Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Treatment Protocol
2.4. Collection of CCs
2.5. Mitochondrial Mass Measurement
2.6. RNA Extraction and Real-Time PCR
2.7. Immunofluorescence Labeling Assay
2.8. Ethics Statement
2.9. Statistical Analysis
3. Results
3.1. Basic Characteristics of Patients Undergoing Ivf Cycles
3.2. Cycle Characteristics and Clinical Outcomes of Patients Undergoing IVF Cycles
3.3. Effects of DHEA Supplementation on Cumulus Cell Mitochondria
3.4. DHEA Restores Mitochondrial Morphology in PORs
3.5. DHEA Supplementation Regulates Mitochondrial Dynamics in CCs
3.6. DHEA Supplementation Prevents Mitophagy in CCs from PORs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saito, H.; Jwa, S.C.; Kuwahara, A.; Saito, K.; Ishikawa, T.; Ishihara, O.; Kugu, K.; Sawa, R.; Banno, K.; Irahara, M. Assisted reproductive technology in Japan: A summary report for 2015 by The Ethics Committee of The Japan Society of Obstetrics and Gynecology. Reprod. Med. Biol. 2018, 17, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Morimoto, N.; Yamanaka, M.; Matsumoto, H.; Yamochi, T.; Goto, H.; Inoue, M.; Nakaoka, Y.; Shibahara, H.; Morimoto, Y. Quantitative and qualitative changes of mitochondria in human preimplantation embryos. J. Assist. Reprod. Genet. 2017, 34, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.T.; Wang, P.H.; Wen, Z.H.; Li, C.J.; Chen, S.N.; Tsai, E.M.; Cheng, J.T.; Tsui, K.H. The application of dehydroepiandrosterone on improving mitochondrial function and reducing apoptosis of cumulus cells in poor ovarian responders. Int. J. Med. Sci. 2017, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.H.; Wang, P.H.; Lin, L.T.; Li, C.J. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction 2017, 154, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ottolenghi, C.; Uda, M.; Hamatani, T.; Crisponi, L.; Garcia, J.E.; Ko, M.; Pilia, G.; Sforza, C.; Schlessinger, D.; Forabosco, A. Aging of oocyte, ovary, and human reproduction. Ann. N. Y. Acad. Sci. 2004, 1034, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; El Shourbagy, S.; St John, J.C. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.T.; Ren, Z.; Yeung, W.S.; Shu, Y.M.; Xu, Y.W.; Zhuang, G.L.; Liang, X.Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunkara, S.K.; Coomarasamy, A.; Faris, R.; Braude, P.; Khalaf, Y. Long gonadotropin-releasing hormone agonist versus short agonist versus antagonist regimens in poor responders undergoing in vitro fertilization: A randomized controlled trial. Fertil. Steril. 2014, 101, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ma, R.; Xia, T.; Afnan, M.; Song, X.; Xu, F.; Hao, G.; Zhu, F.; Han, J.; Zhao, Z. Efficacy and safety of Ding-Kun-Dan for female infertility patients with predicted poor ovarian response undergoing in vitro fertilization/intracytoplasmic sperm injection: Study protocol for a randomized controlled trial. Trials 2018, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Bosdou, J.K.; Venetis, C.A.; Kolibianakis, E.M.; Toulis, K.A.; Goulis, D.G.; Zepiridis, L.; Tarlatzis, B.C. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.I.; Mahmoud, A.A.; Abo-Zeid, F.S.; Fares, N.H. Effects of dehydroepiandrosterone on the ovarian reserve and pregnancy outcomes in perimenopausal rats (DHEA and fertility in perimenopausal rats). Life Sci. 2018, 199, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wiser, A.; Gonen, O.; Ghetler, Y.; Shavit, T.; Berkovitz, A.; Shulman, A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: A randomized prospective study. Hum. Reprod. 2010, 25, 2496–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaenssens, T.; Wathlet, S.; Segers, I.; Verheyen, G.; De Vos, A.; Van der Elst, J.; Coucke, W.; Devroey, P.; Smitz, J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum. Reprod. 2010, 25, 1259–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Turki, H.A. Dehydroepiandrosterone supplementation in women undergoing assisted reproductive technology with poor ovarian response. A prospective case-control study. J. Int. Med. Res. 2018, 46, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: Direct evidence from preimplantation genetic screening (PGS). Reprod. Biol. Endocr. 2010, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Tsui, K.H.; Wang, P.H. Clinical application of dehydroepiandrosterone in reproduction: A review of the evidence. J. Chin. Med. Assoc. 2015, 78, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Cheng, J.T.; Wang, P.H.; Li, C.J.; Tsui, K.H. Dehydroepiandrosterone as a potential agent to slow down ovarian aging. J. Obstet. Gynaecol. Res. 2017, 43, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Steffann, J.; Fallet, C. [Mitochondria and oocyte maturation]. J. Gynecol. Obstet. Biol. Reprod. 2010, 39, 11–13. [Google Scholar] [CrossRef]
- Tsui, K.H.; Lin, L.T.; Horng, H.C.; Chang, R.; Huang, B.S.; Cheng, J.T.; Wang, P.H. Gene expression of cumulus cells in women with poor ovarian response after dehydroepiandrosterone supplementation. Taiwan J. Obstet. Gynecol. 2014, 53, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Sun, L.Y.; Pang, C.Y. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Lin, C.C.; Chen, Y.J.; Kao, L.S.; Liu, Y.C.; Chou, C.C.; Huang, Y.H.; Chang, F.R.; Wu, Y.C.; Tsai, Y.S.; et al. Automatic morphological subtyping reveals new roles of caspases in mitochondrial dynamics. PLoS Comput. Biol. 2011, 7, e1002212. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.A.; Van Wely, M.; Al-Inany, H.; Madani, T.; Jahangiri, N.; Khodabakhshi, S.; Alhalabi, M.; Akhondi, M.; Ansaripour, S.; Tokhmechy, R.; et al. A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: A multicenter randomized non-inferiority trial. Hum. Reprod. 2017, 32, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Abramovich, D.; Irusta, G.; Bas, D.; Cataldi, N.I.; Parborell, F.; Tesone, M. Angiopoietins/TIE2 system and VEGF are involved in ovarian function in a DHEA rat model of polycystic ovary syndrome. Endocrinology 2012, 153, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Hinchliffe, P.M.; Rowlands, P.K.; Borude, G.; Srinivasan, S.; Dhaliwal, S.S.; Yovich, J.L. DHEA supplementation confers no additional benefit to that of growth hormone on pregnancy and live birth rates in ivf patients categorized as poor prognosis. Front. Endocrinol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Prizant, H.; Gleicher, N.; Sen, A. Androgen actions in the ovary: Balance is key. J. Endocrinol. 2014, 222, R141–R151. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Androgen actions and the ovary. Biol. Reprod. 2008, 78, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Fried, G.; Remaeus, K.; Harlin, J.; Krog, E.; Csemiczky, G.; Aanesen, A.; Tally, M. Inhibin B predicts oocyte number and the ratio IGF-I/IGFBP-1 may indicate oocyte quality during ovarian hyperstimulation for in vitro fertilization. J. Assist. Reprod. Genet. 2003, 20, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; He, Z.Y.; Mele, C.A.; Veeck, L.L.; Davis, O.; Rosenwaks, Z. Human endometrial stromal cells improve embryo quality by enhancing the expression of insulin-like growth factors and their receptors in cocultured human preimplantation embryos. Fertil. Steril. 1999, 71, 361–367. [Google Scholar] [CrossRef]
- Mao, K.; Klionsky, D.J. Participation of mitochondrial fission during mitophagy. Cell Cycle 2013, 12, 3131–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Zhao, K.; Jin, S.; Ding, W.X. New methods for monitoring mitochondrial biogenesis and mitophagy in vitro and in vivo. Exp. Biol. Med. 2017, 242, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.T.; Kolesar, J.E.; Kaufman, B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta 2012, 1819, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, V.I.; Charalampopoulos, I.; Panayotopoulou, M.; Kampa, M.; Gravanis, A.; Castanas, E. Dehydroepiandrosterone protects human keratinocytes against apoptosis through membrane binding sites. Exp. Cell Res. 2009, 315, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, D.; Li, L.; Ma, H. Dehydroepiandrosterone ameliorates H2O2-induced Leydig cells oxidation damage and apoptosis through inhibition of ROS production and activation of PI3K/Akt pathways. Int. J. Biochem. Cell Biol. 2016, 70, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Katyare, S.S. Effect of dehydroepiandrosterone (DHEA) treatment on oxidative energy metabolism in rat liver and brain mitochondria. A dose-response study. Clin. Biochem. 2007, 40, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Katyare, S.S. Treatment with dehydroepiandrosterone (DHEA) stimulates oxidative energy metabolism in the cerebral mitochondria. A comparative study of effects in old and young adult rats. Neurosci. Lett. 2006, 402, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Katyare, S.S. Treatment with dehydroepiandrosterone (DHEA) stimulates oxidative energy metabolism in the liver mitochondria from developing rats. Mol. Cell Biochem. 2006, 293, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Schmitt, K.; Lang, U.E.; Mensah-Nyagan, A.G.; Eckert, A. Improvement of neuronal bioenergetics by neurosteroids: Implications for age-related neurodegenerative disorders. Biochim. Biophy. Acta 2014, 1842, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Fragouli, E.; Lalioti, M.D.; Wells, D. The transcriptome of follicular cells: Biological insights and clinical implications for the treatment of infertility. Hum. Reprod. Update 2014, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–965. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanghe, S.; Van Soom, A.; Nauwynck, H.; Coryn, M.; de Kruif, A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol. Reprod. Develop. 2002, 61, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.L.; Albertini, D.F. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J. Assist. Reprod. Genet. 2010, 27, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Cillo, F.; Brevini, T.A.; Antonini, S.; Paffoni, A.; Ragni, G.; Gandolfi, F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 2007, 134, 645–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, P.; Puard, V.; Chevalier, C.; Teusan, R.; Cadoret, V.; Guerif, F.; Houlgatte, R.; Royere, D. Genomic assessment of human cumulus cell marker genes as predictors of oocyte developmental competence: Impact of various experimental factors. PLoS ONE 2012, 7, e40449. [Google Scholar] [CrossRef] [PubMed]
- Boucret, L.; Chao de la Barca, J.M.; Moriniere, C.; Desquiret, V.; Ferre-L′Hotellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.D.; Hsieh, Y.Y.; Hsieh, J.N.; Chang, C.C.; Yang, C.Y.; Yang, J.G.; Cheng, W.L.; Tsai, F.J.; Liu, C.S. Mitochondria DNA deletion and copy numbers of cumulus cells associated with in vitro fertilization outcomes. J. Reprod. Med. 2010, 55, 491–497. [Google Scholar] [PubMed]
- Bentov, Y.; Esfandiari, N.; Burstein, E.; Casper, R.F. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil. Steril. 2010, 93, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Wang, P.H.; Chen, S.N.; Li, C.J.; Wen, Z.H.; Cheng, J.T.; Tsui, K.H. Protection of cumulus cells following dehydroepiandrosterone supplementation. Gynecol. Endocrinol. 2017, 33, 100–104. [Google Scholar] [CrossRef] [PubMed]


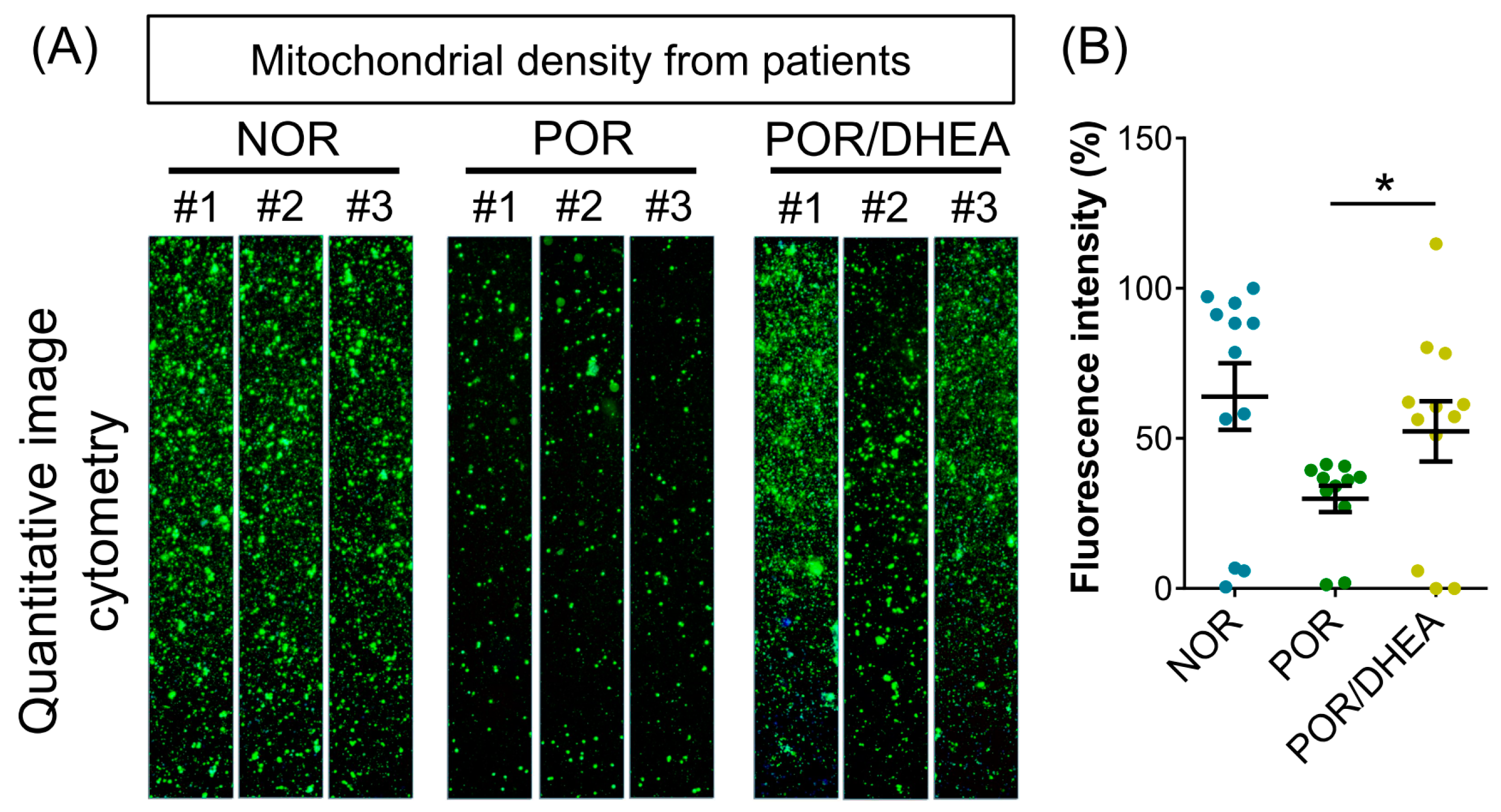
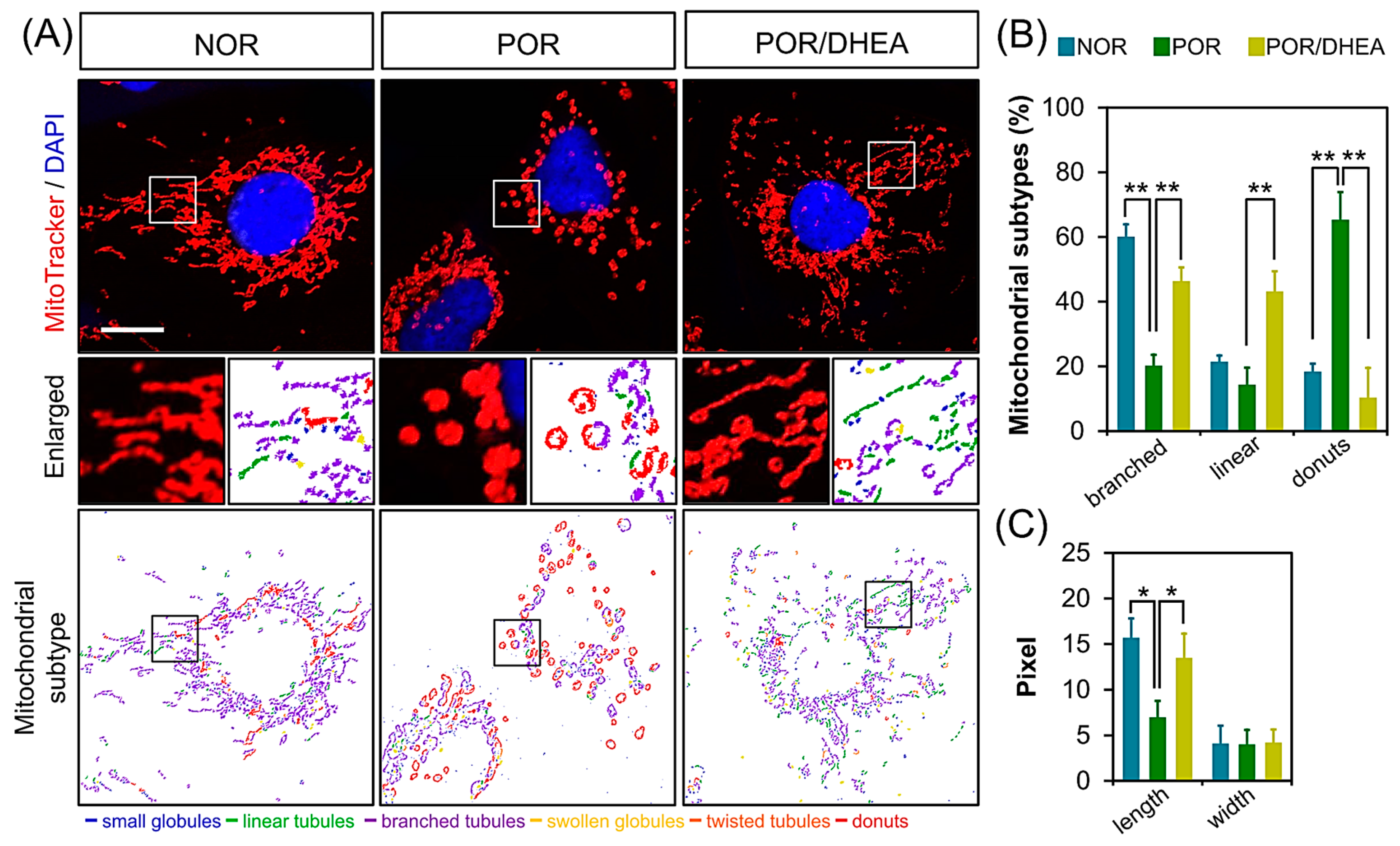
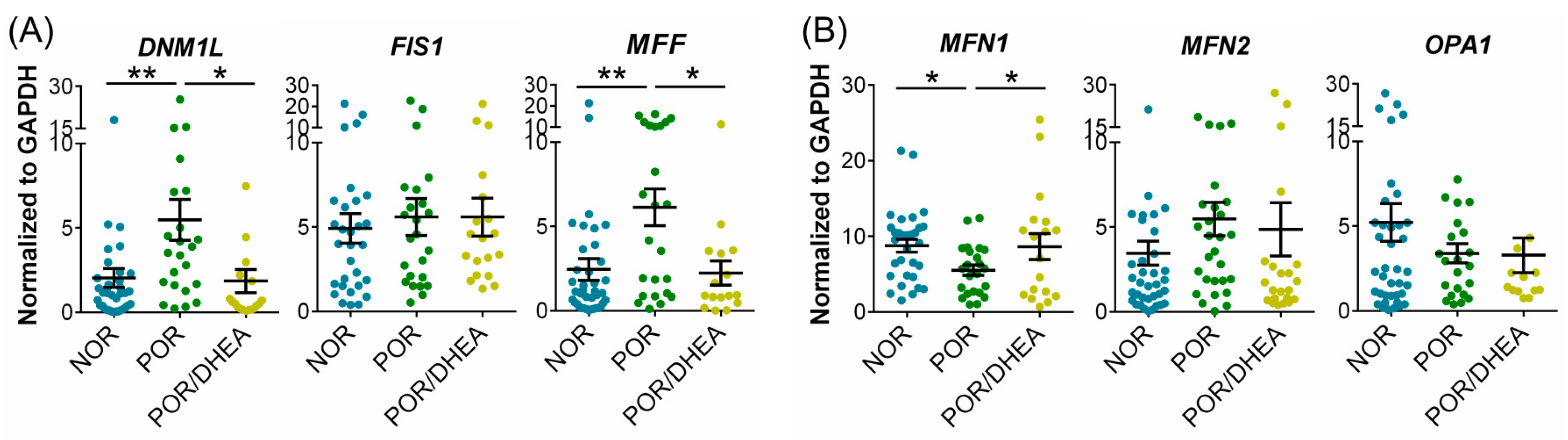

| Parameters | NOR (n = 28) | POR (n = 19) | POR/DHEA (n = 19) |
|---|---|---|---|
| Age (years) | 36.2 ± 3.0 | 40.5 ± 4.3 * | 37.8 ± 3.7 |
| Body mass index (kg/m2) | 24.2 ± 3.8 | 22.1 ± 3.9 | 22.5 ± 3.4 |
| Duration of infertility (years) | 3.8 ± 2.6 | 3.5 ± 2.9 | 6.0 ± 4.5 |
| Previous IVF failure (n) | 1.1 ± 1.1 | 1.7 ± 2.2 | 3.5 ± 2.6 *# |
| Types of infertility (%) | |||
| Primary infertility | 12/28 (43%) | 9/19 (37%) | 10/19 (52%) |
| Secondary infertility | 16/28 (57%) | 12/19 (63%) | 9/19 (47%) |
| Basal FSH (IU/L) | 4.3 ± 1.5 | 6.1 ± 5.3 | 6.1 ± 3.5 |
| Basal E2 (pg/mL) | 94.9 ± 90.8 | 105.6 ± 72.7 | 99.2 ± 78.4 |
| Basal LH (IU/L) | 4.4 ± 2.2 | 4.0 ± 5.3 | 6.3 ± 11.1 |
| Parameters | NOR (n = 28) | POR (n = 19) | POR/DHEA (n = 19) |
|---|---|---|---|
| Stimulation duration (days) | 10.9 ± 1.8 | 10.4 ± 2.2 | 10.6 ± 1.5 |
| HMG/FSH dose (IU) | 3190.3 ± 720.1 | 2775.3 ± 857.5 | 2992.1 ± 577.7 |
| No. of oocytes retrieved (n) | 10.7 ± 5.1 | 3.0 ± 1.9 * | 4.1 ± 3.0 * |
| No. of metaphase II oocytes (n) | 6.6 ± 3.9 | 1.6 ± 1.5 * | 2.0 ± 1.3 * |
| Maturation rate (%) | 60.3 ± 20.1 | 47.4 ± 35.0 | 65.5 ±3 2.3 |
| No. of fertilized oocytes (n) | 7.3 ± 3.4 | 2.3 ± 1.7 * | 2.6 ± 1.6 * |
| Fertilization rate (%) | 69.3 ± 15.6 | 69.8 ± 31.8 | 75.5 ± 22.0 |
| No. of day 3 embryos (n) | 6.2 ± 3.0 | 2.2 ± 1.6 * | 2.2 ± 1.4 * |
| No. of top-quality D3 embryos (n) | 2.6 ± 2.2 | 0.8 ± 1.3 * | 0.8 ± 1.0 * |
| Clinical pregnancy rate % (n) | 50.0% (14/28) | 11.1% (2/18) * | 26.3% (5/19) |
| Ongoing pregnancy rate % (n) | 42.9% (12/28) | 11.1% (2/18) * | 26.3% (5/19) |
| Live birth rate % (n) | 42.9% (12/28) | 11.1% (2/18) * | 16.7% (3/18) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-J.; Chen, S.-N.; Lin, L.-T.; Chern, C.-U.; Wang, P.-H.; Wen, Z.-H.; Tsui, K.-H. Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders. J. Clin. Med. 2018, 7, 293. https://doi.org/10.3390/jcm7100293
Li C-J, Chen S-N, Lin L-T, Chern C-U, Wang P-H, Wen Z-H, Tsui K-H. Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders. Journal of Clinical Medicine. 2018; 7(10):293. https://doi.org/10.3390/jcm7100293
Chicago/Turabian StyleLi, Chia-Jung, San-Nung Chen, Li-Te Lin, Chyi-Uei Chern, Peng-Hui Wang, Zhi-Hong Wen, and Kuan-Hao Tsui. 2018. "Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders" Journal of Clinical Medicine 7, no. 10: 293. https://doi.org/10.3390/jcm7100293







