Genetic Polymorphisms Associated with the Neutrophil–Lymphocyte Ratio and Their Clinical Implications for Metabolic Risk Factors
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.2. Clinical Assessment and Definitions
2.3. Ethics Statement
2.4. Genotyping, Quality Control and Imputation
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
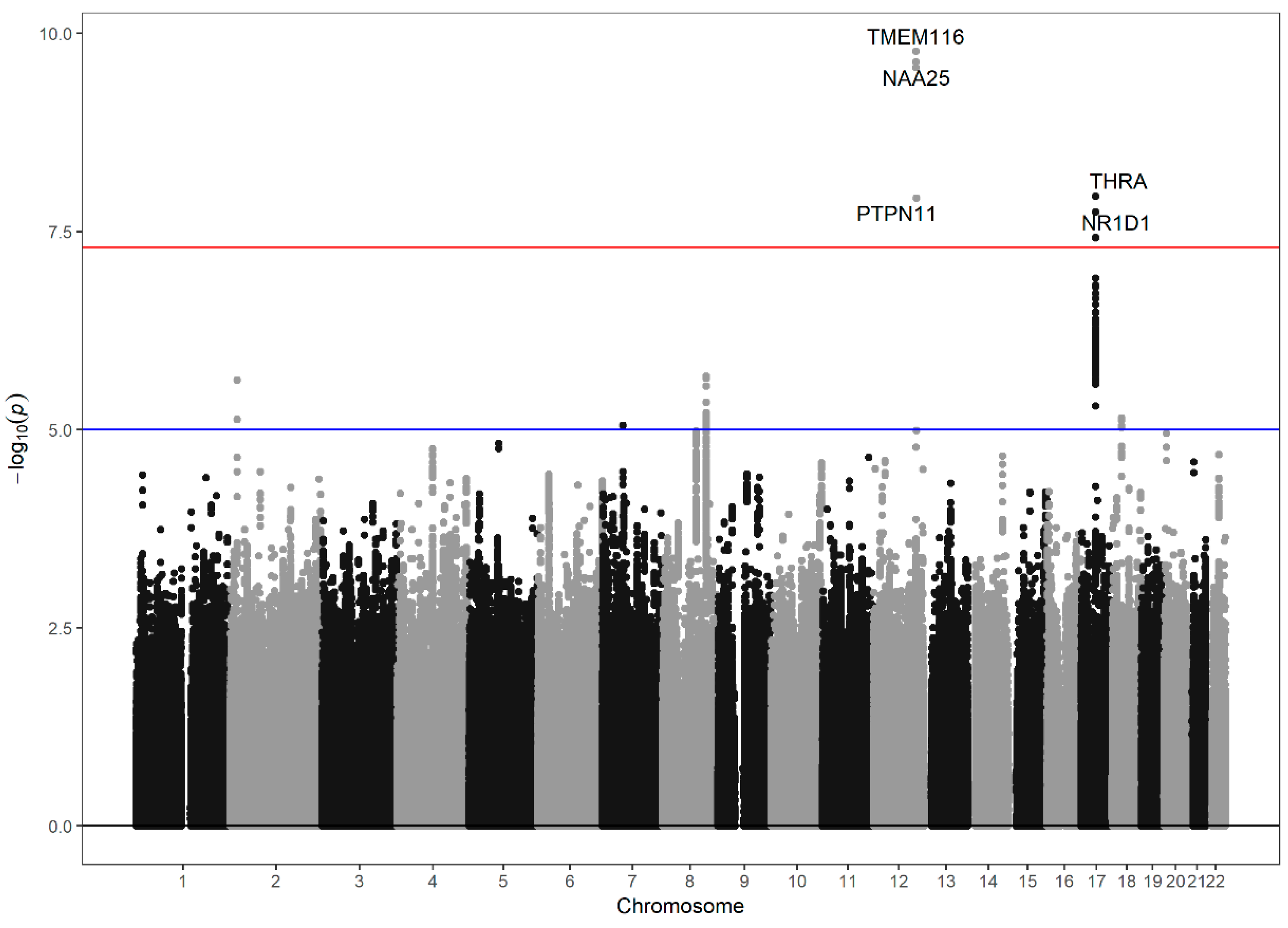
3.2. Genome-Wide Association Study of the Neutrophil–Lymphocyte Ratio
3.3. Case–Control Study for the Presence of Metabolic Syndrome According to the 9 Significant SNPs
3.4. Regression Models for Increased Blood Pressure, Decreased HDL Cholesterol and Metabolic Syndromes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paquissi, F.C. The predictive role of inflammatory biomarkers in atrial fibrillation as seen through neutrophil-lymphocyte ratio mirror. J. Biomark. 2016. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Nash, S.; Adams, R. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: A post hoc analysis of the MRC COIN study. Br. J. Cancer 2016, 114, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lou, L.; Ye, J.; Zhang, S. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A. meta-analysis. BMJ Open 2015, 5, e006404. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.D.; Huang, Y.L.; Qin, B.D. Prognostic value of neutrophil to lymphocyte ratio for gastric cancer. Ann. Transl. Med. 2015, 3, 50. [Google Scholar] [PubMed]
- Surendar, J.; Indulekha, K.; Mohan, V.; Pradeepa, R. Association of neutrophil-lymphocyte ratio with metabolic syndrome and its components in Asian Indians (CURES-143). J. Diabetes Complic. 2016, 30, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.; Shin, D.W.; Kwon, H.M. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS ONE 2017, 12, e0183706. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, M.H.; Li, S. Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of coronary artery disease: A Gensini score assessment. J. Atheroscler. Thromb. 2014, 21, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Parikh, V.; Patel, N. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: Insights from the National Health and Nutrition Examination Survey-III. Int. J. Cardiol. 2014, 171, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choe, E.K.; Choi, J.M. Health and Prevention Enhancement (H-PEACE): A. retrospective, population-based cohort study conducted at the Seoul National University Hospital Gangnam Center: Korea. BMJ Open 2018, 8, e019327. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb. Vasc. Biol. Lung Nat. Heart 2004, 24, e13–e18. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.K.; Lee, Y.; Cho, J.Y. Search for genetic factor association with cancer-free prostate-specific antigen level elevation on the basis of a genome-wide association study in the Korean population. Eur. J. Cancer Prev. 2017, 27, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Delaneau, O.; Zagury, J.F.; Marchini, J. Improved Whole-chromosome phasing for disease and population genetic studies. Nat. Methods 2013, 10, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, H.; Zhu, X. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2017, 130, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Rajakariar, R.; Lawrence, T.; Bystrom, J. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood 2008, 111, 4184–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kebir, D.; Filep, J.G. Targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells 2013, 2, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.R.; Van der Burgt, I.; Brady, A.F. Mapping a gene for Noonan syndrome to the long arm of chromosome 12. Nat. Genet. 1994, 8, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.M.; Plutzky, J.; Neel, B.G. Identification of a human Src homology 2-containing protein-tyrosine-phosphatase: A putative homolog of Drosophila corkscrew. Proc. Natl. Acad. Sci. USA 1992, 89, 11239–11243. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Krueger, N.X.; Serra-Pagès, C. Molecular characterization of the human transmembrane protein-tyrosine phosphatase delta. Evidence for tissue-specific expression of alternative human transmembrane protein-tyrosine phosphatase delta isoforms. J. Biol. Chem. 1995, 270, 6722–6728. [Google Scholar] [CrossRef] [PubMed]
- Dechert, U.; Duncan, A.M.; Bastien, L. Protein-tyrosine phosphatase SH-PTP2 (PTPN11) is localized to 12q24.1–24.3. Hum Genet. 1995, 96, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Banville, D.; Zhao, Z. A widely expressed human protein-tyrosine phosphatase containing Src homology 2 domains. Proc. Natl. Acad. Sci. USA 1993, 90, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, G.; Rivard, N. New and unexpected biological functions for the Src-homology 2 domain-containing phosphatase SHP-2 in the gastrointestinal tract. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bard-Chapeau, E.A.; Li, S.; Ding, J. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011, 19, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Bentires-Alj, M.; Paez, J.G.; David, F.S. Activating mutations of the Noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004, 64, 8816–8820. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Matsuo, K.; Goto, Y. Associations of a PTPN11 G/A polymorphism at intron 3 with Helicobactor pylori seropositivity, gastric atrophy and gastric cancer in Japanese. BMC Gastroenterol. 2009, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, Y.; Gooljar, S.B.; Snieder, H. SHP-2 and PI3-kinase genes PTPN11 and PIK3R1 may influence serum apoB and LDL cholesterol levels in normal women. Atherosclerosis 2007, 194, e26–e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.F.; Cao, X.Y.; Cao, D.H. Polymorphisms of PTPN11 gene could influence serum lipid levels in a sex-specific pattern. Lipids Health Dis. 2013, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starheim, K.K.; Arnesen, T.; Gromyko, D. Identification of the human N(alpha)-acetyltransferase complex B (hNatB): A complex important for cell-cycle progression. Biochem. J. 2008, 415, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Warringer, J.; Blomberg, A. Physiological importance and identification of novel targets for the N-terminal acetyltransferase NatB. Eukaryot. Cell 2006, 5, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Sinner, M.F.; Tucker, N.R.; Lunetta, K.L. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 2014, 130, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Auburger, G.; Gispert, S.; Lahut, S. 12q24 locus association with type 1 diabetes: SH2B3 or ATXN2? World J. Diabetes 2014, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature 2007, 447, 661–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.D.; Walker, N.M.; Smyth, D.J. Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun. 2009, 10, S85–SS94. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.K.; Sim, X.; Jensen, R.A. Four novel Loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS Genet. 2010, 6, e1001184. [Google Scholar] [CrossRef]
- Takaki, S.; Sauer, K.; Iritani, B.M. Control of B cell production by the adaptor protein lnk. Definition of a conserved family of signal-modulating proteins. Immunity 2000, 13, 599–609. [Google Scholar] [CrossRef]
- Bersenev, A.; Wu, C.; Balcerek, J. Lnk constrains myeloproliferative diseases in mice. J. Clin. Investig. 2010, 120, 2058–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.G.; Morin, R.D.; Zhang, J. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 2012, 22, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.A.; Walker, N.M.; Cooper, J.D. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007, 39, 857–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.D.; Walker, N.M.; Healy, B.C. Analysis of 55 autoimmune disease and type II diabetes loci: Further confirmation of chromosomes 4q27, 12q13.2 and 12q24.13 as type I diabetes loci, and support for a new locus, 12q13.3–q14.1. Genes Immun. 2009, 10, S95–S120. [Google Scholar] [CrossRef] [PubMed]
- Hinks, A.; Martin, P.; Flynn, E. Investigation of type 1 diabetes and coeliac disease susceptibility loci for association with juvenile idiopathic arthritis. Ann. Rheum. Dis. 2010, 69, 2169–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, M.J.; Trynka, G.; Heskamp, S. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum. Mol. Genet. 2009, 18, 4195–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köttgen, A.; Albrecht, E.; Teumer, A. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehret, G.B.; Munroe, P.B.; International Consortium for Blood. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, K.P.; Farooqi, S.; Harrup, K. Genetic variance in the spinocerebellar ataxia type 2 (ATXN2) gene in children with severe early onset obesity. PLoS ONE 2009, 4, e8280. [Google Scholar] [CrossRef] [PubMed]
| Total | Discovery Set | Validation Set | ||
|---|---|---|---|---|
| N | (N = 7257) | (N = 4986) | (N = 2271) | P |
| Age | 50.5 ± 10.2 | 50.6 ± 10.4 | 50.2 ± 9.7 | 0.081 |
| NLR | 1.9 ± 0.9 | 1.9 ± 0.9 | 1.9 ± 0.9 | 0.075 |
| * NLR | 0.230 | |||
| Normal | 6657 (91.7%) | 4487 (90.0%) | 2022 (89.0%) | |
| Increased | 600 (8.3%) | 499 (10.0%) | 249 (11.0%) | |
| WBC count (×103/μL) | 5.4 ± 1.5 | 5.3 ± 1.5 | 5.5 ± 1.5 | 0.001 |
| Neutrophil count (×103/μL) | 3.1 ± 1.2 | 3.1 ± 1.2 | 3.2 ± 1.2 | 0.001 |
| Lymphocyte count (×103/μL) | 1.7 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.5 | 0.018 |
| High sensitivity C-reactive protein | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.127 |
| Gender | 0.007 | |||
| Men | 4208 (58.0%) | 2838 (56.9%) | 1370 (60.3%) | |
| Women | 3049 (42.0%) | 2148 (43.1%) | 901 (39.7%) | |
| BMI | 23.1 ± 3.0 | 23.1 ± 3.0 | 23.3 ± 3.0 | 0.009 |
| Smoking | 0.985 | |||
| None or ex-smoker | 3196 (50.4%) | 2184 (50.5%) | 1012 (50.3%) | |
| Current smoker | 1997 (31.5%) | 1363 (31.5%) | 634 (31.5%) | |
| Missing | 1149 (18.1%) | 782 (18.1%) | 367 (18.2%) | |
| Alcohol consumption | 0.026 | |||
| No | 4583 (63.2%) | 3161 (63.4%) | 1422 (62.6%) | |
| Yes | 1559 (21.5%) | 1033 (20.7%) | 526 (23.2%) | |
| Missing | 1115 (15.4%) | 792 (15.9%) | 323 (14.2%) | |
| Diabetes medication | 0.482 | |||
| No | 6896 (95.2%) | 4744 (95.3%) | 2152 (94.9%) | |
| Yes | 350 (4.8%) | 234 (4.7%) | 116 (5.1%) | |
| Hypertension medication | 0.640 | |||
| No | 6030 (83.2%) | 4150 (83.4%) | 1880 (82.9%) | |
| Yes | 1216 (16.8%) | 828 (16.6%) | 388 (17.1%) | |
| Waist circumference | 82.6 ± 8.8 | 82.6 ± 8.8 | 82.5 ± 8.7 | 0.668 |
| Triglyceride | 107.9 ± 72.4 | 107.1 ± 72.1 | 109.6 ± 73.3 | 0.172 |
| High-density lipoprotein cholesterol | 53.9 ± 12.0 | 53.8 ± 12.1 | 53.9 ± 12.0 | 0.709 |
| Fasting glucose | 98.2 ± 16.6 | 97.7 ± 15.6 | 99.5 ± 18.5 | 0.000 |
| Systolic blood pressure | 115.4 ± 13.2 | 115.2 ± 13.4 | 115.7 ± 12.9 | 0.198 |
| Diastolic blood pressure | 75.9 ± 10.3 | 75.6 ± 10.4 | 76.6 ± 10.0 | 0.000 |
| Metabolic syndrome | 1320 (18.2%) | 890 (17.9%) | 430 (19.0%) | 0.284 |
| SNP | a Elevated Blood Pressure | b Increased Waist Circumference | Metabolic Syndrome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| rs76181728 | 1.348 | 1.111–1.635 | 0.002 | 1.42 | 1.063–1.897 | 0.017 | 1.253 | 0.972–1.615 | 0.082 |
| rs79945097 | 1.348 | 1.112–1.635 | 0.002 | 1.424 | 1.067–1.901 | 0.016 | 1.263 | 0.98–1.626 | 0.071 |
| rs76596471 | 1.341 | 1.105–1.627 | 0.003 | 1.429 | 1.071–1.908 | 0.015 | 1.266 | 0.983–1.631 | 0.068 |
| rs7977554 | 1.209 | 0.987–1.48 | 0.067 | 1.44 | 1.071–1.935 | 0.016 | 1.178 | 0.901–1.54 | 0.231 |
| rs7502539 | 1.072 | 0.974–1.181 | 0.156 | 1.05 | 0.907–1.214 | 0.515 | 1.09 | 0.96–1.237 | 0.184 |
| rs7502233 | 1.07 | 0.972–1.179 | 0.167 | 1.057 | 0.914–1.222 | 0.458 | 1.093 | 0.963–1.24 | 0.168 |
| rs1879265 | 1.046 | 0.95–1.15 | 0.361 | 0.929 | 0.805–1.073 | 0.318 | 0.89 | 0.784–1.009 | 0.069 |
| rs62065216 | 1.111 | 1.013–1.219 | 0.025 | 0.984 | 0.856–1.131 | 0.822 | 0.993 | 0.88–1.121 | 0.912 |
| rs2102928 | 1.007 | 0.878–1.155 | 0.918 | 0.964 | 0.785–1.183 | 0.724 | 0.956 | 0.799–1.143 | 0.621 |
| Predicting Feature | a Model 1 | b Model 2 | c Model 3 | d Model 4 |
|---|---|---|---|---|
| OR (95% CI), P-Value | OR (95% CI), P-Value | OR (95% CI), P-Value | OR (95% CI), P-Value | |
| e Increased NLR | 0.584 (0.413–0.827), 0.002 | 0.616 (0.415–0.913), 0.015 | ||
| Increased blood pressure | 1.308 (1.072–1.597), 0.008 | 1.169 (0.928–1.474), 0.186 | 1.342 (1.098–1.641), 0.004 | 1.204 (0.954–1.52), 0.118 |
| Increased waist circumference | 1.418 (1.054–1.908), 0.021 | 1.506 (1.075–2.11), 0.017 | 1.427 (1.059–1.924), 0.019 | 1.563 (1.114–2.193), 0.009 |
| Metabolic syndrome | 1.242 (0.957–1.612), 0.103 | 1.418 (1.057–1.901), 0.019 | 1.27 (0.977–1.651), 0.073 | 1.465 (1.091–1.969), 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Choe, E.K.; Kang, H.Y.; Shin, E.; Lee, S.; Won, S. Genetic Polymorphisms Associated with the Neutrophil–Lymphocyte Ratio and Their Clinical Implications for Metabolic Risk Factors. J. Clin. Med. 2018, 7, 204. https://doi.org/10.3390/jcm7080204
Park B, Choe EK, Kang HY, Shin E, Lee S, Won S. Genetic Polymorphisms Associated with the Neutrophil–Lymphocyte Ratio and Their Clinical Implications for Metabolic Risk Factors. Journal of Clinical Medicine. 2018; 7(8):204. https://doi.org/10.3390/jcm7080204
Chicago/Turabian StylePark, Boram, Eun Kyung Choe, Hae Yeon Kang, Eunsoon Shin, Sangwoo Lee, and Sungho Won. 2018. "Genetic Polymorphisms Associated with the Neutrophil–Lymphocyte Ratio and Their Clinical Implications for Metabolic Risk Factors" Journal of Clinical Medicine 7, no. 8: 204. https://doi.org/10.3390/jcm7080204





