Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Liver Diseases
2.1. Non-Alcoholic Fatty Liver Disease (NAFLD)
2.2. Alcoholic Steatohepatitis (ASH), Liver Cirrhosis, and Acute Liver Failure
3. Pancreatic Diseases
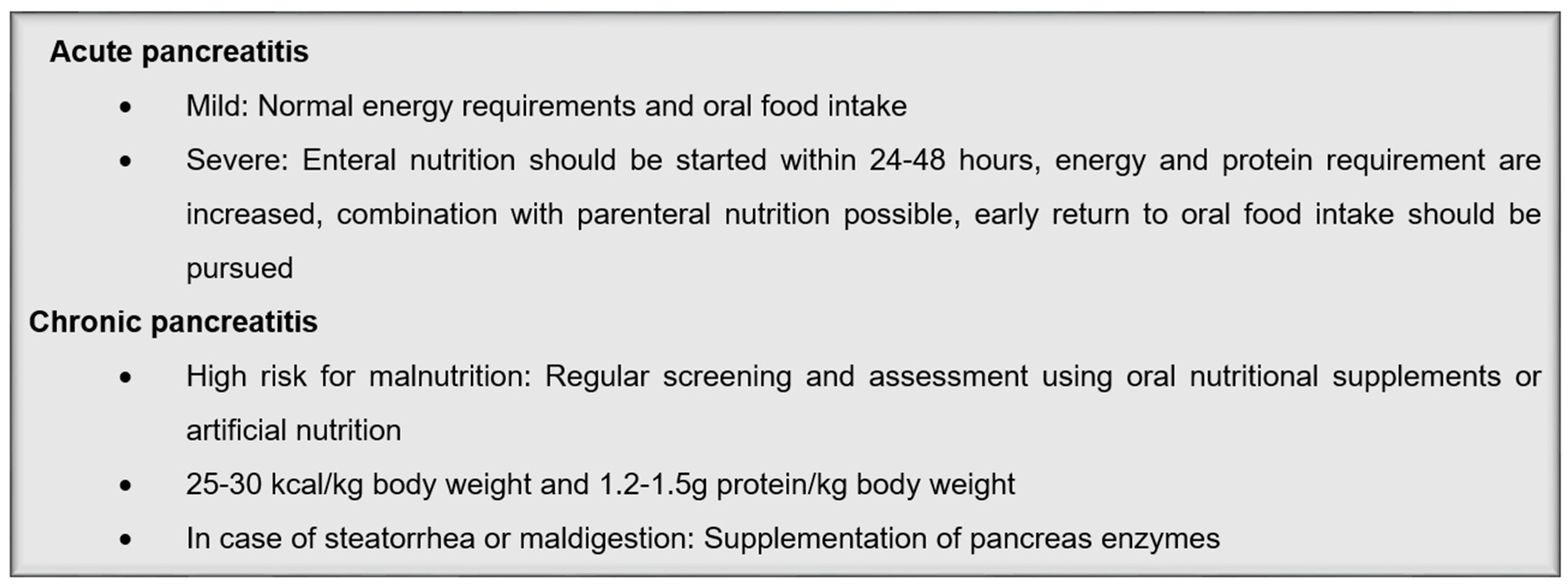
3.1. Acute Pancreatitis
3.2. Chronic Pancreatitis
4. Inflammatory Bowel Diseases (IBD)
5. Future Perspectives
Author Contributions
Conflicts of Interest
References
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: A meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Abraham, M.; Unalp, A.; Wilson, L.; Lavine, J.; Doo, E.; Bass, N.M. Nonalcoholic steatohepatitis clinical research, N. association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012, 56, 943–951. [Google Scholar] [CrossRef] [PubMed]
- European association for the study of the liver, european association for the study of diabetes, european association for the study of obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 2016, 9, 65–90. [CrossRef] [PubMed]
- Ullah, R.; Rauf, N.; Nabi, G.; Ullah, H.; Shen, Y.; Zhou, Y.D.; Fu, J. Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: Recent updates. Int. J. Biol. Sci. 2019, 15, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharm. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, A.; Mayerle, J.; Beglinger, C.; Büchler, M.; Bufler, P.; Dathe, K.; Fölsch, U.; Friess, H.; Izbicki, J.; Kahl, S.; et al. S3-Leitlinie chronische pankreatitis: Definition, ätiologie, diagnostik, konservative, interventionell endoskopische und operative therapie der chronischen pankreatitis. leitlinie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten (DGVS). Zeitschrift für Gastroenterol. 2012, 50, 1176–1224. [Google Scholar]
- Hanauer, S.B. Inflammatory Bowel Disease: Epidemiology, Pathogenesis, and Therapeutic Opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef]
- Wedrychowicz, A.; Zajac, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mummadi, R.R.; Kasturi, K.S.; Chennareddygari, S.; Sood, G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2008, 6, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic fatty liver disease and insulin resistance: New insights and potential new treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Li, D.; Ikaga, R. Effective food ingredients for fatty liver: Soy protein beta-conglycinin and fish oil. Int. J. Mol. Sci. 2018, 19, 4107. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Baratta, F.; Pastori, D.; Angelico, F. The role of nutraceuticals for the treatment of non-alcoholic fatty liver disease. Br. J. Clin. Pharm. 2017, 83, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Boque, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Yaskolka Meir, A.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of distinct lifestyle Interventions on mobilization of fat storage pools: Central magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef]
- Mendenhall, C.L.; Anderson, S.; Weesner, R.E.; Goldberg, S.J.; Crolic, K.A. Protein-calorie malnutrition associated with alcoholic hepatitis. Am. J. Med. 1984, 76, 211–222. [Google Scholar] [CrossRef]
- Merli, M.; Riggio, O.; Dally, L. Italian Multicenter Cooperative Project on nutrition in liver cirrhosis. Nutritional status in cirrhosis. J. Hepatol. 1994, 21, 317–325. [Google Scholar]
- Lautz, H.U.; Selberg, O.; Körber, J.; Bürger, M.; Müller, M.J. Protein-calorie malnutrition in liver cirrhosis. Clin. Investig. 1992, 70, 478–486. [Google Scholar] [CrossRef]
- Anand, A.C. Nutrition and muscle in cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Borhofen, S.M.; Gerner, C.; Lehmann, J.; Fimmers, R.; Gortzen, J.; Hey, B.; Geiser, F.; Strassburg, C.P.; Trebicka, J. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig. Dis. Sci. 2016, 61, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A.; Fitness, L.E. Exercise in liver transplantation, C. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017, 23, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Jhangiani, S.S.; Agarwal, N.; Holmes, R.; Cayten, C.G.; Pitchumoni, C.S. Energy expenditure in chronic alcoholics with and without liver disease. Am. J. Clin. Nutr. 1986, 44, 323–329. [Google Scholar] [CrossRef]
- Schneeweiss, B.; Pammer, J.; Ratheiser, K.; Schneider, B.; Maldl, C.; Kramer, L.; Kranz, A.; Ferenci, P.; Druml, W.; Grimm, G.; et al. Energy metabolism in acute hepatic failure. Gastroenterology 1993, 105, 1515–1521. [Google Scholar] [CrossRef]
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, J.; Lopez-Hellin, J.; Planas, M.; Sabin, P.; Sanpedro, F.; Castro, F.; Esteban, R.; Guardia, J. Normal protein diet for episodic hepatic encephalopathy: Results of a randomized study. J. Hepatol. 2004, 41, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ney, M.; Vandermeer, B.; van Zanten, S.J.; Ma, M.M.; Gramlich, L.; Tandon, P. Meta-analysis: Oral or enteral nutritional supplementation in cirrhosis. Aliment. Pharm. 2013, 37, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Clemmesen, J.O.; Kondrup, J.; Ott, P. Splanchnic and leg exchange of amino acids and ammonia in acute liver failure. Gastroenterology 2000, 118, 1131–1139. [Google Scholar] [CrossRef]
- Rosen, H.M.; Yoshimura, N.; Hodgman, J.M.; Fischer, J.E. Plasma amino acid patterns in hepatic encepathalopathy of differing etiology. Gastroenterology 1977, 72, 483–487. [Google Scholar] [PubMed]
- Walsh, T.S.; Wigmore, S.J.; Hopton, P.; Richardson, R.; Lee, A. Energy expenditure in acetaminophen-induced fulminant hepatic failure. Crit. Care. Med. 2000, 28, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J.; Löser, C.; Kraft, M.; Madl, C. S3-Leitlinie der Deutschen Gesellschaft für Ernährungsmedizin (DGEM) in Zusammenarbeit mit der GESKES, der AKE und der DGVS. Aktuelle Ernährungsmedizin 2014, 39, e43–e56. [Google Scholar] [CrossRef]
- Meier, R.; Ockenga, J.; Pertkiewicz, M.; Pap, A.; Milinic, N.; Macfie, J.; Dgem; Loser, C.; Keim, V. ESPEN guidelines on enteral nutrition: Pancreas. Clin. Nutr. 2006, 25, 275–284. [Google Scholar] [CrossRef]
- Larino-Noia, J.; Lindkvist, B.; Iglesias-Garcia, J.; Seijo-Rios, S.; Iglesias-Canle, J.; Dominguez-Munoz, J.E. Early and/or immediately full caloric diet versus standard refeeding in mild acute pancreatitis: A randomized open-label trial. Pancreatology 2014, 14, 167–173. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Shuster, D.; Rogers, M.A.M.; Mann, J.; Conte, M.L.; Saint, S.; Chopra, V. Early versus delayed feeding in patients with acute pancreatitis: A systematic review. Ann. Intern. Med. 2017, 166, 883–892. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M. The european organization for research and treatment of cancer qlq-c30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Zaloga, G.P. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004, 328, 1407. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Chang, W.K.; Dhaliwal, R.; Heyland, D.K. Nutrition support in acute pancreatitis: A systematic review of the literature. J. Parenter. Enter. Nutr. 2006, 30, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Al-Omran, M.; Albalawi, Z.H.; Tashkandi, M.F.; Al-Ansary, L.A. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst. Rev. 2010, CD002837. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.W.; Carter, C.R.; McKay, C.J. Enteral and parenteral nutrition in acute pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H.F.; Wolfe, R.R. Glucose, fatty acid, and urea kinetics in patients with severe pancreatitis—The response to substrate infusion and total parenteral nutrition. Ann. Surg. 1986, 204, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, K.; He, X.; Tian, J.; Ma, B.; Jiang, L. Probiotics in patients with severe acute pancreatitis: A meta-analysis. Langenbecks Arch. Surg. 2009, 394, 171–177. [Google Scholar] [CrossRef]
- Petrov, M.S.; Atduev, V.A.; Zagainov, V.E. Advanced enteral therapy in acute pancreatitis: Is there a room for immunonutrition? A meta-analysis. Int. J. Surg. 2008, 6, 119–124. [Google Scholar]
- Asrani, V.; Chang, W.K.; Dong, Z.; Hardy, G.; Windsor, J.A.; Petrov, M.S. Glutamine supplementation in acute pancreatitis: A meta-analysis of randomized controlled trials. Pancreatology 2013, 13, 468–474. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Dominguez-Munoz, J.E.; Phillips, M. Nutritional therapy in chronic pancreatitis. Gastroenterol. Clin. North. Am. 2018, 47, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J. Importance of nutritional management in diseases with exocrine pancreatic insufficiency. HPB 2009, 11 (Suppl. S3), 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lévy, P.; Mathurin, P.; Roqueplo, A.; Rueff, B.; Bernades, P. A Multidimensional case-control study of dietary, alcohol, and tobacco habits in alcoholic men with chronic pancreatitis. Pancreas 1995, 10, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Smyth, N.D.; O’Sullivan, M.; Feehan, S.; Ridgway, P.F.; Conlon, K.C. The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis. Nutr. Clin. Pr. 2014, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Dominguez-Munoz, J.E.; Luaces-Regueira, M.; Castineiras-Alvarino, M.; Nieto-Garcia, L.; Iglesias-Garcia, J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Phillips, M.E.; Dominguez-Munoz, J.E. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology 2015, 15, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Montalto, G.; Soresi, M.; Carroccio, A.; Scafidi, E.; Barbagallo, C.M.; Ippolito, S.; Notarbartolo, A. Lipoproteins and chronic pancreatitis. Pancreas 1994, 9, 137–138. [Google Scholar] [CrossRef]
- Dominguez-Munoz, J.E. Pancreatic exocrine insufficiency: Diagnosis and treatment. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S2), 12–16. [Google Scholar] [CrossRef]
- Lohr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United european gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef]
- Saez-Gonzalez, E.; Mateos, B.; Lopez-Munoz, P.; Iborra, M.; Moret, I.; Nos, P.; Beltran, B. Bases for the adequate development of nutritional recommendations for patients with inflammatory bowel disease. Nutrients 2019, 11, 1062. [Google Scholar] [CrossRef]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The role of vitamin D in inflammatory bowel disease: Mechanism to management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The role of dietary nutrients in inflammatory bowel disease. Front. Immunol. 2018, 9, 3183. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharm. 2003, 17, 307–320. [Google Scholar]
- Hill, R.J.; Cleghorn, G.J.; Withers, G.D.; Lewindon, P.J.; Ee, L.C.; Connor, F.; Davies, P.S. Resting energy expenditure in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Wiskin, A.E.; Wootton, S.A.; Culliford, D.J.; Afzal, N.A.; Jackson, A.A.; Beattie, R.M. Impact of disease activity on resting energy expenditure in children with inflammatory bowel disease. Clin. Nutr. 2009, 28, 652–656. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Sandhu, A.; Mosli, M.; Yan, B.; Wu, T.; Gregor, J.; Chande, N.; Ponich, T.; Beaton, M.; Rahman, A. Self-screening for malnutrition risk in outpatient inflammatory bowel disease patients using the malnutrition universal screening tool (MUST). J. Parenter Enter. Nutr. 2016, 40, 507–510. [Google Scholar] [CrossRef]
- Gajendran, M.; Umapathy, C.; Loganathan, P.; Hashash, J.G.; Koutroubakis, I.E.; Binion, D.G. Analysis of hospital-based emergency department visits for inflammatory bowel disease in the USA. Dig. Dis. Sci. 2016, 61, 389–399. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; McGinley, E.L. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J. Crohns. Colitis. 2013, 7, 107–112. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Ogden, J.; Rund, J.; Potter, P. Steroids and bowel rest versus elemental diet in the treatment of patients with Crohn’s disease: The effects on protein metabolism and immune function. J. Parenter. Enter. Nutr. 1989, 13, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.; Greenberg, G.R.; Allard, J.P.; Baker, J.P.; Jeejeebhoy, K.N. Total enteral nutrition support improves body composition of patients with active Crohn’s disease. J. Parenter. Enter. Nutr. 1995, 19, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Ross, V.; Mahadevan, U. Micronutrient deficiencies in inflammatory bowel disease: From A to zinc. Inflamm. Bowel Dis. 2012, 18, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or sarcopenia? A practical guide. Gastroenterol. Res. Pr. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Staun, M.; Bhandari, S.; Munoz, M. State of the iron: How to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 429–440. [Google Scholar] [CrossRef]
- Cantorna, M.T. Vitamin D and its role in immunology: Multiple sclerosis, and inflammatory bowel disease. Prog. Biophys. Mol. Biol. 2006, 92, 60–64. [Google Scholar] [CrossRef]
- Fujiya, M.; Ueno, N.; Kohgo, Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: A meta-analysis of randomized controlled trials. Clin. J. Gastroenterol. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.; van Limbergen, J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: results of a propensity-score matched cohort analysis. J. Crohns Colitis 2017, 11, 1063–1070. [Google Scholar] [CrossRef]
- Levine, A.; Turner, D.; Pfeffer Gik, T.; Amil Dias, J.; Veres, G.; Shaoul, R.; Staiano, A.; Escher, J.; Kolho, K.L.; Paerregaard, A.; et al. Comparison of outcomes parameters for induction of remission in new onset pediatric Crohn’s disease: Evaluation of the porto IBD group “growth relapse and outcomes with therapy” (GROWTH CD) study. Inflamm. Bowel Dis. 2014, 20, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; Martin de Carpi, J.; Staiano, A.; Shaoul, R.; Lionetti, P.; et al. Differences in outcomes over time with exclusive enteral nutrition compared with steroids in children with mild to moderate Crohn’s disease: Results from the GROWTH CD study. J. Crohns Colitis 2018, 12, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Shah, P.S.; Steinhart, A.H.; Zlotkin, S.; Griffiths, A.M. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflamm. Bowel Dis. 2011, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Paradis, A.M.; Fontaine-Bisson, B.; Bosse, Y.; Robitaille, J.; Lemieux, S.; Jacques, H.; Lamarche, B.; Tchernof, A.; Couture, P.; Vohl, M.C. The peroxisome proliferator-activated receptor alpha Leu162Val polymorphism influences the metabolic response to a dietary intervention altering fatty acid proportions in healthy men. Am. J. Clin. Nutr. 2005, 81, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1441. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharm. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhong, X.; Kim, S.J.; Kim, D.H.; Kim, H.S.; Lee, J.S.; Yum, H.W.; Lee, J.; Na, H.K.; Surh, Y.J. Comparative effects of curcumin and tetrahydrocurcumin on dextran sulfate sodium-induced colitis and inflammatory signaling in mice. J. Cancer Prev. 2018, 23, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Hanai, H.; Tozawa, K.; Aoshi, T.; Uchijima, M.; Nagata, T.; Koide, Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002, 123, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, F.D.; Versalovic, J. Development of the pediatric gut microbiome: Impact on health and disease. Am. J. Med. Sci. 2018, 356, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mandato, C.; Di Nuzzi, A.; Vajro, P. Nutrition and liver disease. Nutrients 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, J.; Laukkarinen, J.; Lahtela, J.; Seppanen, H.; Jarvinen, S.; Nordback, I.; Sand, J. The long-term prospective follow-up of pancreatic function after the first episode of acute alcoholic pancreatitis: Recurrence predisposes one to pancreatic dysfunction and pancreatogenic diabetes. J. Clin. Gastroenterol. 2017, 51, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Das, S.L.; Kennedy, J.I.; Murphy, R.; Phillips, A.R.; Windsor, J.A.; Petrov, M.S. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. World J. Gastroenterol. 2014, 20, 17196–17205. [Google Scholar] [CrossRef] [PubMed]
- Hollemans, R.A.; Hallensleben, N.D.L.; Mager, D.J.; Kelder, J.C.; Besselink, M.G.; Bruno, M.J.; Verdonk, R.C.; van Santvoort, H.C. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology 2018, 18, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Das, S.L.; Singh, P.P.; Phillips, A.R.; Murphy, R.; Windsor, J.A.; Petrov, M.S. Newly diagnosed diabetes mellitus after acute pancreatitis: A systematic review and meta-analysis. Gut 2014, 63, 818–831. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storck, L.J.; Imoberdorf, R.; Ballmer, P.E. Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease. J. Clin. Med. 2019, 8, 1098. https://doi.org/10.3390/jcm8081098
Storck LJ, Imoberdorf R, Ballmer PE. Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease. Journal of Clinical Medicine. 2019; 8(8):1098. https://doi.org/10.3390/jcm8081098
Chicago/Turabian StyleStorck, Lena J., Reinhard Imoberdorf, and Peter E. Ballmer. 2019. "Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease" Journal of Clinical Medicine 8, no. 8: 1098. https://doi.org/10.3390/jcm8081098




