Use of Estimating Equations for Dosing Antimicrobials in Patients with Acute Kidney Injury Not Receiving Renal Replacement Therapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Estimation of GFR Using Cockroft-Gault, MDRD, Jelliffe and Modified Jelliffe Equations
2.2. Evaluation of the Discordance in Drug Dosing Among Estimating Equations
Total #Episodes
3. Results
3.1. Overall Impact on Drug Dosing Based on Individual Equations
3.2. Breakdown of Impact on Drug Dosing Based on Drug Administered
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M.; Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyriere, H.; Branger, B.; Bengler, C.; Vecina, F.; Pinzani, V.; Hillaire-Buys, D.; Blayac, J.P. Neurologic toxicity caused by zelitrex (valaciclovir) in 3 patients with renal failure. Is overdose associated with improvement of product bioavailability improvement? Rev. Med. Int. 2001, 22, 297–303. [Google Scholar]
- Matzke, G.R.; McGory, R.W.; Halstenson, C.E.; Keane, W.F. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob. Agents Chemother. 1984, 25, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Moore, R.D.; Lietman, P.S. Studies of risk factors for aminoglycoside nephrotoxicity. Am. J. Kidney Dis. 1986, 8, 308–313. [Google Scholar] [CrossRef]
- Matzke, G.R.; Frye, R.F. Drug administration in patients with renal insufficiency. Minimising renal and extrarenal toxicity. Drug Saf. 1997, 16, 205–231. [Google Scholar] [CrossRef] [PubMed]
- Rosborough, T.K.; Shepherd, M.F.; Couch, P.L. Selecting an equation to estimate glomerular filtration rate for use in renal dosage adjustment of drugs in electronic patient record systems. Pharmacotherapy 2005, 25, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Goerdt, P.J.; Heim-Duthoy, K.L.; Macres, M.; Swan, S.K. Predictive performance of renal function estimate equations in renal allografts. Br. J. Clin. Pharmacol. 1997, 44, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Int. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Eiland, E.H., 3rd; Hamm, W.; English, T.M.; Phillippe, H.M. Comparison of the modification of diet in renal disease and Cockcroft-Gault equations for antimicrobial dosage adjustments. Ann. Pharmacother. 2006, 40, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Knight, E.L.; Hogan, M.L.; Singh, A.K. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J. Am. Soc. Nephrol. 2003, 14, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Poggio, E.D.; Nef, P.C.; Wang, X.; Greene, T.; Van Lente, F.; Dennis, V.W.; Hall, P.M. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am. J. Kidney Dis. 2005, 46, 242–252. [Google Scholar] [CrossRef] [PubMed]
- le Riche, M.; Zemlin, A.E.; Erasmus, R.T.; Davids, M.R. An audit of 24-hour creatinine clearance measurements at Tygerberg Hospital and comparison with prediction equations. S. Afr. Med. J. 2007, 97, 968–970. [Google Scholar] [PubMed]
- Kuan, Y.; Hossain, M.; Surman, J.; El Nahas, A.M.; Haylor, J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol. Dial. Transplant. 2005, 20, 2394–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, S.; Zarowitz, B.J.; Peterson, E.L.; Dumler, F. Predictability of creatinine clearance estimates in critically ill patients. Crit. Care Med. 1993, 21, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J., Jr.; Bennett, W.M.; Decker, B.S.; Eckardt, K.U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney disease—A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am. J. Nephrol. 2002, 22, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.; Macedo, E.; Soroko, S.; Chertow, G.M.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Mehta, R.L.; Program to Improve Care in Acute Renal Disease. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol. Dial. Transplant. 2010, 25, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Malyuk, R.; Djurdjev, O.; Levin, A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group—A cautionary tale. Nephrol. Dial. Transplant. 2007, 22, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Damen, J.; Vanholder, R.C.; Lameire, N.H.; Delanghe, J.R.; Van den Hauwe, K.; Colardyn, F.A. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol. Dial. Transplant. 2005, 20, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragadottir, G.; Redfors, B.; Ricksten, S.E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury—True GFR versus urinary creatinine clearance and estimating equations. Crit. Care 2013, 17, R108. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.M.; Blake, G.M.; Galani, E.; Steer, C.B.; Harper, S.E.; Adamson, K.L.; Bailey, D.L.; Harper, P.G. Evaluation of the Cockroft-Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients. Ann. Oncol. 2004, 15, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirwan, C.J.; Philips, B.J.; Macphee, I.A. Estimated glomerular filtration rate correlates poorly with four-hour creatinine clearance in critically ill patients with acute kidney injury. Crit. Care Res. Pract. 2013, 2013, 406075. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Fay, M.F.; Udy, A.; Roberts, J.; Kirkpatrick, C.; Ungerer, J.; Lipman, J. Pitfalls of using estimations of glomerular filtration rate in an intensive care population. Int. Med. J. 2011, 41, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Lyndon, W.D.; Wille, K.M.; Tolwani, A.J. Solute clearance in CRRT: Prescribed dose versus actual delivered dose. Nephrol. Dial. Transplant. 2012, 27, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Nolin, T.D.; Appiah, K.; Kendrick, S.A.; Le, P.; McMonagle, E.; Himmelfarb, J. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J. Am. Soc. Nephrol. 2006, 17, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Lane, K.; Macphee, I.; Philips, B. Xenobiotic metabolism: The effect of acute kidney injury on non-renal drug clearance and hepatic drug metabolism. Int. J. Mol. Sci. 2014, 15, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Vilay, A.M.; Churchwell, M.D.; Mueller, B.A. Clinical review: Drug metabolism and nonrenal clearance in acute kidney injury. Crit. Care 2008, 12, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyler, R.F.; Mueller, B.A.; Medscap. Antibiotic dosing in critically ill patients with acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.L.; Nolin, T.D. Lack of drug dosing guidelines for critically ill patients receiving continuous renal replacement therapy. Clin. Pharmacol. Ther. 2014, 96, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]

| Name | Equation |
|---|---|
| Cockcroft Gault | CLcr = ((140 – age) × weight (kg))/(72 × Scr (mg/dL)) Multiply by 0.85 if female |
| MDRD | GFR = 186 × (SCr (mg/dL))–1.154 × (age (years))–0.203 × (0.742 if patient is female) × (1.21 if patient is black) |
| MDRD adjusted for BSA | GFR = MDRD × BSA / 1.73 m2 |
| Jelliffe | (((Volume of distribution × (Scr on day 1 – Scr on day 2)) + creatinine production) × 100/1440/average Scr |
| Modified Jelliffe | Substitute Adjusted SCr into Jelliffe equation Adjusted SCr = SCr (measured) × Correction Factor Correction Factor = ((admit weight (kg) × 0.6) + Sum (Daily fluid balance))/admit weight × 0.6 |
| Variable | n (%) or Median (Range) |
|---|---|
| Age (years) | 49.5 (31–89) |
| Gender Male Female | 14 (44%) 18 (56%) |
| Weight (kg) | 73.9 (45–99) |
| Height (cm) | 169 (152–191) |
| BSA (m2) | 1.81 (1.38–2.26) |
| APACHE III Score * | 90 (38–151) |
| History of CKD | 4 (12.5) |
| Etiology of AKI ATN Nephrotoxicity Multifactorial Hepatorenal Prerenal | 14 (44%) 2 (6%) 11 (34%) 3 (10%) 2 (6%) |
| Parameter | Timed Urine Collection | CG (mL/min) | MDRD (mL/min/1.73 m2) | MDRD – Adj BSA (mL/min) | Jelliffe (mL/min) | Modified Jelliffe (mL/min) |
|---|---|---|---|---|---|---|
| CL peak Scr Median (range) | - | 27.4 (9.3–66.3) | 19.8 (9.8–47.0) | 21.2 (9.9–60.4) | 21.2 (5.2–56.4) | 20.5 (4.9–49.6) |
| CL Nadir Sc rMedian (range) | - | 58.8 (18.4–88.9) | 46.9 (24.8–85.8) | 50.8 (22.0–92.1) | 52.8 (17.3–79.0) | 47.2 (14.3–79.0) |
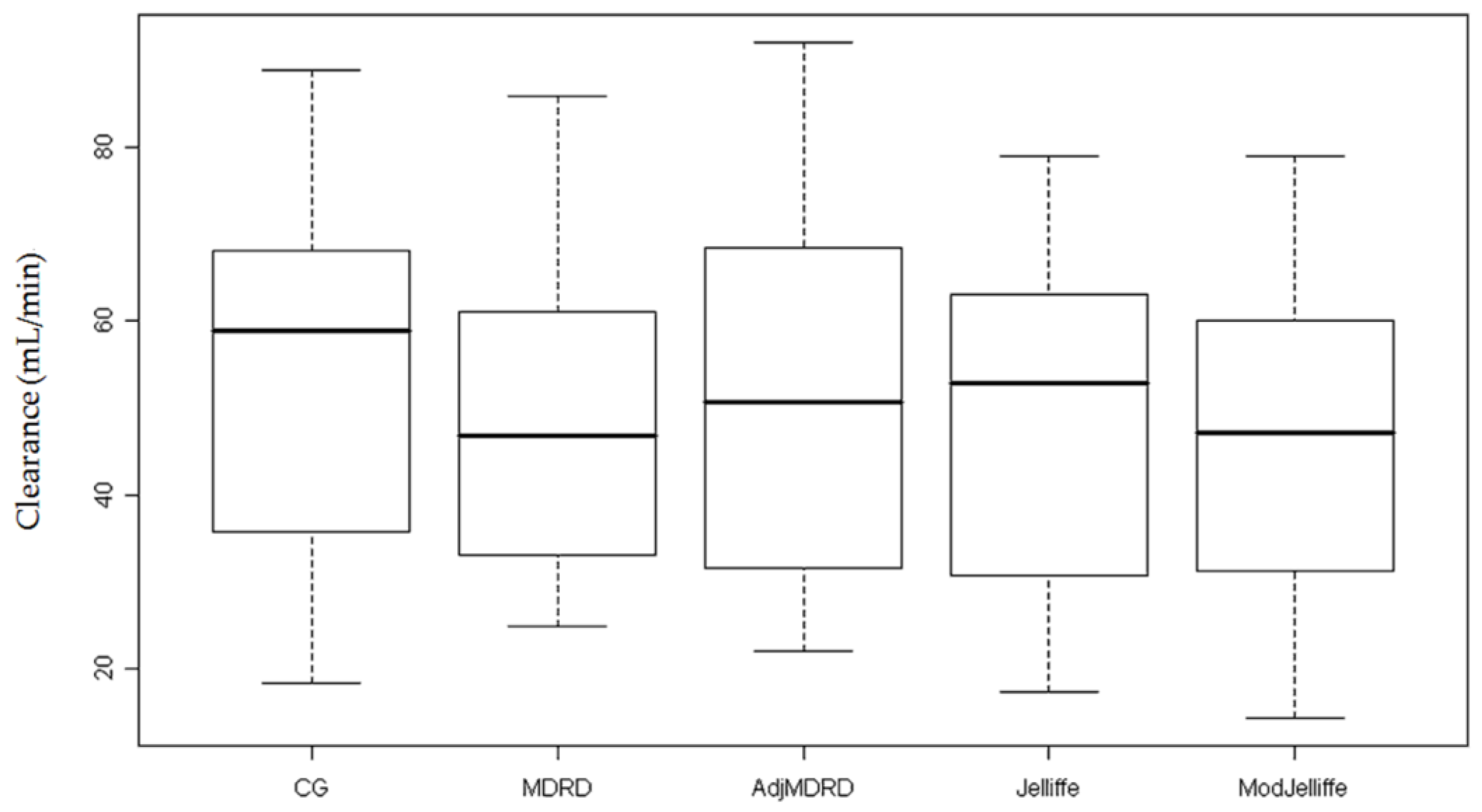
| Median CL (range) | 22.8 (13.4–26.2) | 34.4 (9.3–88.9) | 28.6 (9.8–85.8) | 29.3 (9.9–92.1) | 30.3 (4.5–78.9) | 26.7 (4.6–78.9) |
| Estimating Equation | Number Dosed Correct (%) n = 719 episodes (32 patients) | Discordance Rate (%) | Number Dosed Correct (%) n = 580 episodes (30 patients) | Discordance Rate (%) |
|---|---|---|---|---|
| CG | 580 (81) | - | 580 (100) | - |
| MDRD | 529 (74) | 7 | 515 (89) | 11 |
| MDRD BSA | 531 (74) | 7 | 526 (91) | 9 |
| Jelliffe | 531 (74) | 7 | 530 (91) | 9 |
| Mod Jelliffe | 488 (68) | 12 | 485 (84) | 16 |
| Drug | # Patients Received (%) | # Correct CG (%) | # Correct Mod-Jelliffe (%) | Discordance Rate (%) | p Value |
|---|---|---|---|---|---|
| All drugs | - | 580/719 | 488/719 | 13 | <0.001 |
| Ceftazidime | 22 (69) | 140/200 (70) | 107/200 (54) | 16 | 0.009 |
| Ciprofloxacin | 21 (66) | 164/170 (96) | 153/170 (90) | 6 | - |
| Fluconazole | 15 (47) | 104/129 (81) | 91/129 (71) | 10 | - |
| Metronidazole | 11 (34) | 52/52 (100) | 45/52 (87) | 14 | - |
| Cefazolin | 7 (22) | 31/36 (86) | 23/36 (64) | 22 | - |
| Ganciclovir | 7 (22) | 59/92 (64) | 41/92 (45) | 20 | - |
| Ampicillin | 4 (13) | 10/16 (63) | 9/16 (56) | 6 | - |
| Piperacillin/Tazobactam | 4 (13) | 16/16 (100) | 15/16 (94) | 6 | - |
| Antimicrobial | Number of Patients | Number of Dosing Episodes | Number of Overdosing Episodes | Median Daily Dose (Range) | Median Overdoseper Day (Range) |
|---|---|---|---|---|---|
| Acyclovir | 2 | 4 | 0 | 2400 mg | 0 mg |
| Ampicillin | 3 | 10 | 1 | 3500 mg (3000–8000) | 5000 mg |
| Cefazolin | 7 | 31 | 7 | 3000 mg (2000–3000) | 1000 mg |
| Ceftazidime | 20 | 140 | 33 | 2000 mg (500–3000) | 1000 mg (500–3000) |
| Ciprofloxacin | 24 | 164 | 11 | 500 mg (400–1500) | 400 mg (200–500) |
| Fluconazole | 17 | 104 | 16 | 100 mg (50–400) | 50 mg (50-100) |
| Ganciclovir | 6 | 59 | 18 | Oral: 3000 mg (1000–3000) IV: 100 mg (75–400) | Oral: 2000 mg (1000–2000) IV: 110 mg (45–200) |
| Metronidazole | 11 | 52 | 7 | 1500 mg (1000–1500) | 500 mg |
| Piperacillin/Tazobactam | 4 | 16 | 1 | 11,250 mg (6750–1,3500) | 4500 mg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awdishu, L.; Connor, A.I.; Bouchard, J.; Macedo, E.; Chertow, G.M.; Mehta, R.L. Use of Estimating Equations for Dosing Antimicrobials in Patients with Acute Kidney Injury Not Receiving Renal Replacement Therapy. J. Clin. Med. 2018, 7, 211. https://doi.org/10.3390/jcm7080211
Awdishu L, Connor AI, Bouchard J, Macedo E, Chertow GM, Mehta RL. Use of Estimating Equations for Dosing Antimicrobials in Patients with Acute Kidney Injury Not Receiving Renal Replacement Therapy. Journal of Clinical Medicine. 2018; 7(8):211. https://doi.org/10.3390/jcm7080211
Chicago/Turabian StyleAwdishu, Linda, Ana Isabel Connor, Josée Bouchard, Etienne Macedo, Glenn M. Chertow, and Ravindra L. Mehta. 2018. "Use of Estimating Equations for Dosing Antimicrobials in Patients with Acute Kidney Injury Not Receiving Renal Replacement Therapy" Journal of Clinical Medicine 7, no. 8: 211. https://doi.org/10.3390/jcm7080211





