Development of Recombinant Flagellar Antigens for Serological Detection of Salmonella enterica Serotypes Enteritidis, Hadar, Heidelberg, and Typhimurium in Poultry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Expression and Purification of Recombinant Salmonella H1 and H2 Flagellin Antigens


2.2. Development and Evaluation of S. enterica Enteritidis, Specific ELISA Using Recombinant FliCg,m Protein Fusion as the Detecting Antigen
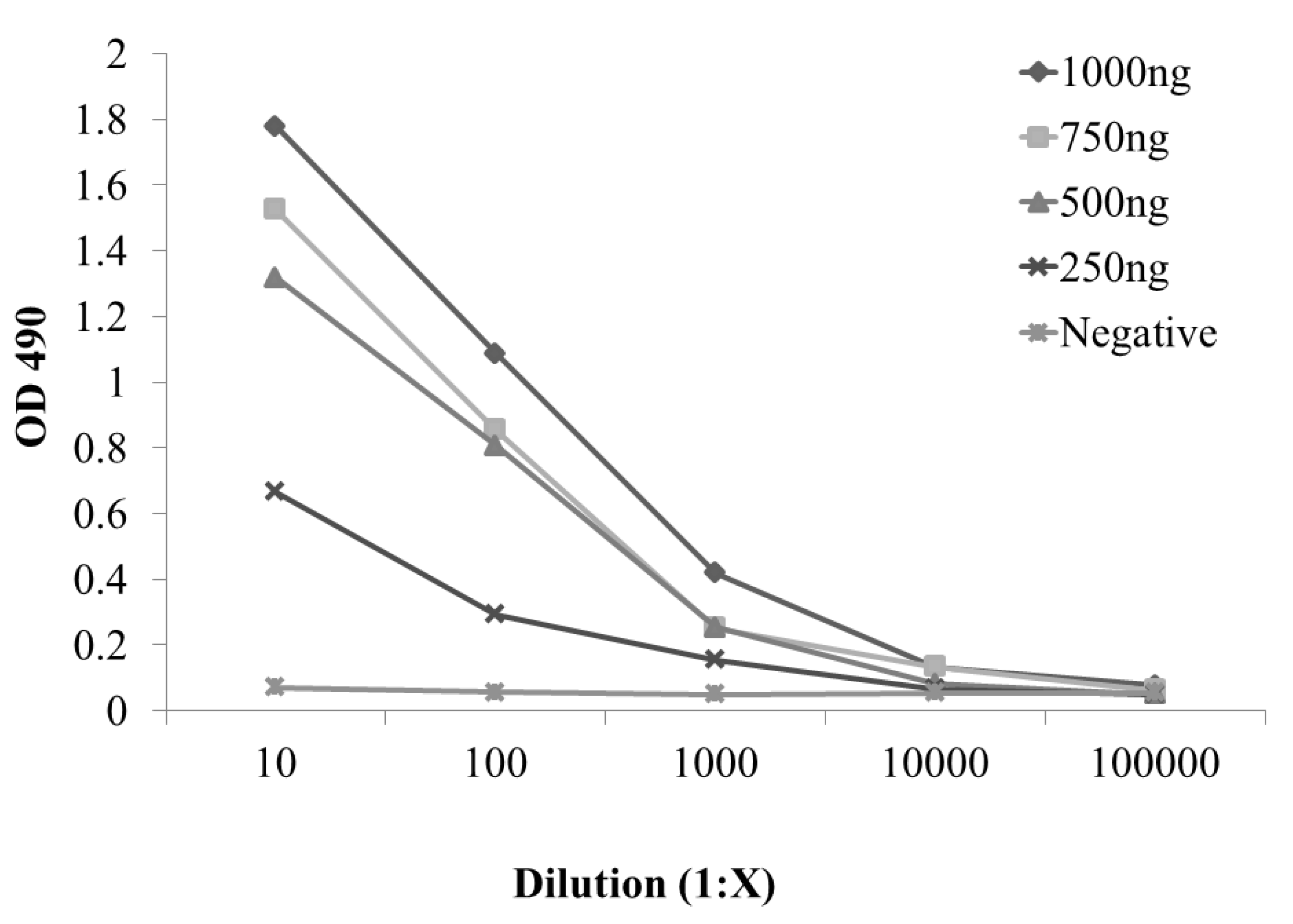
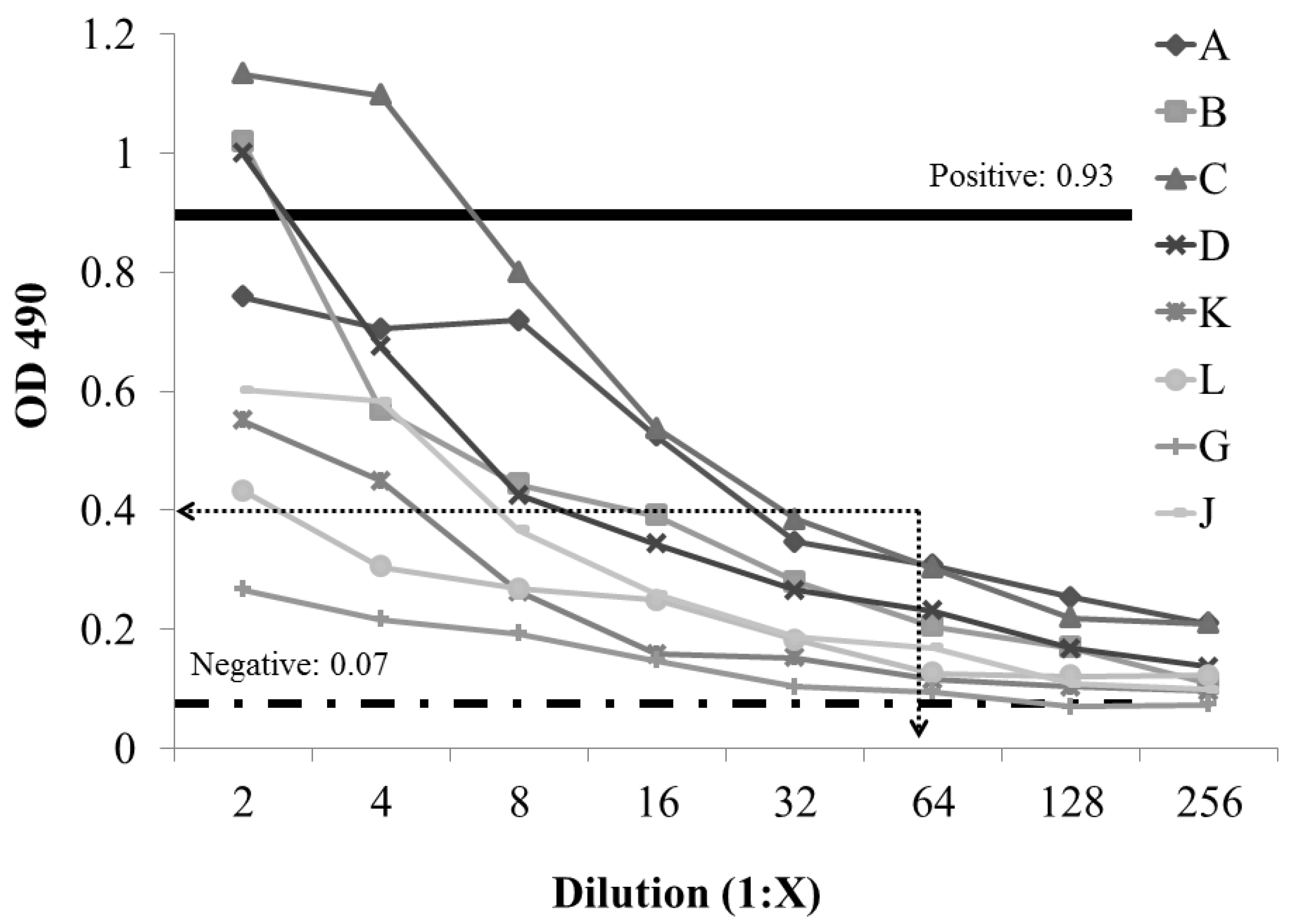

3. Experimental Section
3.1. PCR amplification and Cloning of S. enterica Flagellin Genes, fliC and fljB from Salmonella Serotypes Enteritidis, Hadar, Heidelberg, and Typhimurium
| Target/Name | Sequence1 | Expected Size (bp) | Distance from Fusion2 |
|---|---|---|---|
| fliC | F: ACA AGT CAT TAA TAC AAA CAG CC | 1500 | |
| R :GGA TCC AGT AAA GAG AGG ACG TTT TG | |||
| fljB | F: GGC ACA AGT AAT CAA CAC | 1500 | |
| R: GGA TCC TTA ACT TAA CAG AGA CAG CA | |||
| 3′BPT | GGA GAC CGA GAT CAA CGC TC | −24 | |
| 5′ fliC | GGA AGA CAG ACG CTC GAT AG | 419 | |
| 5′ fljB | GGT CAG CAG CGA CAG ACT GT | 445 |
| Strain | Genotype or Description | Source |
|---|---|---|
| E. coli BL21 (DE3) | F- dcm, ompT, hsdS(rBmB), gal λ(DE3) | [54] |
| E. coli JM 109 | endA1, recA1, gyrA96, thi, hsdR17 (rk–, mk+), relA1, supE44, Δ(lac-proAB), [F’, traD36, proAB, lacIq, ZM15] | [55] |
| S. Typhimurium | Poultry Isolate | [56] |
| S. Enteritidis | Poultry Isolate | [56] |
| S. Heidelberg | Poultry Isolate | [56] |
| S. Hadar | Poultry Isolate | [56] |
| Plasmid | ||
| PinPoint™ Xa-1 T | blaTEM, pUC based vector with a biotin purification tag for protein fusions (Promega, Madison, WI) | [57] |
| pJMZ 63 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Typhimurium fliC (i) | This Study |
| pJMZ 42 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Enteritidis fliC (g,m) | This Study |
| pJMZ 2 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Heidelberg fliC (r) | This Study |
| pJMZ 47 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Hadar fliC (z10) | This Study |
| pJMZ 5 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Typhimurium fljB (1,2) | This Study |
| pJMZ 18 | PinPoint™ Xa-1 T with in-frame biotin fusion with S. Hadar fljB (e,n,x) | This Study |
3.2. Expression S. enterica Flagellin, Protein Fusions in E. coli Cloning Host BL21.
3.3. Purification of Recombinant Salmonella Flagellin Proteins with Biotin Tag
3.4. Enzyme-Linked Immunosorbent Assay
4. Conclusions
Acknowledgments
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar]
- Batz, M.B.; Hoffmann, S.; Morris, J.G., Jr. Ranking the disease burden of 14 pathogens in food sources in the united states using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef]
- Pathogen reduction; hazard analysis and critical control point (haccp) systems; final rule. Fed. Regist. 1996, 61, 38805–38989.
- Anonymous. Fsis notice 54-12: Performance standards for Salmonella and Campylobacter in chilled carcasses at young chicken and turkey slaughter establishments. USDA Food Safety and Inspection Service. 2012. Available online: http://www.fsis.usda.gov/OPPDE/rdad/FSISNotices/54-12.pdf (accessed on 4 April 2013).
- Dorea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Thayer, S.G.; Maurer, J.J.; Hofacre, C.L. Effect of vaccinating breeder chickens with a killed Salmonella vaccine on Salmonella prevalences and loads in breeder and broiler chicken flocks. J. Food Prot. 2011, 74, 727–734. [Google Scholar] [CrossRef]
- Toyota-Hanatani, Y.; Ekawa, T.; Ohta, H.; Igimi, S.; Hara-Kudo, Y.; Sasai, K.; Baba, E. Public health assessment of Salmonella enterica serovar Enteritidis inactivated-vaccine treatment in layer flocks. Appl. Environ. Microbiol. 2009, 75, 1005–1010. [Google Scholar] [CrossRef]
- Anonymous. National poultry improvement plan and auxiliary provisions. Fed. Regist. 2000, 65, 8014–8023. [Google Scholar]
- Popoff, M.Y.; Bockemuhl, J.; Gheesling, L.L. Supplement 2001 (no. 45) to the kauffmann-white scheme. Res. Microbiol. 2003, 154, 173–174. [Google Scholar] [CrossRef]
- Anonymous. Salmonella annual summary 2009. Centers for Disease Control and Prevention; National Salmonella Surveillance Data. 2009. Available online: http://www.cdc.gov/ncezid/PDFs/SalmonellaAnnualSummaryTables2009.pdf (accessed on 25 March 2013).
- Anonymous. Serotypes profile of Salmonella isolates from meat and poultry products: January 1998 through december 2010. USDA Food Safety and Inspection Service. 2010. Available online: http://www.fsis.usda.gov/PDF/Serotypes_Profile_Salmonella_2010.pdf (accessed on 25 March 2013).
- Anonymous. Fsis notice 22-13: Historical Salmonella serotype information letters for establishments producing ground chicken and ground turkey. USDA Food Safety and Inspection Service. 2013. Available online: http://www.fsis.usda.gov/OPPDE/rdad/FSISNotices/22-13.pdf (accessed on 4 April 2013).
- CDC. Salmonella-serotype of isolate by year, United States, 1973–1998. Morbid. Mortal. Wkly. Rep. 1999, 47, 60.
- Lin, F.Y.; Morris, J.G., Jr.; Trump, D.; Tilghman, D.; Wood, P.K.; Jackman, N.; Israel, E.; Libonati, J.P. Investigation of an outbreak of Salmonella Enteritidis gastroenteritis associated with consumption of eggs in a restaurant chain in maryland. Am. J. Epidemiol. 1988, 128, 839–844. [Google Scholar]
- Ebel, E.D.; David, M.J.; Mason, J. Occurrence of Salmonella Enteritidis in the U.S. Commercial egg industry: Report on a national spent hen survey. Avian Dis. 1992, 36, 646–654. [Google Scholar] [CrossRef]
- Blivet, D.; Salvat, G.; Humbert, F.; Colin, P. Evaluation of a new enrichment broth for the isolation of Salmonella spp. from poultry products. Int. J. Food Microbiol. 1997, 38, 211–216. [Google Scholar] [CrossRef]
- D’Aoust, J.Y.; Sewell, A.M.; Warburton, D.W. A comparison of standard cultural methods for the detection of foodborne Salmonella. Int. J. Food Microbiol. 1992, 16, 41–50. [Google Scholar] [CrossRef]
- Hajna, A.A.; Damon, S.R. New enrichment and plating media for the isolation of Salmonella and Shigella organisms. Appl. Microbiol. 1956, 4, 341–345. [Google Scholar]
- Miller, R.G.; Tate, C.R.; Mallinson, E.T.; Scherrer, J.A. Xylose-lysine-tergitol 4: An improved selective agar medium for the isolation of Salmonella. Poult. Sci. 1991, 70, 2429–2432. [Google Scholar] [CrossRef]
- Liu, T.; Liljebjelke, K.; Bartlett, E.; Hofacre, C.; Sanchez, S.; Maurer, J.J. Application of nested polymerase chain reaction to detection of Salmonella in poultry environment. J. Food Prot. 2002, 65, 1227–1232. [Google Scholar]
- Hong, Y.; Liu, T.; Hofacre, C.; Maier, M.; White, D.; Ayers, S.; Wang, L.; Maurer, J. A restriction fragment length polymorphism-based polymerase chain reaction as analternative to serotyping for identifying Salmonella serotypes. Avian Dis. 2003, 47, 387–395. [Google Scholar] [CrossRef]
- Thorns, C.J.; McLaren, I.M.; Sojka, M.G. The use of latex particle agglutination to specifically detect Salmonella Enteritidis. Int. J. Food Microbiol. 1994, 21, 47–53. [Google Scholar] [CrossRef]
- Davies, R.H.; Nicholas, R.A.; McLaren, I.M.; Corkish, J.D.; Lanning, D.G.; Wray, C. Bacteriological and serological investigation of persistent Salmonella Enteritidis infection in an integrated poultry organisation. Vet. Microbiol. 1997, 58, 277–293. [Google Scholar] [CrossRef]
- Gast, R.K.; Porter, R.E., Jr.; Hold, P.S. Applying tests for specific yolk antibodies to predict contamination by Salmonella Enteritidis in eggs from experimentally infected laying hens. Avian Dis. 1997, 41, 195–202. [Google Scholar] [CrossRef]
- Harvey, R.W.; Price, T.H. A comparison of two modifications of rappaport’s enrichment medium (r25 and rv) for the isolation of salmonellas from sewage polluted natural water. J. Hyg. Lond. 1983, 91, 451–458. [Google Scholar] [CrossRef]
- Osborne, W.W.; Stokes, J.L. A modified selenite brilliant-green medium for the isolation of Salmonella from egg products. Appl. Microbiol. 1955, 3, 295–299. [Google Scholar]
- Baylis, C.L.; MacPhee, S.; Betts, R.P. Comparison of two commercial preparations of buffered peptone water for the recovery and growth of Salmonella bacteria from foods. J. Appl. Microbiol. 2000, 89, 501–510. [Google Scholar] [CrossRef]
- Pourciau, S.S.; Springer, W.T. Evaluation of secondary enrichment for detecting salmonellae in bobwhite quail. Avian Dis. 1978, 22, 42–45. [Google Scholar] [CrossRef]
- Rigby, C.E.; Pettit, J.R.; Bentley, A.H.; Spencer, J.L.; Salomons, M.O.; Lior, H. The relationships of salmonellae from infected broiler flocks, transport crates or processing plants to contamination of eviscerated carcases. Can. J. Comp. Med. 1982, 46, 272–278. [Google Scholar]
- Waltman, W.D.; Horne, A.M.; Pirkle, C. Influence of enrichment incubation time on the isolation of Salmonella. Avian Dis. 1993, 37, 884–887. [Google Scholar] [CrossRef]
- Blankenship, L.C.; Bailey, J.S.; Cox, N.A.; Stern, N.J.; Brewer, R.; Williams, O. Two-step mucosal competitive exclusion flora treatment to diminish salmonellae in commercial broiler chickens. Poult. Sci. 1993, 72, 1667–1672. [Google Scholar] [CrossRef]
- Nicholas, R.A. Serological response of chickens naturally infected with Salmonella Typhimurium detected by elisa. Br. Vet. J. 1992, 148, 241–248. [Google Scholar] [CrossRef]
- Edel, W. Salmonella Enteritidis eradication programme in poultry breeder flocks in the netherlands. Int. J. Food Microbiol. 1994, 21, 171–178. [Google Scholar] [CrossRef]
- Veling, J.; van Zijderveld, F.G.; van Zijderveld-van Bemmel, A.M.; Barkema, H.W.; Schukken, Y.H. Evaluation of three newly developed enzyme-linked immunosorbent assays and two agglutination tests for detecting Salmonella enterica subsp. enterica serovar dublin infections in dairy cattle. J. Clin. Microbiol. 2000, 38, 4402–4407. [Google Scholar]
- Nicholas, R.A.; Cullen, G.A. Development and application of an elisa for detecting antibodies to Salmonella Enteritidis in chicken flocks. Vet. Rec. 1991, 128, 74–76. [Google Scholar]
- Barrow, P.A. Serological diagnosis of Salmonella serotype Enteritidis infections in poultry by elisa and other tests. Int. J. Food Microbiol. 1994, 21, 55–68. [Google Scholar] [CrossRef]
- Barrow, P.A. Further observations on the serological response to experimental Salmonella Typhimurium in chickens measured by elisa. Epidemiol. Infect. 1992, 108, 231–241. [Google Scholar] [CrossRef]
- Desmidt, M.; Ducatelle, R.; Haesebrouck, F.; de Groot, P.A.; Verlinden, M.; Wijffels, R.; Hinton, M.; Bale, J.A.; Allen, V.M. Detection of antibodies to Salmonella Enteritidis in sera and yolks from experimentally and naturally infected chickens. Vet. Rec. 1996, 138, 223–226. [Google Scholar] [CrossRef]
- Rasolofo-Razanamparany, V.; Cassel-Beraud, A.M.; Roux, J.; Sansonetti, P.J.; Phalipon, A. Predominance of serotype-specific mucosal antibody response in shigella flexneri-infected humans living in an area of endemicity. Infect. Immun. 2001, 69, 5230–5234. [Google Scholar] [CrossRef]
- Van Zijderveld, F.G.; van Zijderveld-van Bemmel, A.M.; Anakotta, J. Comparison of four different enzyme-linked immunosorbent assays for serological diagnosis of Salmonella Enteritidis infections in experimentally infected chickens. J. Clin. Microbiol. 1992, 30, 2560–2566. [Google Scholar]
- Baay, M.F.; Huis in’t Veld, J.H. Alternative antigens reduce cross-reactions in an elisa for the detection of Salmonella Enteritidis in poultry. J. Appl. Bacteriol 1993, 74, 243–247. [Google Scholar] [CrossRef]
- Kilger, G.; Grimont, P.A. Differentiation of Salmonella phase 1 flagellar antigen types by restriction of the amplified flic gene. J. Clin. Microbiol. 1993, 31, 1108–1110. [Google Scholar]
- Newton, S.M.; Wasley, R.D.; Wilson, A.; Rosenberg, L.T.; Miller, J.F.; Stocker, B.A. Segment iv of a Salmonella flagellin gene specifies flagellar antigen epitopes. Mol. Microbiol. 1991, 5, 419–425. [Google Scholar] [CrossRef]
- Joys, T.M. The covalent structure of the phase-1 flagellar filament protein of Salmonella Typhimurium and its comparison with other flagellins. J. Biol. Chem. 1985, 260, 15758–15761. [Google Scholar]
- Rosenberg, I.M. Protein Analysis and Purification: Benchtop Techniques; Birkhauser: Boston, MA, USA, 1996. [Google Scholar]
- Balfour, A.H.; Harford, J.P. Quality Control and Standardization in Elisa in the Clinical Laboratory; Public Health Service Laboratory: London, UK, 1990; pp. 36–47. [Google Scholar]
- United States. Animal and Plant Health Inspection Service. National Poultry Improvement Plan and Auxiliary Provisions. Amendments to Provisions. Fed. Regist. 2001, 66, 37919–37932.
- Rigby, C.E.; Pettit, J.R. Delayed secondary enrichment for the isolation of salmonellae from broiler chickens and their environment. Appl. Environ. Microbiol. 1980, 40, 783–786. [Google Scholar]
- Anonymous. Fda food safety modernization act. U.S. Food and Drug Administration, 2011. Available online: http://www.fda.gov/Food/GuidanceRegulation/FSMA/ucm247548.htm (accessed on 4 April 2013).
- Hilton, A.C.; Banks, J.G.; Penn, C.W. Optimization of rapd for fingerprinting Salmonella. Lett. Appl. Microbiol. 1997, 24, 243–248. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Fillmore, G.C.; Hillyard, D.R. Automated polymerase chain reaction in capillary tubes with hot air. Nucleic Acids Res. 1989, 17, 4353–4357. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Ausubel, F.M. Current Protocols in Molecular Biology; John Wiley & Sons: New York, NY, USA, 2001; p. 4v. [Google Scholar]
- Weiner, M.P.; Anderson, C.; Jerpseth, B.; Wells, S.; Johnson-Browne, B. Sturdier pET system vectors and hosts. Strategies 1994, 7, 41–43. [Google Scholar]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved m13 phage cloning vectors and host strains: Nucleotide sequences of the m13mp18 and puc19 vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Swamy, S.C.; Barnhart, H.M.; Lee, M.D.; Dreesen, D.W. Virulence determinants inva and spvc in salmonellae isolated from poultry products, wastewater, and human sources. Appl. Environ. Microbiol. 1996, 62, 3768–3771. [Google Scholar]
- Cronan, J.E., Jr. Biotination of proteins in vivo. a post-translational modification to label, purify, and study proteins. J. Biol. Chem. 1990, 265, 10327–10333. [Google Scholar]
- Lammeli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, T.; Lee, M.D.; Hofacre, C.L.; Maier, M.; White, D.G.; Ayers, S.; Wang, L.; Berghaus, R.; Maurer, J.J. Rapid screening of Salmonella enterica serovars Enteritidis, hadar, heidelberg and Typhimurium using a serologically-correlative allelotyping pcr targeting the o and h antigen alleles. BMC Microbiol. 2008, 8, 178. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Minicozzi, J.; Sanchez, S.; Lee, M.D.; Holt, P.S.; Hofacre, C.L.; Maurer, J.J. Development of Recombinant Flagellar Antigens for Serological Detection of Salmonella enterica Serotypes Enteritidis, Hadar, Heidelberg, and Typhimurium in Poultry. Agriculture 2013, 3, 381-397. https://doi.org/10.3390/agriculture3030381
Minicozzi J, Sanchez S, Lee MD, Holt PS, Hofacre CL, Maurer JJ. Development of Recombinant Flagellar Antigens for Serological Detection of Salmonella enterica Serotypes Enteritidis, Hadar, Heidelberg, and Typhimurium in Poultry. Agriculture. 2013; 3(3):381-397. https://doi.org/10.3390/agriculture3030381
Chicago/Turabian StyleMinicozzi, Joseph, Susan Sanchez, Margie D. Lee, Peter S. Holt, Charles L. Hofacre, and John J. Maurer. 2013. "Development of Recombinant Flagellar Antigens for Serological Detection of Salmonella enterica Serotypes Enteritidis, Hadar, Heidelberg, and Typhimurium in Poultry" Agriculture 3, no. 3: 381-397. https://doi.org/10.3390/agriculture3030381




