatt, R.D. Variation with age in response of broilers to aflatoxin. Poult. Sci. 1980, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Quezada, T.; Cuéllar, H.; Jaramillo-Juárez, F.; Valdivia, A.G.; Reyes, J.L. Effects of aflatoxin B1 on the liver and kidney of broiler chickens during development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 265–272. [Google Scholar] [CrossRef]
- Barraud, L.; Douki, T.; Guerret, S.; Chevallier, M.; Jamard, C.; Trepo, C.; Wild, C.P.; Cadet, J.; Cova, L. The role of duck hepatitis B virus and aflatoxin B1 in the induction of oxidative stress in the liver. Cancer Detect. Prev. 2001, 25, 192–201. [Google Scholar] [PubMed]
- Kim, J.E.; Bauer, M.M.; Mendoza, K.M.; Reed, K.M.; Coulombe, R.A., Jr. Comparative genomics identifies new alpha class genes within the avian glutathione S-transferase gene cluster. Gene 2010, 452, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Bunderson, B.R.; Croasdell, A.; Coulombe, R.A., Jr. Functional characterization of alpha-class glutathione S-transferases from the turkey (Meleagris gallopavo). Toxicol. Sci. 2011, 124, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Quist, C.F.; Bounous, D.I.; Kilburn, J.V.; Nettles, V.F.; Wyatt, R.D. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 2000, 36, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M. Turkey. In Genome Mapping and Genomics in Domestic Animals; Cockett, N.E., Kole, C., Eds.; Springer-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2005, 169, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Bayyari, G.R.; Huff, W.E.; Rath, N.C.; Balog, J.M.; Newberry, L.A.; Villines, J.D.; Skeeles, J.K.; Anthony, N.B.; Nestor, K.E. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997, 76, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nestor, K.E.; Saif, Y.M.; Luhtala, M. Flow cytometric analysis of T lymphocyte subpopulations in large-bodied turkey lines and a randombred control population. Poult. Sci. 2000, 79, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Nestor, K.E.; Saif, Y.M.; Anderson, J.W.; Patterson, R.A. Effect of selection for increased body weight in turkeys on lymphoid organ weights, phagocytosis, and antibody responses to fowl cholera and Newcastle disease-inactivated vaccines. Poult. Sci. 2001, 80, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Van der Most, P.J.; de Jong, B.; Parmentier, H.K.; Verhulst, S. Trade-off between growth and immune function: A meta-analysis of selection experiments. Funct. Ecol. 2011, 25, 74–80. [Google Scholar] [CrossRef]
- Kim, J.E.; Bunderson, B.R.; Croasdell, A.; Reed, K.M.; Coulombe, R.A., Jr. Alpha-class glutathione S-transferases in wild turkeys (Meleagris gallopavo): Characterization and role in resistance to the carcinogenic mycotoxin aflatoxin B1. PLoS ONE 2013, 8, e60662. [Google Scholar] [CrossRef] [PubMed]
- Bunderson, B.R.; Kim, J.E.; Croasdell, A.; Mendoza, K.M.; Reed, K.M.; Coulombe, R.A., Jr. Heterologous expression and functional characterization of avian mu-class glutathione S-transferases. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Smela, M.E.; Hamm, M.L.; Henderson, P.T.; Harris, C.M.; Harris, T.M.; Essigmann, J.M. The aflatoxin B1 formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2002, 99, 6655–6660. [Google Scholar] [CrossRef] [PubMed]
- Corrier, D.E. Mycotoxicosis: Mechanisms of immunosuppression. Vet. Immunol. Immunopathol. 1991, 30, 73–87. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Arneson, K.O.; Williams, K.M.; Deng, Z.; Harris, T.M. Reaction of aflatoxin B1 oxidation products with lysine. Chem. Res. Toxicol. 2002, 15, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Kitagawa, T.; Akamatsu, Y.; Aibara, K. Cytotoxic effects of aflatoxin B1 and its association with cellular components in chicken embryo primary cultured cells. Biochim. Biophys. Acta 1990, 1035, 146–153. [Google Scholar] [CrossRef]
- IARC. Aflatoxins. In A Review of Human Carcinogens. Part. F: Chemical Agents and Related Occupations; International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012. [Google Scholar]
- Aguilar, F.; Hussain, S.P.; Cerutti, P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 8586–8590. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Ong, C.N. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat. Res. 1996, 366, 23–44. [Google Scholar] [CrossRef]
- Wild, C.P.; Montesano, R. A model of interaction: Aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009, 286, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.H.; Greenblatt, M.; Lijinsky, W. Carcinogenesis in rats by aflatoxins B1, G1, and B2. Cancer Res. 1969, 29, 2206–2211. [Google Scholar] [PubMed]
- Wogan, G.N.; Newberne, P.M. Dose-response characteristics of aflatoxin B1 carcinogenesis in the rat. Cancer Res. 1967, 27, 2370–2376. [Google Scholar] [PubMed]
- Wogan, G.N.; Paglialunga, S.; Newberne, P.M. Carcinogenic effects of low dietary levels of aflatoxin B1 in rats. Food Cosmet. Toxicol. 1974, 12, 681–685. [Google Scholar] [CrossRef]
- Cova, L.; Wild, C.P.; Mehrotra, R.; Turusov, V.; Shirai, T.; Lambert, V.; Jacquet, C.; Tomatis, L.; Trépo, C.; Montesano, R. Contribution of aflatoxin B1 and hepatitis B virus infection in the induction of liver tumors in ducks. Cancer Res. 1990, 50, 2156–2163. [Google Scholar] [PubMed]
- Cullen, J.M.; Marion, P.L.; Sherman, G.J.; Hong, X.; Newbold, J.E. Hepatic neoplasms in aflatoxin B1-treated, congenital duck hepatitis B virus-infected, and virus-free Pekin ducks. Cancer Res. 1990, 50, 4072–4080. [Google Scholar] [PubMed]
- Uchida, T.; Suzuki, K.; Esumi, M.; Arii, M.; Shikata, T. Influence of aflatoxin B1 intoxication on duck livers with duck hepatitis B virus infection. Cancer Res. 1988, 48, 1559–1565. [Google Scholar] [PubMed]
- Chen, X.; Horn, N.; Applegate, T.J. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult. Sci. 2014, 93, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of purified aflatoxin on broiler chickens. Poult. Sci. 1985, 64, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Corrier, D.E.; Mollenhauer, H.H. Progression of aflatoxicosis in broiler chickens. Poult. Sci. 1986, 65, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Rauber, R.H.; Dilkin, P.; Giacomini, L.Z.; Araújo de Almeida, C.A.; Mallmann, C.A. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult. Sci. 2007, 86, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L.; Pier, A.C.; Cysewski, S.J.; Graham, C.K. Effect of aflatoxin and aspergillosis on turkey poults. Avian Dis. 1973, 17, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Hamilton, P.B. Aflatoxicosis in the broiler chicken. Poult. Sci. 1970, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sims, W.M., Jr.; Kelley, D.C.; Sanford, P.E. A study of aflatoxicosis in laying hens. Poult. Sci. 1970, 49, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Johri, T.S.; Swain, B.K.; Ameena, S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Br. Poult. Sci. 2004, 45, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Yarru, L.P.; Settivari, R.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Toxicological and gene expression analysis of the impact of aflatoxin B1 on hepatic function of male broiler chicks. Poult. Sci. 2009, 88, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Jessen, K.A.; Beltran, R.; Starkl, V.; Schatzmayr, G.; Borutova, R.; Caldwell, D.J. Mycotoxin-contaminated diets and deactivating compound in laying hens: 1. Effects on performance characteristics and relative organ weight. Poult. Sci. 2012, 91, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Rosmaninho, J.F.; Butkeraitis, P.; Corrêa, B.; Reis, T.A.; Guerra, J.L.; Albuquerque, R.; Moro, M.E. Effect of low levels of dietary aflatoxin B1 on laying Japanese quail. Poult. Sci. 2002, 81, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Horn, N.; Cotter, P.F.; Applegate, T.J. Growth, serum biochemistry, complement activity, and liver gene expression responses of Pekin ducklings to graded levels of cultured aflatoxin B1. Poult. Sci. 2014, 93, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.H.; Gabal, M.A. Aflatoxin and immunity in layer hens. Avian Pathol. 1998, 27, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Garlich, J.D.; Tung, H.T.; Hamilton, P.B. The effects of short term feeding of aflatoxin on egg production and some plasma constituents of the laying hen. Poult. Sci. 1973, 52, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Howarth, B., Jr.; Wyatt, R.D. Effect of dietary aflatoxin on fertility, hatchability, and progeny performance of broiler breeder hens. Appl. Environ. Microbiol. 1976, 31, 680–684. [Google Scholar] [PubMed]
- Khan, W.A.; Khan, M.Z.; Khan, A.; Hassan, Z.U.; Rafique, S.; Saleemi, M.K.; Ahad, A. Dietary vitamin E in White Leghorn layer breeder hens: A strategy to combat aflatoxin B1-induced damage. Avian Pathol. 2014, 43, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Jessen, K.A.; Beltran, R.; Starkl, V.; Schatzmayr, G.; Borutova, R.; Caldwell, D.J. Effects of mycotoxin-contaminated diets and deactivating compound in laying hens: 2. Effects on white shell egg quality and characteristics. Poult. Sci. 2012, 91, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.F.; Rosmaninho, J.F.; Castro, A.L.; Butkeraitis, P.; Alves Reis, T.; Corrêa, B. Aflatoxin residues in eggs of laying Japanese quail after long-term administration of rations containing low levels of aflatoxin B1. Food Addit. Contam. 2003, 20, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Ortatatli, M.; Oğuz, H. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. Res. Vet. Sci. 2001, 71, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Merkley, J.W.; Maxwell, R.J.; Phillips, J.G.; Huff, W.E. Hepatic fatty acid profiles in aflatoxin-exposed broiler chickens. Poult. Sci. 1987, 66, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Siloto, E.V.; Oliveira, E.F.; Sartori, J.R.; Fascina, V.B.; Martins, B.A.; Ledoux, D.R.; Rottinghaus, G.E.; Sartori, D.R. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult. Sci. 2013, 92, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.J.; van Vleet, T.R.; Hall, J.O.; Coulombe, R.A., Jr. Dietary butylated hydroxytoluene protects against aflatoxicosis in turkeys. Toxicol. Appl. Pharmacol. 2002, 182, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ortatatli, M.; Oğuz, H.; Hatipoğlu, F.; Karaman, M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005, 78, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fang, J.; Peng, X.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; Lai, W.; Zhou, Y. Effect of selenium supplementation on aflatoxin B1-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem. Toxicol. 2014, 74, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Harvey, R.B.; Bailey, R.H.; Buckley, S.A.; Rottinghaus, G.E. Effects of a hydrated sodium calcium aluminosilicate (T-Bind) on mycotoxicosis in young broiler chickens. Poult. Sci. 1998, 77, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.T.; Wyatt, R.D.; Thaxton, P.; Hamilton, P.B. Concentrations of serum proteins during aflatoxicosis. Toxicol. Appl. Pharmacol. 1975, 34, 320–326. [Google Scholar] [CrossRef]
- Fernández, A.; Verde, M.T.; Gomez, J.; Gascon, M.; Ramos, J.J. Changes in the prothrombin time, haematology and serum proteins during experimental aflatoxicosis in hens and broiler chickens. Res. Vet. Sci. 1995, 58, 119–122. [Google Scholar] [CrossRef]
- Doerr, J.A.; Huff, W.E.; Tung, H.T.; Wyatt, R.D.; Hamilton, P.B. A survey of T-2 toxin, ochratoxin, and aflatoxin for their effects on the coagulation of blood in young broiler chickens. Poult. Sci. 1974, 53, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Doerr, J.A.; Wyatt, R.D.; Hamilton, P.B. Impairment of coagulation function during aflatoxicosis in young chickens. Toxicol. Appl. Pharmacol. 1976, 35, 437–446. [Google Scholar] [CrossRef]
- Doerr, J.A.; Hamilton, P.B. Aflatoxicosis and intrinsic coagulation function in broiler chickens. Poult. Sci. 1981, 60, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Witlock, D.R.; Wyatt, R.D. Effect of dietary aflatoxin on hemostasis of young turkey poults. Poult. Sci. 1981, 60, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, W.E.; Tung, H.T.; Hamilton, P.B. Depression of fatty acid synthesis in chick liver (Gallus domesticus) by aflatoxin. Comp. Biochem. Physiol. B 1972, 41, 843–847. [Google Scholar] [CrossRef]
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shu, G.; Peng, X.; Fang, J.; Cui, H.; Chen, J.; Wang, F.; Chen, Z.; Zuo, Z.; Deng, J.; Geng, Y.; Lai, W. Protective role of sodium selenite on histopathological lesions decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem. Toxicol. 2013, 59, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Peng, X.; Rang, J.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Geng, Y.; Lai, W.; Tang, L.; Yang, Q. Effects of dietary selenium on histopathological changes and T cells of spleen in broilers exposed to aflatoxin B1. Int. J. Environ. Res. Public Health 2014, 11, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Pier, A.C.; Heddleston, K.L.; Cysewski, S.J.; Patteron, J.M. Effect of aflatoxin on immunity in turkeys. II. Reversal of impaired resistance to bacterial infection by passive transfer of plasma. Avian Dis. 1972, 16, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, J.P.; Tung, H.T.; Hamilton, P.B. Immunosuppression in chickens by aflatoxin. Poult. Sci. 1974, 53, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Edrington, T.S.; Kubena, L.F.; Harvey, R.B.; Rottinghaus, G.E. Influence of a superactivated charcoal on the toxic effects of aflatoxin or T-2 toxin in growing broilers. Poult. Sci. 1997, 76, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Hagler, W.M., Jr.; Swanson, S.P.; Phillips, T.D.; Creger, C.R. Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poult. Sci. 1986, 65, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988, 67, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, K.; Bai, S.; Ding, X.; Zeng, Q.; Yang, J.; Fang, J.; Chen, K. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. Int. J. Environ. Res. Public Health 2014, 11, 8567–8580. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hamilton, P.B. Impaired phagocytosis by heterophils from chickens during aflatoxicosis. Toxicol. Appl. Pharmacol. 1979, 48, 459–466. [Google Scholar] [CrossRef]
- Ibrahim, I.K.; Shareef, A.M.; Al-Joubory, K.M. Ameliorative effects of sodium bentonite on phagocytosis and Newcastle disease antibody formation in broiler chickens during aflatoxicosis. Res. Vet. Sci. 2000, 69, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.C.; Chauhan, H.V.; Jha, G.J. Suppression of cell-mediated immunity by purified aflatoxin B1 in broiler chicks. Vet. Immunol. Immunopathol. 1991, 28, 165–172. [Google Scholar] [CrossRef]
- Neldon-Ortiz, D.L.; Qureshi, M.A. Effects of AFB1 embryonic exposure on chicken mononuclear phagocytic cell functions. Dev. Comp. Immunol. 1992, 16, 187–196. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Brake, J.; Hamilton, P.B.; Hagler, W.M., Jr.; Nesheim, S. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult. Sci. 1998, 77, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hamilton, P.B. Impairment of phagocytosis in chicken monocytes during aflatoxicosis. Poult. Sci. 1979, 58, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Neldon-Ortiz, D.L.; Qureshi, M.A. Direct and microsomal activated aflatoxin B1 exposure and its effects on turkey peritoneal macrophage functions in vitro. Toxicol. Appl. Pharmacol. 1991, 109, 432–442. [Google Scholar] [CrossRef]
- Neldon-Ortiz, D.L.; Qureshi, M.A. The effects of direct and microsomal activated aflatoxin B1 on chicken peritoneal macrophages in vitro. Vet. Immunol. Immunopathol. 1992, 31, 61–76. [Google Scholar] [CrossRef]
- Chang, C.F.; Hamilton, P.B. Refractory phagocytosis by chicken thrombocytes during aflatoxicosis. Poult. Sci. 1979, 58, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.C.; Chauhan, H.V.; Roy, S. Immunosuppression in broilers under experimental aflatoxicosis. Br. Vet. J. 1990, 146, 457–462. [Google Scholar] [CrossRef]
- Giambrone, J.J.; Ewert, D.L.; Wyatt, R.D.; Eidson, C.S. Effect of aflatoxin on the humoral and cell-mediated immune systems of the chicken. Am. J. Vet. Res. 1978, 39, 305–308. [Google Scholar] [PubMed]
- Chen, K.; Yuan, S.; Chen, J.; Peng, X.; Wang, F.; Cui, H.; Fang, J. Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-γ induced by aflatoxin B1 in broilers. Res. Vet. Sci. 2013, 95, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, K.; Yuan, S.; Peng, X.; Fang, J.; Wang, F.; Cui, H.; Chen, Z.; Yuan, J.; Geng, Y. Effects of aflatoxin B1 on oxidative stress markers and apoptosis in spleens in broilers. Toxicol. Ind. Health 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shu, G.; Peng, X.; Fang, J.; Chen, K.; Cui, H.; Chen, Z.; Zuo, Z.; Deng, J.; Gene, Y.; Lai, W. Protective effects of sodium selenite against aflatoxin-B1 induced oxidative stress and apoptosis in broiler spleen. Int. J. Environ. Res. Public Health 2013, 10, 2834–2844. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fang, J.; Peng, X.; Cui, H.; Zuo, Z.; Deng, J.; Chen, Z.; Lai, W.; Shu, G.; Tang, L. Effects of sodium selenite on aflatoxin B1-induced decrease of ileac T cell and the mRNA contents of IL-2, IL-6, and TNF-α in broilers. Biol. Trace Elem. Res. 2014, 159, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Peng, X.; Fang, J.; Cui, H.; Yu, Z.; Chen, Z. Effects of aflatoxin B1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int. J. Mol. Sci. 2015, 16, 6945–6959. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.L., Jr.; May, J.D.; Huff, W.E.; Doerr, J.A. Evaluation of immunity of young broiler chickens during simultaneous aflatoxicosis and ochratoxicosis. Poult. Sci. 1983, 62, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Pier, A.C.; Heddleston, K.L.; Boney, W.A.; Lukert, P.K. The effect of aflatoxin on immunity. Proc. XIX Con. Mund. Med. Vet. Zootech. 1971, 1, 216–219. [Google Scholar]
- Stewart, R.G.; Skeeles, J.K.; Wyatt, R.D.; Brown, J.; Page, R.K.; Russell, I.D.; Lukert, P.D. The effect of aflatoxin on complement activity in broiler chickens. Poult. Sci. 1985, 64, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.H.; Gabal, M.A. Interaction of aflatoxin in the feed and immunization against selected infectious diseases. I. Infectious bursal disease. Avian Pathol. 1997, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gabal, M.A.; Azzam, A.H. Interaction of aflatoxin in the feed and immunization against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease, infectious bronchitis and infectious bursal disease. Avian Pathol. 1998, 27, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Boulton, S.L.; Dick, J.W.; Hughes, B.L. Effects of dietary aflatoxin and ammonia-inactivated aflatoxin on Newcastle disease antibody titers in layer-breeders. Avian Dis. 1982, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.M.; Azzam, A.; Gabal, M.A. Interaction of naturally occurring aflatoxins in poultry feed and immunization against fowl cholera. Poult. Sci. 1991, 70, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Pier, A.C.; Heddleston, K.L. The effect of aflatoxin on immunity in turkeys. I. Impairment of actively acquired resistance to bacterial challenge. Avian Dis. 1970, 14, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Pier, A.C. Effects of aflatoxin on immunity. J. Am. Vet. Med. Assoc. 1973, 163, 1268–1269. [Google Scholar] [PubMed]
- Boonchuvit, B.; Hamilton, P.B. Interaction of aflatoxin and paratyphoid infection in broiler chickens. Poult. Sci. 1975, 54, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.M.; Adachi, Y. Comparison of the effects of dietary selenium, zinc, and selenium and zinc supplementation on growth and immune response between chick groups that were inoculated with Salmonella and aflatoxin or Salmonella. Poult. Sci. 2000, 79, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.D.; Hamilton, P.B. Interaction between aflatoxicosis and a natural infection of chickens with Salmonella. Appl. Microbiol. 1975, 30, 870–872. [Google Scholar] [PubMed]
- Edds, G.T.; Nair, K.P.; Simpson, C.F. Effect of aflatoxin B1 on resistance in poultry against cecal coccidiosis and Marek’s disease. Am. J. Vet. Res. 1973, 34, 819–826. [Google Scholar] [PubMed]
- Edds, G.T.; Simpson, C.F. Cecal coccidiosis in poultry as affected by prior exposure to aflatoxin B1. Am. J. Vet. Res. 1976, 37, 65–68. [Google Scholar] [PubMed]
- Witlock, D.R.; Wyatt, R.D.; Anderson, W.I. Relationship between Eimeria adenoeides infection and aflatoxicosis in turkey poults. Poult. Sci. 1982, 61, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.D.; Ruff, M.D.; Page, R.K. Interaction of aflatoxin with Eimeria tenella infection and monensin in young broiler chickens. Avian Dis. 1975, 19, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Harris, J.R. Interaction of aflatoxicosis with Candida albicans infections and other stresses in chickens. Poult. Sci. 1971, 50, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hamilton, P.B. Increased severity and new symptoms of infectious bursal disease during aflatoxicosis in broiler chickens. Poult. Sci. 1982, 61, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Giambrone, J.J.; Partadiredja, M.; Eidson, C.S.; Kleven, S.H.; Wyatt, R.D. Interaction of aflatoxin with infectious bursal disease virus infection in young chickens. Avian Dis. 1978, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Pruthi, A.K.; Sadana, J.R. Effect of aflatoxin B1 on the efficacy of turkey herpesvirus vaccine against Marek’s disease. Res. Vet. Sci. 1991, 51, 115–119. [Google Scholar] [CrossRef]
- Tessari, E.N.; Oliveira, C.A.; Cardoso, A.L.; Ledoux, D.R.; Rottinghaus, G.E. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Br. Poult. Sci. 2006, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Ghareeb, K.; Abd-El-Fattah, A.A.; Twaruzek, M.; Böhm, J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult. Sci. 2011, 90, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [PubMed]
- Applegate, T.J.; Schatzmayr, G.; Prickel, K.; Troche, C.; Jiang, Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult. Sci. 2009, 88, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, F.; Zhang, K.; Lv, X.; Bai, S.; Zhao, L.; Peng, X.; Ding, X.; Li, Y.; Zhang, J. Effects of feeding corn naturally contaminated with AFB1 and AFB2 on performance and aflatoxin residues in broilers. Czech. J. Anim. Sci. 2012, 57, 506–515. [Google Scholar]
- Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Alonso-Debolt, M. Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult. Sci. 1999, 78, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Garlich, J.D. Failure of vitamin supplementation to alter the fatty liver syndrome caused by aflatoxin. Poult. Sci. 1972, 51, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Tung, H.T.; Wyatt, R.D.; Donaldson, W.E. Interaction of dietary aflatoxin with some vitamin deficiencies. Poult. Sci. 1974, 53, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.R.; Pesti, G.M.; Wyatt, R.D. Effect of tryptophan supplementation on aflatoxicosis in laying hens. Poult. Sci. 1991, 70, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Tung, H.T.; Harris, J.R.; Gainer, J.H.; Donaldson, W.E. The effect of dietary fat on aflatoxicosis in turkeys. Poult. Sci. 1972, 51, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.E.; Nelson, L.A.; Hamilton, P.B. Effect of dietary fat level on dose response relationships during aflatoxicosis in young chickens. Poult. Sci. 1987, 66, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Hill, C.H.; Hamilton, P.B. The effect of dietary modifications on aflatoxicosis in the broiler chicken. Poult. Sci. 1971, 50, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi, H.; Akbari, M.R.; Maleki, M.; Behgar, M. Effect of prolonged low level inclusion of aflatoxin B1 into diet on performance, nutrient digestibility, histopathology and blood enzymes of broiler chickens. J. Anim. Vet. Adv. 2007, 6, 686–692. [Google Scholar]
- Verma, J.; Swain, B.K.; Johri, T.S. Effect of various levels of aflatoxin and ochratoxin A and combinations thereof on protein and energy utilisation in broilers. J. Sci. Food Agric. 2002, 82, 1412–1417. [Google Scholar] [CrossRef]
- Verma, J.; Johri, T.S.; Swain, B.K. Effect of aflatoxin, ochratoxin and their combination on protein and energy utilisation in white leghorn laying hens. J. Sci. Food Agric. 2007, 87, 760–764. [Google Scholar] [CrossRef]
- Ruff, M.D.; Wyatt, R.D. Intestinal absorption of l-methionine and glucose in chickens with aflatoxicosis. Toxicol. Appl. Pharmacol. 1976, 37, 257–262. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Kobashigawa, E.; Reis, T.A.; Mestieri, L.; Albuquerque, R.; Corrêa, B. Aflatoxin B1 residues in eggs of laying hens fed a diet containing different levels of the mycotoxin. Food Addit. Contam. 2000, 17, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Oguz, H.; Demet, O.; Boydak, M.; Donmez, H.H.; Sur, E.; Nizamlioglu, F. Embryotoxicity assay of aflatoxin produced by Aspergillus parasiticus NRRL 2999. Br. Poul. Sci. 2000, 41, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Qureshi, M.A.; Nanna, U.C.; Bloom, S.E. Embryonic exposure to aflatoxin-B1: Mutagenicity and influence on development and immunity. Environ. Mutagen. 1985, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Edrington, T.S.; Harvey, R.B.; Kubena, L.F. Toxic effects of aflatoxin B1 and ochratoxin A, alone and in combination, on chicken embryos. Bull. Environ. Contam. Toxicol. 1995, 54, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Oznurlu, Y.; Celik, I.; Sur, E.; Ozaydın, T.; Oğuz, H.; Altunbaş, K. Determination of the effects of aflatoxin B1 given in ovo on the proximal tibial growth plate of broiler chickens: Histological, histometric and immunohistochemical findings. Avian Pathol. 2012, 41, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sur, E.; Celik, I. Effects of aflatoxin B1 on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. Br. Poult. Sci. 2003, 44, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Deschl, U.; William, G.M. DNA damage in fetal liver cells of turkey and chicken eggs dosed with aflatoxin B1. Arch. Toxicol. 2011, 85, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Perrone, C.E.; Ahr, H.J.; Duan, J.D.; Jeffrey, A.M.; Schmidt, U.; Williams, G.M.; Enzmann, H.H. Embryonic turkey liver: Activities of biotransformation enzymes and activation of DNA-reactive carcinogens. Arch. Toxicol. 2004, 78, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sur, E.; Celik, I.; Oznurlu, Y.; Aydin, M.F.; Oguz, H.; Kurtoglu, V.; Ozaydin, T. Enzyme histochemical and serological investigations on the immune system from chickens treated in ovo with aflatoxin B1 (AFB1). Revue Méd. Vét. 2011, 162, 443–448. [Google Scholar]
- Ul-Hassan, Z.; Khan, M.Z.; Khan, A.; Javed, I. Immunological status of the progeny of breeder hens kept on ochratoxin A (OTA)- and aflatoxin B1 (AFB1)-contaminated feeds. J. Immunotoxicol. 2012, 9, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Lilleberg, S.L.; Cabonce, M.A.; Raju, N.R.; Wagner, L.M.; Kier, L.D. Alterations in the structural gene and the expression of p53 in rat liver tumors induced by aflatoxin B1. Mol. Carcinog. 1992, 6, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.J.; Shaddock, J.G.; Manjanatha, M.G.; Lisenbey, J.A.; Casciano, D.A. Identification of differentially expressed genes in aflatoxin B1-treated cultured primary rat hepatocytes and Fischer 344 rats. Carcinogenesis 1998, 19, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Merrick, B.A.; Phadke, D.P.; Auerbach, S.S.; Mav, D.; Stiegelmeyer, S.M.; Shah, R.R.; Tice, R.R. RNA-Seq profiling reveals novel hepatic gene expression pattern in aflatoxin B1 treated rats. PLoS ONE 2013, 8, e61768. [Google Scholar] [CrossRef] [PubMed]
- Dugyala, R.R.; Sharma, R.P. The effect of aflatoxin B1 on cytokine mRNA and corresponding protein levels in peritoneal macrophages and splenic lymphocytes. Int. J. Immunopharmacol. 1996, 18, 599–608. [Google Scholar] [CrossRef]
- Han, S.H.; Jeon, Y.J.; Yea, S.S.; Yang, K.H. Suppression of the interleukin-2 gene expression by aflatoxin B1 is mediated through the down-regulation of the NF-AT and AP-1 transcription factors. Toxicol. Lett. 1999, 108, 1–10. [Google Scholar] [CrossRef]
- Hinton, D.M.; Myers, M.J.; Raybourne, R.A.; Francke-Carroll, S.; Sotomayor, R.E.; Shaddock, J.; Warbritton, A.; Chou, M.W. Immunotoxicity of aflatoxin B1 in rats: Effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol. Sci. 2003, 73, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Murarolli, R.A. Effects of Aflatoxin B1 (AFB1) on Hepatic Gene Expression in Pigs and Turkeys. Doctoral Dissertation, University of Missouri, Columbia, MO, USA, 2013. [Google Scholar]
- Tilton, S.C.; Gerwick, L.G.; Hendricks, J.D.; Rosato, C.S.; Corley-Smith, G.; Givan, S.A.; Bailey, G.S.; Bayne, C.J.; Williams, D.E. Use of a rainbow trout oligonucleotide microarray to determine transcriptional patterns in aflatoxin B1-induced hepatocellular carcinoma compared to adjacent liver. Toxicol. Sci. 2005, 88, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Afanassieff, M.; Goto, R.M.; Ha, J.; Sherman, M.A.; Zhong, L.; Auffray, C.; Coudert, F.; Zoorob, R.; Miller, M.M. At least one class I gene in restriction fragment pattern-Y (Rfp.-Y.), the second MHC gene cluster in the chicken, is transcribed, polymorphic, and shows divergent specialization in antigen binding region. J. Immunol. 2001, 166, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.M.; Reed, K.M. Extended sequence of the turkey MHC B-locus and sequence variation in the highly polymorphic B-G loci. Immunogenetics 2011, 63, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.D.; Krueth, S.B.; Bauer, M.M.; Reed, K.M. Sequence of a turkey BAC clone identifies MHC class III orthologs and supports ancient origins of immunological gene clusters. Cytogenet. Genome Res. 2011, 132, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.D.; Krueth, S.B.; Reed, K.M. Characterization of the turkey MHC chromosome through genetic and physical mapping. Cytogenet. Genome Res. 2007, 117, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.D.; Krueth, S.B.; Reed, K.M. Defining the turkey MHC: Sequence and genes of the B locus. J. Immunol. 2009, 183, 6530–6537. [Google Scholar] [CrossRef] [PubMed]
- Delany, M.E.; Robinson, C.M.; Goto, R.M.; Miller, M.M. Architecture and organization of chicken microchromosome 16: Order of the NOR, MHC-Y, and MHC-B subregions. J. Hered. 2009, 100, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Milne, S.; Göbel, T.W.F.; Walker, B.A.; Jacob, J.P.; Auffray, C.; Zoorob, R.; Beck, S. The chicken B locus is a minimal essential major histocompatibility complex. Nature 1999, 401, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Goto, R.; Bernot, A.; Zoorob, R.; Auffray, C.; Bumstead, N.; Briles, W.E. Two Mhc class I and two Mhc class II genes map to the chicken Rfp.-Y. system outside the B complex. Proc. Natl. Acad. Sci. USA 1994, 91, 4397–4401. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M.; Bauer, M.M.; Monson, M.S.; Benoit, B.; Chaves, L.D.; O’Hare, T.H.; Delany, M.E. Defining the turkey MHC: Identification of expressed class I- and class IIB-like genes independent of the MHC-B. Immunogenetics 2011, 63, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Settlage, R.E.; Mendoza, K.M.; Rawal, S.; El-Nezami, H.S.; Coulombe, R.A.; Reed, K.M. Modulation of the spleen transcriptome in domestic turkey (Meleagris gallopavo) in response to aflatoxin B1 and probiotics. Immunogenetics 2015, 67, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Rustemeyer, S.M.; Lamberson, W.R.; Ledoux, D.R.; Wells, K.; Austin, K.J.; Cammack, K.M. Effects of dietary aflatoxin on the hepatic expression of apoptosis genes in growing barrows. J. Anim. Sci. 2005, 89, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Settlage, R.E.; McMahon, K.W.; Mendoza, K.M.; Rawal, S.; el-Nezami, H.S.; Coulombe, R.A.; Reed, K.M. Response of the hepatic transcriptome to aflatoxin B1 in domestic turkey (Meleagris gallopavo). PLoS ONE 2014, 9, e100930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bothast, R.J.; Nofsinger, G.W.; Lagoda, A.A.; Black, L.T. Integrated process for ammonia inactivation of aflatoxin-contaminated corn and ethanol fermentation. Appl. Environ. Microbiol. 1982, 43, 961–963. [Google Scholar] [PubMed]
- Brekke, O.L.; Sinnhuber, R.O.; Peplinski, A.J.; Wales, J.H.; Putnam, G.B.; Lee, D.J.; Ciegler, A. Aflatoxin in corn: Ammonia inactivation and bioassay with rainbow trout. Appl. Environ. Microbiol. 1977, 34, 34–37. [Google Scholar] [PubMed]
- Jalili, M.; Jinap, S.; Son, R. The effect of chemical treatment on reduction of aflatoxins and ochratoxin a in black and white pepper during washing. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Gardner, H.K.; Booth, A.N.; Gumbmann, M.R. Aflatoxin inactivation. Chemical and biological properties of ammonia and methylamine treated cottonseed meal. J. Agric. Food Chem. 1971, 19, 1155–1158. [Google Scholar] [PubMed]
- Vesonder, R.F.; Beckwith, A.C.; Ciegler, A.; Dimler, R.J. Ammonium hydroxide treatment of aflatoxin B1. Some chemical characteristics and biological effects. J. Agric. Food Chem. 1975, 23, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.Y.; Martinez, A.J.; Park, D.L. Efficacy and permanency of ammonia treatment in reducing aflatoxin levels in corn. Food Addit. Contam. 1994, 11, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Codifer, L.P., Jr.; Mann, G.E.; Dollear, F.G. Aflatoxin inactivation: Treatment of peanut meal with formaldehyde and calcium hydroxide. J. Am. Oil Chem. Soc. 1976, 53, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.D.; Govindarajan, P.; Dave, P.J. Inactivation of aflatoxin B1 by using the synergistic effect of hydrogen peroxide and gamma radiation. Appl. Environ. Microbiol. 1989, 55, 465–467. [Google Scholar] [PubMed]
- Moerck, K.E.; McElfresh, P.; Wohlman, A.; Hilton, B.W. Aflatoxin destruction in corn using sodium bisulfite, sodium hydroxide and aqueous ammonia. J. Food Prot. 1980, 43, 571–574. [Google Scholar]
- Yang, C.Y. Comparative studies on the detoxification of aflatoxins by sodium hypochlorite and commercial bleaches. Appl. Microbiol. 1972, 24, 885–890. [Google Scholar] [PubMed]
- Gregory, J.F., 3rd; Edds, G.T. Effect of dietary selenium on the metabolism of aflatoxin B1 in turkeys. Food Chem. Toxicol. 1984, 22, 637–642. [Google Scholar] [CrossRef]
- Diaz, G.J.; Cortés, A.; Botero, L. Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in turkey poults. Br. Poult. Sci. 2009, 50, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Harvey, R.B.; Huff, W.E.; Corrier, D.E.; Phillips, T.D.; Rottinghaus, G.E. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T-2 toxin. Poult. Sci. 1990, 69, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Harvey, R.B.; Phillips, T.D.; Corrier, D.E.; Huff, W.E. Diminution of aflatoxicosis in growing chickens by the dietary addition of a hydrated, sodium calcium aluminosilicate. Poult. Sci. 1990, 69, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Huff, W.E.; Harvey, R.B.; Yersin, A.G.; Elissalde, M.H.; Witzel, D.A.; Giroir, L.E.; Phillips, T.D.; Petersen, H.D. Effects of a hydrated sodium calcium aluminosilicate on growing turkey poults during aflatoxicosis. Poult. Sci. 1991, 70, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Harvey, R.B.; Phillips, T.D.; Clement, B.A. Effect of hydrated sodium calcium aluminosilicates on aflatoxicosis in broiler chicks. Poult. Sci. 1993, 72, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shirley, R.B.; Dibner, J.D.; Uraizee, F.; Officer, M.; Kitchell, M.; Vazquez-Anon, M.; Knight, C.D. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010, 89, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, A.P.; Monge, M.P.; Miazzo, R.D.; Cavaglieri, L.R.; Magnoli, C.E.; Merkis, C.I.; Cristofolini, A.L.; Dalcero, A.M.; Chiacchiera, S.M. Effect of low levels of aflatoxin B1 on performance, biochemical parameters, and aflatoxin B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011, 90, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, R.A.; Guarisco, J.A.; Klein, P.J.; Hall, J.O. Chemoprevention of aflatoxicosis in poultry by dietary butylated hydroxytoluene. Anim. Feed Sci. Technol. 2005, 121, 217–225. [Google Scholar] [CrossRef]
- Guarisco, J.A.; Hall, J.O.; Coulombe, R.A., Jr. Mechanisms of butylated hydroxytoluene chemoprevention of aflatoxicosis-inhibition of aflatoxin B1 metabolism. Toxicol. Appl. Pharmacol. 2008, 227, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Guarisco, J.A.; Hall, J.O.; Coulombe, R.A., Jr. Butylated hydroxytoluene chemoprevention of aflatoxicosis—Effects on aflatoxin B1 bioavailability, hepatic DNA adduct formation, and biliary excretion. Food Chem. Toxicol. 2008, 46, 3727–3731. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.J.; van Vleet, T.R.; Hall, J.O.; Coulombe, R.A., Jr. Effects of dietary butylated hydroxytoluene on aflatoxin B1-relevant metabolic enzymes in turkeys. Food Chem. Toxicol. 2003, 41, 671–678. [Google Scholar] [CrossRef]
- Larsen, C.; Ehrich, M.; Driscoll, C.; Gross, W.B. Aflatoxin-antioxidant effects on growth of young chicks. Poult. Sci. 1985, 64, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Gowda, N.K.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 2009, 102, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh Kasmani, F.; Karimi Torshizi, M.A.; Allameh, A.; Shariatmadari, F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poult. Sci. 2012, 91, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.T.; Rarick, M.D.; Ji, G.E.; Linz, J.E. Binding of aflatoxin B1 to Bifidobacteria in vitro. J. Food Prot. 2000, 63, 1133–1136. [Google Scholar] [PubMed]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and Bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- Shahin, A.A.M. Removal of aflatoxin B1 from contaminated liquid media by dairy lactic acid bacteria. Int. J. Agric. Biol. 2007, 9, 71–75. [Google Scholar]
- Topcu, A.; Bulat, T.; Wishah, R.; Boyac, I.H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Deabes, M.M.; Darwish, H.R.; Abdel-Aziz, K.B.; Farag, I.M.; Nada, S.A.; Tawfek, N.S. Protective effects of Lactobacillus rhamnosus GG on aflatoxins-induced toxicities in male albino mice. J. Environ. Anal. Toxicol. 2012, 2, 132. [Google Scholar]
- El-Nezami, H.; Salminen, S.J.; Ahokas, J. Biological control of food carcinogens with use of Lactobacillus GG. Nutr. Today 1996, 21, 41S–42S. [Google Scholar]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [PubMed]
- El-Nezami, H.; Mykkänen, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B1 from the chicken duodenum. J. Food Prot. 2000, 63, 549–552. [Google Scholar] [PubMed]
- Fazell, M.R.; Hajimohammadali, M.; Moshkani, A.; Samadi, N.; Jamalifar, H.; Khoshayand, M.R.; Vaghari, E.; Pouragahi, S. Aflatoxin B1 binding capacity of autochthonous strains of lactic acid bacteria. J. Food Prot. 2009, 72, 189–192. [Google Scholar]
- Gratz, S.; Mykkänen, H.; Ouwehand, A.C.; Juvonen, R.; Salminen, S.; el-Nezami, H. Intestinal mucus alters the ability of probiotic bacteria to bind aflatoxin B1 in vitro. Appl. Environ. Microbiol. 2004, 70, 6306–6308. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.; Täubel, M.; Juvonen, R.O.; Viluksela, M.; Turner, P.C.; Mykkänen, H.; el-Nezami, H. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion and toxicity of aflatoxin B1 in rats. Appl. Environ. Microbiol. 2006, 72, 7398–7400. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.; Wu, Q.K.; el-Nezami, H.; Juvonen, R.O.; Mykkänen, H.; Turner, P.C. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl. Environ. Microbiol. 2007, 73, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.; Binnion, C.; Ahokas, J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 2000, 128, 39–49. [Google Scholar] [CrossRef]
- Haskard, C.A.; el-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mendoza, A.; Garcia, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mendoza, A.; Guzman de Peña, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mendoza, A.; Rivas-Jimenez, L.; Garcia, H.S. Assessment of aflatoxin B1 binding to Lactobacillus reuteri by microscopy and fluorescence techniques. Food Biotechnol. 2011, 25, 140–150. [Google Scholar] [CrossRef]
- Kankaanpää, P.; Tuomola, E.; El-Nezami, H.; Ahokas, J.; Salminen, S.J. Binding of aflatoxin B1 alters the adhesion properties of Lactobacillus rhamnosus strain GG in a Caco-2 model. J. Food Prot. 2000, 63, 412–414. [Google Scholar] [PubMed]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; el-Nezami, H.; Haskard, C.A.; Gratz, S.; Puong, K.Y.; Salminen, S.; Mykkänen, H. Kinetics of adsorption and desorption of aflatoxin B1 by viable and nonviable bacteria. J. Food Prot. 2003, 66, 426–430. [Google Scholar] [PubMed]
- Peltonen, K.D.; el-Nezami, H.S.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 by probiotic bacteria. J. Sci. Food Agric. 2000, 80, 1942–1945. [Google Scholar] [CrossRef]
- Turbic, A.; Ahokas, J.T.; Haskard, C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002, 19, 144–152. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Mykkänen, H.; Kankaanpää, P.; Suomalaine, T.; Salminen, S.; Ahokas, J. Ability of a mixture of Lactobacillus and Propionibacterium to influence the faecal aflatoxin content in healthy Egyptian volunteers: A pilot clinical study. Biosci. Microflora 2000, 19, 41–45. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Polychronaki, N.N.; Ma, J.; Zhu, H.; Ling, W.; Salminen, E.K.; Juvonen, R.O.; Salminen, S.J.; Poussa, T.; Mykkänen, H.M. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am. J. Clin. Nutr. 2006, 83, 1199–1203. [Google Scholar] [PubMed]
- Lanza, G.M.; Washburn, K.W.; Wyatt, R.D.; Marks, H.L. Genetic variation of physiological response to aflatoxin in Gallus domesticus. Theor. Appl. Genet. 1982, 63, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.M.; Washburn, K.W.; Wyatt, R.D.; Marks, H.L. Effect of dietary aflatoxin concentration on the assessment of genetic variability of response in a randombred population of chickens. Genetics 1983, 104, 123–131. [Google Scholar] [PubMed]
- Manning, R.O.; Wyatt, R.D.; Marks, H.L.; Fletcher, O.J. Effects of dietary aflatoxin in aflatoxin-resistant and control lines of chickens. Poult. Sci. 1990, 69, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.R.; Rowland, S.M.; Rodgers, R.S.; Bodine, A.B. Genetic selection for aflatoxin B1 resistance influences chicken T-cell and thymocyte proliferation. Dev. Comp. Immunol. 1991, 15, 383–391. [Google Scholar] [CrossRef]
- Wyatt, R.D.; Marks, H.L.; Manning, R.O. Selection for resistance to aflatoxin in chickens. Poult. Sci. 1987, 66, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.L.; Wyatt, R.D. Genetic resistance to aflatoxin in Japanese quail. Science 1979, 206, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Pegram, R.A.; Wyatt, R.D.; Marks, H.L. Comparative responses of genetically resistant and nonselected Japanese quail to dietary aflatoxin. Poult. Sci. 1985, 64, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Pegram, R.A.; Wyatt, R.D.; Marks, H.L. The relationship of certain blood parameters to aflatoxin resistance in Japanese quail. Poult. Sci. 1986, 65, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Rodeheaver, D.P.; Wyatt, R.D.; Marks, H.L. Relation of serum alpha-amylase to aflatoxin resistance in Japanese quail. Avian Dis. 1986, 30, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S. Hepatotoxic and Immunomodulatory Transcriptome Responses to Aflatoxin B1 in the Turkey (Meleagris Gallopavo). Ph.D. Thesis, University of Minnesota, Saint Paul, MN, USA, May 2015. [Google Scholar]
- Coulombe, R.A. USDA project: Functional Genomics to Enhance Aflatoxin Resistance in Poultry. Utah State University: Logan, UT, USA, 2015. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monson, M.S.; Coulombe, R.A.; Reed, K.M. Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry. Agriculture 2015, 5, 742-777. https://doi.org/10.3390/agriculture5030742
Monson MS, Coulombe RA, Reed KM. Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry. Agriculture. 2015; 5(3):742-777. https://doi.org/10.3390/agriculture5030742
Chicago/Turabian StyleMonson, Melissa S., Roger A. Coulombe, and Kent M. Reed. 2015. "Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry" Agriculture 5, no. 3: 742-777. https://doi.org/10.3390/agriculture5030742






