Adverse Effects of Larkspur (Delphinium spp.) on Cattle
Abstract
:1. Introduction
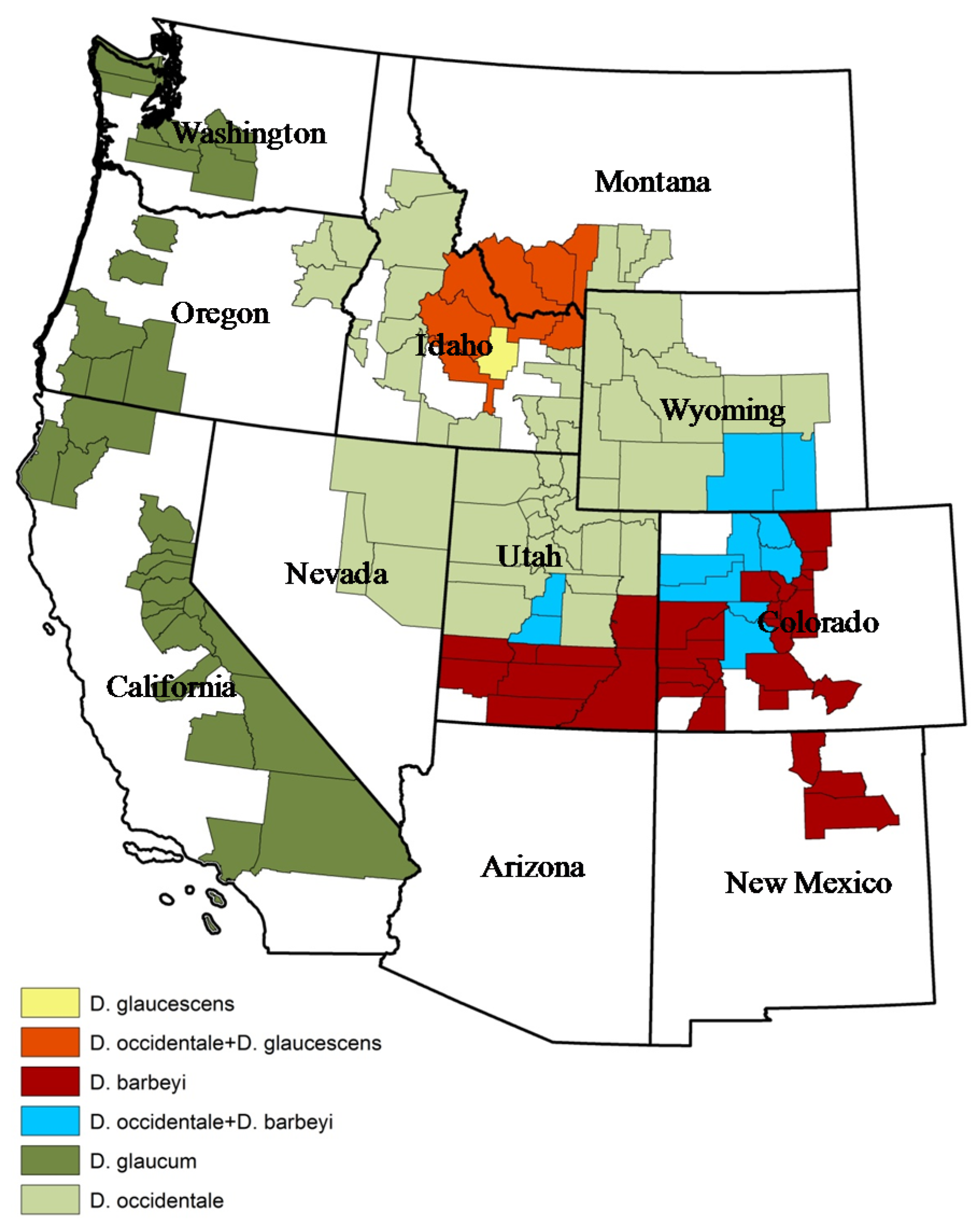
2. Plant Ecology and Distribution
| Larkspur Class | Species | Major Alkaloids | Plant Communities |
|---|---|---|---|
| Tall Larkspurs | D. barbeyi | Deltaline | Alpine, Aspen, Conifer, Mountain Brush, Mountain Meadows, Sagebrush |
| D. glaucescens | 14-O-Acetyldictyocarpine | ||
| D. glaucum | |||
| D. occidentale | Methyllycaconitine | ||
| D. ramosum | |||
| Low Larkspurs | D. andersonii | Methyllycaconitine | Aspen, Conifer, Desert Shrub, Mountain Brush, Mountain Meadows, Pinion-Juniper, Sagebrush |
| D. bicolor | Geyerline | ||
| D. nuttallianum | Nudicauline | ||
| D. parishii | 14-Deacetylnudicauline | ||
| D. scaposum | |||
| Plains Larkspur | D. geyeri | Methyllycaconitine | Desert Shrub, Mountain Brush, Sagebrush, Shortgrass Prairie |

3. Analytical Techniques
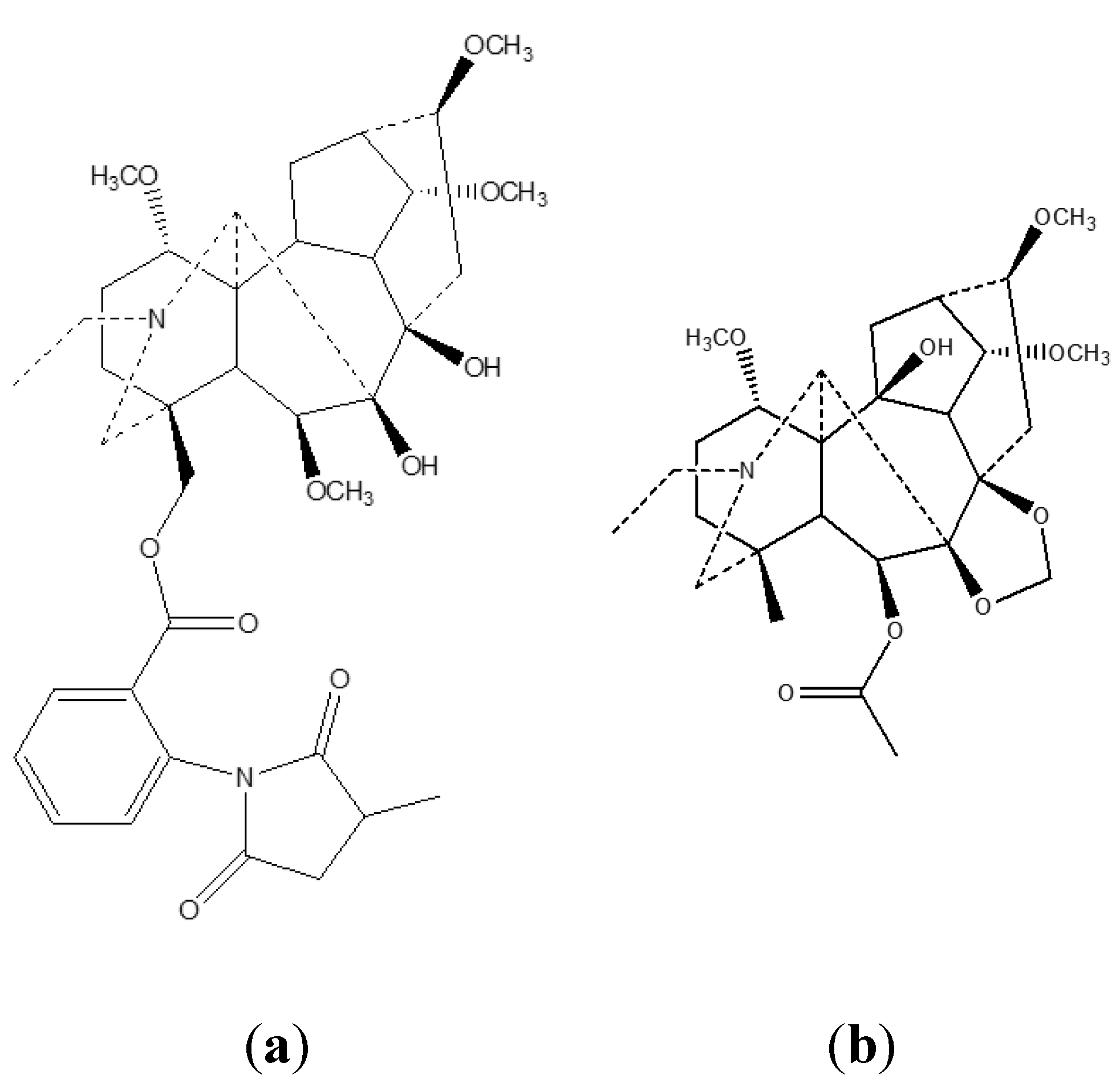
4. Toxicology

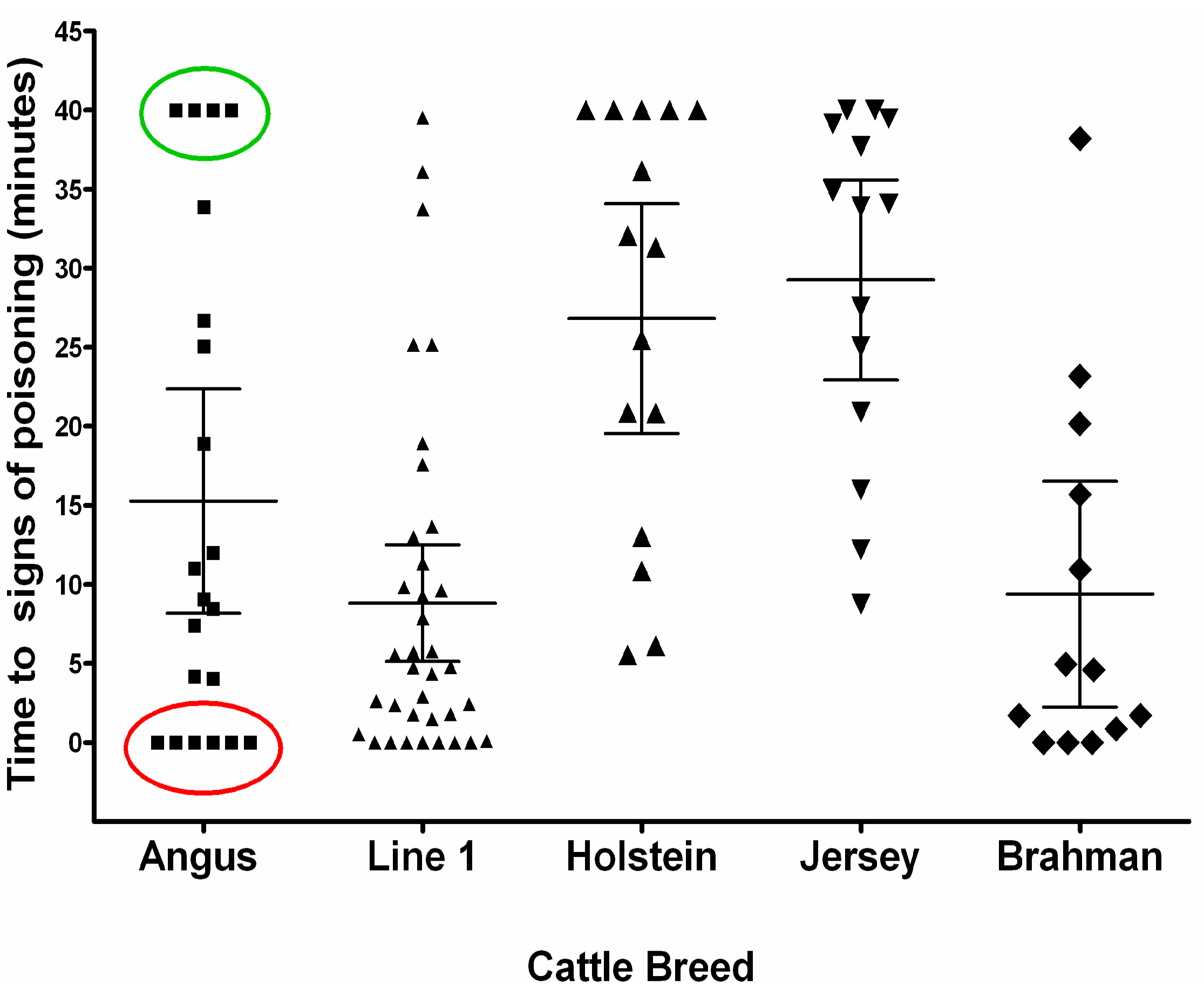
5. Genetic Susceptibility
6. Food Safety
7. Management Recommendations
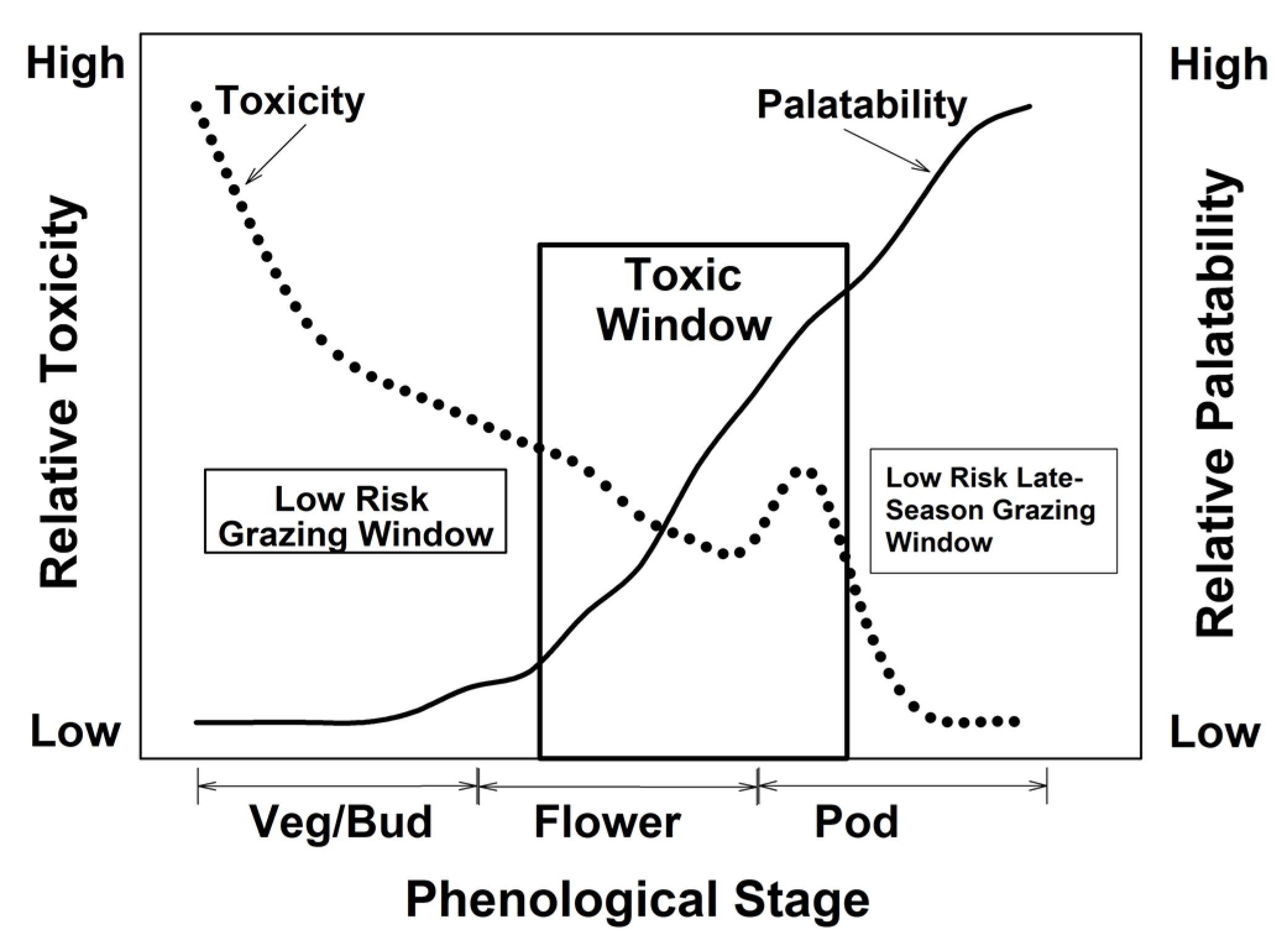
8. Grazing and other Management Options
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kingsbury, J.M. Poisonous Plants of the United States and Canada; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1964. [Google Scholar]
- Burrows, G.E.; Tyrl, R.J. Toxic Plants of North America; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Nielsen, D.B.; Ralphs, M.R. Larkspur: Ecconomic considerations. In The Ecology and Ecconomic Impact of Poisonous Plants on Livestock Production; James, L.F., Ralphs, M.H., Nielsen, D.B., Eds.; Westview Press: Boulder and London, UK, 1988; pp. 119–129. [Google Scholar]
- Nielsen, D.B.; Ralphs, M.H.; Evans, J.O.; Call, C.A. Economic feasibility of controlling tall larkspur on rangelands. J. Range Manag. 1994, 47, 369–372. [Google Scholar] [CrossRef]
- Pfister, J.A.; Ralphs, M.H.; Gardner, D.R.; Stegelmeier, B.L.; Manners, G.D.; Panter, K.E.; Lee, S.T. Management of three toxic Delphinium species based on alkaloid concentrations. Biochem. Syst. Ecol. 2002, 30, 129–138. [Google Scholar] [CrossRef]
- Marsh, C.D.; Clawson, A.B. Larkspur poisoning of livestock. USDA Bull. 1916, 365, 1–91. [Google Scholar]
- Pfister, J.A.; Gardner, D.R.; Panter, K.E.; Manners, G.D.; Ralphs, M.H.; Stegelmeier, B.L.; Schoch, T.K. Larkspur (Delphinium spp.) poisoning in livestock. J. Nat. Toxins 1999, 8, 81–94. [Google Scholar] [PubMed]
- Olsen, J.D. Tall larkspur poisoning in cattle and sheep. J. Am. Vet. Med. Assoc. 1978, 173, 762–765. [Google Scholar] [PubMed]
- Panter, K.E.; Manners, G.D.; Stegelmeier, B.L.; Lee, S.; Gardner, D.R.; Ralphs, M.H.; Pfister, J.A.; James, L.F. Larkspur poisoning: Toxicology and alkaloid structure—Activity relationships. Biochem. Syst. Ecol. 2002, 30, 113–128. [Google Scholar] [CrossRef]
- Ewan, J. Synopsis of the North American Species of Delphinium; University of Colorado: Boulder, CO, USA, 1945. [Google Scholar]
- Warnock, M. A taxonomic conspectus of North American Delphinium. Phytologia 1995, 78, 73–101. [Google Scholar]
- Warnock, M. Delphinium. Flora N. Am. North Mex. 1997, 3, 196–240. [Google Scholar]
- Manners, G.D.; Panter, K.E.; Ralphs, M.H.; Pfister, J.A.; Olsen, J.D.; James, L.F. Toxicity and chemical phenology of norditerpenoid alkaloids in the tall larkspurs (Delphinium species). J. Agric. Food Chem. 1993, 41, 96–100. [Google Scholar] [CrossRef]
- Manners, G.D.; Panter, K.E.; Pelletier, S.W. Structure-activity relationships of norditerpenoid alkaloids occurring in toxic larkspur (Delphinium) species. J. Nat. Prod. 1995, 58, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Panter, K.E.; Gardner, D.R.; Green, B.T.; Pfister, J.A.; Cook, D.; Stegelmeier, B.L. The effect of 7,8-methylenedioxylycoctonine-type diterpenoid alkaloids on the toxicity of methyllycaconitine in mice. J. Anim. Sci. 2008, 86, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Green, B.T.; Gardner, D.R.; Cook, D.; Pfister, J.A.; Stegelmeier, B.L.; Panter, K.E.; Davis, T.Z. Influence of 7,8-methylenedioxylycoctonine-type alkaloids on the toxic effects associated with ingestion of tall larkspur (Delphinium spp.) in cattle. Am. J. Vet. Res. 2010, 71, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Ralphs, M.H.; Turner, D.L.; Welsh, S.L. Taxonomic implications of diterpene alkaloids in three toxic tall larkspur species (Delphinium spp.). Biochem. Syst. Ecol. 2002, 30, 77–90. [Google Scholar] [CrossRef]
- Cook, D.; Welch, K.D.; Green, B.T.; Gardner, D.R.; Pfister, J.A.; Constantino, J.R.; Stonecipher, C.A. The relative toxicity of Delphinium stachydeum in mice and cattle. Toxicon 2015, 99, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Gardner, D.R.; Pfister, J.A.; Welch, K.D.; Green, B.T.; Lee, S.T. The biogeographical distribution of duncecap larkspur (Delphinium occidentale) chemotypes and their potential toxicity. J. Chem. Ecol. 2009, 35, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Pfister, J.A. HPLC-MS analysis of toxic norditerpenoid alkaloids: Refinement of toxicity assessment of low larkspurs (Delphinium spp.). Phytochem. Anal. 2009, 20, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Green, B.T.; Welch, K.D.; Gardner, D.R.; Pfister, J.A.; Panter, K.E. Comparison of the toxic effects of two duncecap larkspur (Delphinium occidentale) chemotypes in mice and cattle. Am. J. Vet. Res. 2011, 72, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Green, B.T.; Gardner, D.R.; Cook, D.; Pfister, J.A.; Panter, K.E. The effect of 7,8-methylenedioxylycoctonine-type diterpenoid alkaloids on the toxicity of tall larkspur (Delphinium spp.) in cattle. J. Anim. Sci. 2012, 90, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Manners, G.D.; Panter, K.E.; Lee, S.T.; Pfister, J.A. Three new toxic norditerpenoid alkaloids from the low larkspur Delphinium nuttallianum. J. Nat. Prod. 2000, 63, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Pfister, J.A. Toxic alkaloid concentrations in Delphinium nuttallianum, Delphinium andersonii, and Delphinium geyeri in the intermountain region. Rangel. Ecol. Manag. 2007, 60, 441–446. [Google Scholar] [CrossRef]
- Ralphs, M.H.; Gardner, D.R. Alkaloid levels in duncecap (Delphinium occidentale) and tall larkspur (D. barbeyi) grown in reciprocal gardens: Separating genetic from environmental influences. Biochem. Syst. Ecol. 2001, 29, 117–124. [Google Scholar] [CrossRef]
- Ralphs, M.H.; Gardner, D.R. Distribution of norditerpene alkaloids in tall larkspur plant parts through the growing season. J. Chem. Ecol. 2003, 29, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Pfister, J.A. Late season toxic alkaloid concentrations in tall larkspur (Delphinium spp.). J. Range Manag. 2000, 53, 329–334. [Google Scholar] [CrossRef]
- Pfister, J.; Cook, D. Influence of weather on low larkspur (Delphinium nuttallianum) density. J. Agric. Sci. 2011, 3, 36. [Google Scholar] [CrossRef]
- Saavedra, F.; Inouye, D.W.; Price, M.V.; Harte, J. Changes in flowering and abundance of Delphinium nuttallianum (ranunculaceae) in response to a subalpine climate warming experiment. Glob. Chang. Biol. 2003, 9, 885–894. [Google Scholar] [CrossRef]
- Cundiff, R.H.; Markunas, P.C. Determination of nicotine, nornicotine, and total alkaloids in tobacco. Anal. Chem. 1955, 27, 1650–1653. [Google Scholar] [CrossRef]
- Williams, M.C.; Cronin, E.H. Effect of silvex and 2,4,5-T on alkaloid content of tall larkspur. Weeds 1963, 11, 317–319. [Google Scholar] [CrossRef]
- Pfister, J.A.; Manners, G.D.; Ralphs, M.H.; Hong, Z.X.; Lane, M.A. Effects of phenology, site, and rumen fill on tall larkspur consumption by cattle. J. Range Manag. 1988, 41, 509–514. [Google Scholar] [CrossRef]
- Manners, G.D.; Pfister, J.A.; Ralphs, M.H.; Panter, K.E.; Olsen, J.D. Larkspur chemistry: Toxic alkaloids in tall larkspurs. J. Range Manag. 1992, 45, 63–67. [Google Scholar] [CrossRef]
- Pelletier, S.W.; Dailey, O.D., Jr.; Mody, N.V.; Olsen, J.D. Isolation and structure elucidation of the alkaloids of Delphinium glaucescens rybd. J. Org. Chem. 1981, 46, 3284–3293. [Google Scholar] [CrossRef]
- Clark, D.; Ralphs, M.; Lamb, R. Total alkaloid determinations in larkspur and lupine with near infrared reflectance spectroscopy. Agron. J. 1987, 79, 481–485. [Google Scholar] [CrossRef]
- Gardner, D.R.; Manners, G.D.; Ralphs, M.H.; Pfister, J.A. Quantitative analysis of norditerpenoid alkaloids in larkspur (Delphinium spp.) by fourier transform infrared spectroscopy. Phytochem. Anal. 1997, 8, 55–62. [Google Scholar] [CrossRef]
- Ralphs, M.H.; Gardner, D.R.; Turner, D.L.; Pfister, J.A.; Thacker, E. Predicting toxicity of tall larkspur (Delphinium barbeyi): Measurement of the variation in alkaloid concentration among plants and among years. J. Chem. Ecol. 2002, 28, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.D.; Ralphs, M.H. Capillary gas chromatography of Delphinium diterpenoid alkaloids. J. Chromatogr. 1989, 466, 427–432. [Google Scholar] [CrossRef]
- Majak, W.; McDiarmid, R.E.; Benn, M.H. Isolation and HPLC determination of methyllycaconitine in a species of low larkspur (Delphinium nuttallianum). J. Agric. Food Chem. 1987, 35, 800–802. [Google Scholar] [CrossRef]
- Coates, P.A.; Blagbrough, I.S.; Lewis, T.; Potter, B.V.; Rowan, M.G. An HPLC assay for the norditerpenoid alkaloid methyllycaconitine, a potent nicotinic acetylcholine receptor antagonist. J. Pharm. Biomed. Anal. 1995, 13, 1541–1544. [Google Scholar] [CrossRef]
- Turek, J.W.; Kang, C.H.; Campbell, J.E.; Arneric, S.P.; Sullivan, J.P. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J. Neurosci. Methods 1995, 61, 113–118. [Google Scholar] [CrossRef]
- Gardner, D.R.; Panter, K.E.; Pfister, J.A.; Knight, A.P. Analysis of toxic norditerpenoid alkaloids in Delphinium species by electrospray, atmospheric pressure chemical ionization, and sequential tandem mass spectrometry. J. Agric. Food Chem. 1999, 47, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Bando, H.; Kawahara, N.; Mori, T.; Murayama, M. Determination and quantitative analysis of alkaloids in Aconitum japonicum by liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Biol. Mass Spectrom. 1994, 23, 97–102. [Google Scholar] [CrossRef]
- Wada, K.; Mori, T.; Kawahara, N. Stereochemistry of norditerpenoid alkaloids by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. J. Mass Spectrom. 2000, 35, 432–439. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Hall, J.O.; Gardner, D.R.; Panter, K.E. The toxicity and kinetics of larkspur alkaloid, methyllycaconitine, in mice. J. Anim. Sci. 2003, 81, 1237–1241. [Google Scholar] [PubMed]
- Green, B.T.; Welch, K.D.; Gardner, D.R.; Stegelmeier, B.L.; Pfister, J.A.; Cook, D.; Davis, T.Z. A toxicokinetic comparison of norditerpenoid alkaloids from Delphinium barbeyi and D. glaucescens in cattle. J. Appl. Toxicol. 2011, 31, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, V.N.; Benn, M.H.; Hanna, T.; Jacyno, J.; Roth, S.H.; Wilkens, J.L. The principal toxin of Delphinium brownii Rydb., and its mode of action. Experientia 1979, 35, 1367–1368. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.H.; Jacyno, J.M. The toxicology and pharmacology of diternpenoid alkaloids. In Alkaloids: Chemical and Biological Perspective; Pelletier, S.W., Ed.; John Wiley and Sons: New York, NY, USA, 1983; pp. 153–210. [Google Scholar]
- Dobelis, P.; Madl, J.E.; Pfister, J.A.; Manners, G.D.; Walrond, J.P. Effects of Delphinium alkaloids on neuromuscular transmission. J. Pharmacol. Exp. Ther. 1999, 291, 538–546. [Google Scholar] [PubMed]
- Kukel, C.F.; Jennings, K.R. Delphinium alkaloids as inhibitors of alpha-bungarotoxin binding to rat and insect neural membranes. Can. J. Physiol. Pharmacol. 1994, 72, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Cockcroft, V.B.; Lunt, G.G.; Smillie, F.S.; Wonnacott, S. Methyllycaconitine: A selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990, 270, 45–48. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Panter, K.E.; Pfister, J.A.; James, L.F.; Manners, G.D.; Ralphs, M.R.; Olsen, J.D. Experimental modification of larkspur (Delphinium spp.) poisoning in livestock. In Toxic Plants and Other Natural Toxicants; Garland, T.R., Barr, A.C., Eds.; CABI: Wallingford, Oxforshire, UK, 1998; pp. 205–210. [Google Scholar]
- Panter, K.E.; Welch, K.D.; Gardner, D.R.; Lee, S.T.; Green, B.T.; Pfister, J.A.; Cook, D.; Davis, T.Z.; Stegelmeier, B.L. Poisonous plants of the United States. In Veterinary Toxicology, Basic and Clinical Principles, 2nd ed.; Gupta, R.C., Ed.; Elsevier: New York, NY, USA, 2012; pp. 1031–1079. [Google Scholar]
- Green, B.T.; Pfister, J.A.; Cook, D.; Welch, K.D.; Stegelmeier, B.L.; Lee, S.T.; Gardner, D.R.; Knoppel, E.L.; Panter, K.E. Effects of larkspur (Delphinium barbeyi) on heart rate and electrically evoked electromyographic response of the external anal sphincter in cattle. Am. J. Vet. Res. 2009, 70, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.; Provenza, F.; Manners, G.; Gardner, D.; Ralphs, M. Tall larkspur ingestion: Can cattle regulate intake below toxic levels? J. Chem. Ecol. 1997, 23, 759–777. [Google Scholar] [CrossRef]
- Welch, K.D.; Green, B.T.; Gardner, D.R.; Cook, D.; Pfister, J.A. The effect of administering multiple doses of tall larkspur (Delphinium barbeyi) to cattle. J. Anim. Sci. 2015, in press. [Google Scholar]
- Goddard, M.E.; Hayes, B.J. Mapping genes for complex traits in domestic animals and their use in breeding programmes. Nat. Rev. Genet. 2009, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Green, B.T.; Panter, K.E.; Gardner, D.R.; Pfister, J.A.; Cook, D.; Stegelmeier, B.L. Investigation of the susceptibility of various strains of mice to methyllycaconitine toxicosis. J. Anim. Sci. 2009, 87, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.D.; Green, B.T.; Panter, K.E.; Pfister, J.A.; Gardner, D.R. The role of the α7 subunit of the nicotinic acetylcholine receptor in the acute toxicosis of methyllycaconitine in mice. J. Appl. Toxicol. 2013, 33, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Welch, K.D.; Pfister, J.A.; Chitko-McKown, C.G.; Gardner, D.R.; Panter, K.E. Mitigation of larkspur poisoning on rangelands through the selection of cattle. Rangelands 2014, 36, 10–15. [Google Scholar] [CrossRef]
- Green, B.T.; Welch, K.D.; Gardner, D.R.; Stegelmeier, B.L.; Davis, T.Z.; Cook, D.; Lee, S.T.; Pfister, J.A.; Panter, K.E. Serum elimination profiles of methyllycaconitine and deltaline in cattle following oral administration of larkspur (Delphinium barbeyi). Am. J. Vet. Res. 2009, 70, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Welch, K.D.; Gardner, D.R.; Stegelmeier, B.L.; Pfister, J.A.; Cook, D.; Panter, K.E. Toxicokinetics of norditerpenoid alkaloids from low larkspur (Delphinium andersonii) orally administered to cattle. Am. J. Vet. Res. 2012, 73, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Welch, K.D.; Gardner, D.R.; Stegelmeier, B.L.; Lee, S.T. A toxicokinetic comparison of two species of low larkspur (Delphinium spp.) in cattle. Res. Vet. Sci. 2013, 95, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Ralphs, M.H.; Jensen, D.T.; Pfister, J.A.; Nielsen, D.B.; James, L.F. Storms influence cattle to graze larkspur: An observation. J. Range Manag. 1994, 47, 275–278. [Google Scholar] [CrossRef]
- Pfister, J.A.; Ralphs, M.H.; Manners, G.D. Cattle grazing tall larkspur on Utah mountain rangeland. J. Range Manag. 1988, 41, 118–122. [Google Scholar] [CrossRef]
- Pfister, J.A.; Cook, D.; Gardner, D.R.; Baker, S.D. Early season grazing by cattle of waxy larkspur (Delphinium glaucescens) in central Idaho. Rangelands 2013, 35, 2–5. [Google Scholar] [CrossRef]
- Pfister, J.A.; Gardner, D.R. Consumption of low larkspur (Delphinium nuttallianum) by cattle. J. Range Manag. 1999, 63, 378–383. [Google Scholar] [CrossRef]
- Pfister, J.A.; Manners, G.D.; Gardner, D.R.; Price, K.W.; Ralphs, M.H. Influence of alkaloid concentration on acceptability of tall larkspur (Delphinium spp.) to cattle and sheep. J. Chem. Ecol. 1996, 22, 1147–1168. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.A.; Provenza, F.D.; Manners, G.D. Ingestion of tall larkspur by cattle: Separating effects of flavor from postingestive consequences. J. Chem. Ecol. 1990, 16, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.A.; Gardner, D.R.; Stegelmeier, B.L.; Hackett, K.; Secrist, G. Catastrophic cattle loss to low larkspur (Delphinium nuttallianum) in Idaho. Vet. Hum. Toxicol. 2003, 45, 137–139. [Google Scholar] [PubMed]
- Cook, D.; Pfister, J.A.; Constantino, J.R.; Roper, J.M.; Gardner, D.R.; Welch, K.D.; Hammond, Z.J.; Green, B.T. Development of a PCR-based method for detection of Delphinium species in poisoned cattle. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef]
- Ralphs, M.H. Toxic alkaloid concentration in tall larkspur species in the western U.S. J. Range Manag. 1997, 50, 497–502. [Google Scholar] [CrossRef]
- Chesnut, V.; Wilcox, E. The stock poisoning plants of Montana. USDA Div. Bot. Bull. 1901, 26, 1–150. [Google Scholar]
- Ralphs, M.H.; Olsen, J.D. Comparison of larkspur alkaloid extract and lithium chloride in maintaining cattle aversion to larkspur in the field. J. Anim. Sci. 1992, 70, 1116–1120. [Google Scholar] [PubMed]
- Pfister, J.A.; Gardner, D.R.; Cheney, C.C.; Panter, K.E.; Hall, J.O. The capability of several toxic plants to condition taste aversions in sheep. Small Rumin. Res. 2010, 90, 114–119. [Google Scholar] [CrossRef]
- Knight, A.P.; Pfister, J.A. Larkspur poisoning in livestock: Myths and misconceptions. Rangelands 1997, 19, 10–13. [Google Scholar]
- Nation, P.N.; Benn, M.H.; Roth, S.H.; Wilkens, J.L. Clinical signs and studies of the site of action of purified larkspur alkaloid, methyllycaconitine, administered parenterally to calves. Can. Vet. J. 1982, 23, 264–266. [Google Scholar] [PubMed]
- Pfister, J.A.; Panter, K.E.; Manners, G.D.; Cheney, C.D. Reversal of tall larkspur (Delphinium barbeyi) poisoning in cattle with physostigmine. Vet. Hum. Toxicol. 1994, 36, 511–514. [Google Scholar] [PubMed]
- Ralphs, M.H.; Evans, J.O.; Dewey, S.A. Timing of herbicide applications for control of larkspurs (Delphinium spp.). Weed Sci. 1992, 40, 264–269. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welch, K.D.; Cook, D.; Green, B.T.; Gardner, D.R.; Pfister, J.A.; McDaneld, T.G.; Panter, K.E. Adverse Effects of Larkspur (Delphinium spp.) on Cattle. Agriculture 2015, 5, 456-474. https://doi.org/10.3390/agriculture5030456
Welch KD, Cook D, Green BT, Gardner DR, Pfister JA, McDaneld TG, Panter KE. Adverse Effects of Larkspur (Delphinium spp.) on Cattle. Agriculture. 2015; 5(3):456-474. https://doi.org/10.3390/agriculture5030456
Chicago/Turabian StyleWelch, Kevin D., Daniel Cook, Benedict T. Green, Dale R. Gardner, James A. Pfister, Tara G. McDaneld, and Kip E. Panter. 2015. "Adverse Effects of Larkspur (Delphinium spp.) on Cattle" Agriculture 5, no. 3: 456-474. https://doi.org/10.3390/agriculture5030456





