Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases
Abstract
:1. Introduction
2. IL-17 Producing Cells
2.1. Characterization of Th17 Cells
2.2. Pathogenic Th17 Cells
2.3. γδT17 Cells
2.4. Invariant Natural Killer T (iNKT) Cells
2.5. Innate Lymphoid Cells (ILC)
3. Mechanisms Involved in Th17 Cell Polarization in Obese AT, or Periphery
3.1. Obesity and Adipose Tissue Inflammation: Role of Macrophages
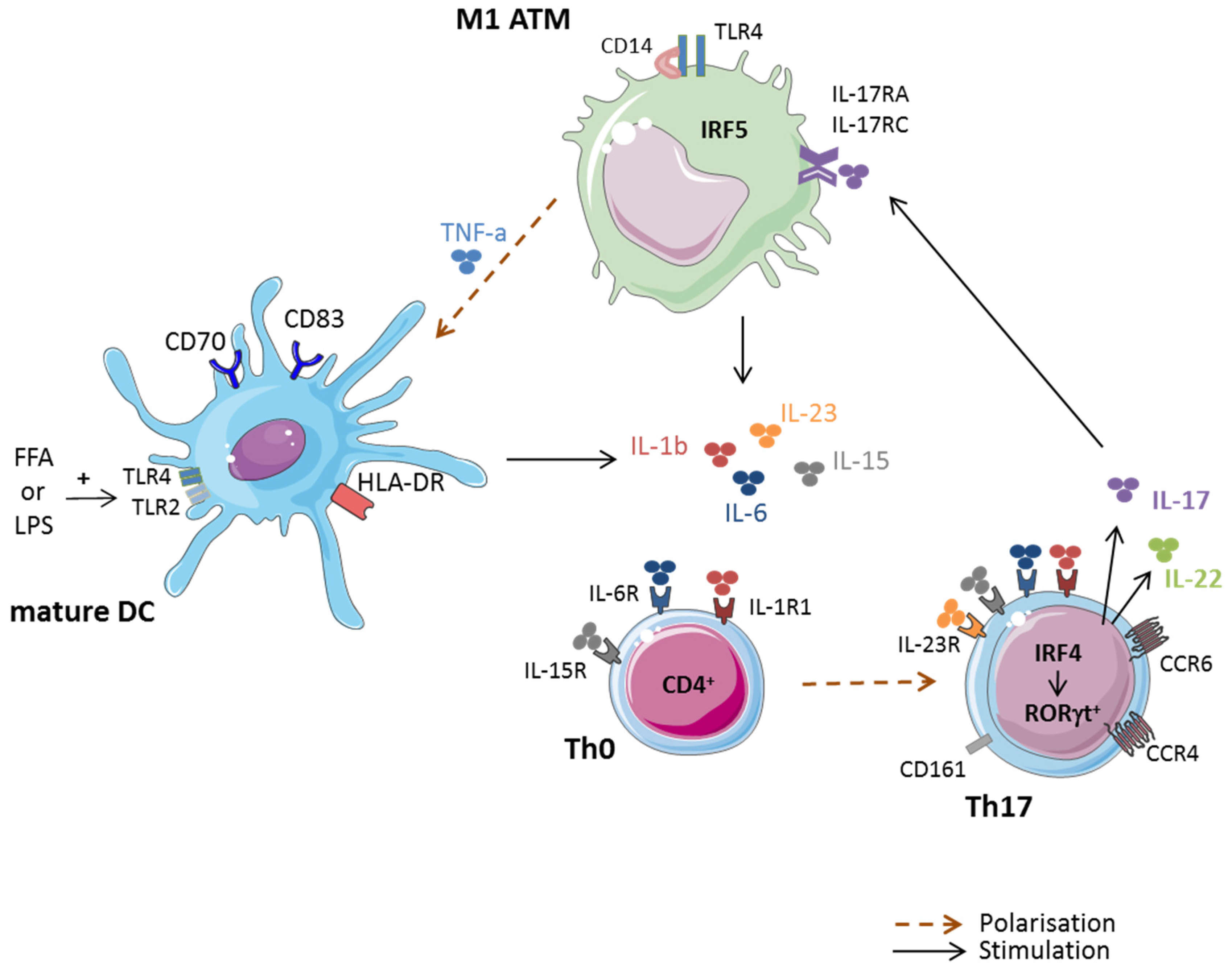
3.2. Macrophages and Th17 Cell Polarization in Obese AT
3.3. Contribution of ASC and Adipocytes in Th17 Cell Polarization
3.4. Immunometabolism and Th17 Cell Differentiation
4. Obesity and Th17 Cell-Driven Inflammatory Diseases
4.1. Rheumatoid Arthritis
4.2. Multiple Sclerosis
4.3. Psoriasis
4.4. Cancer
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chan, Z.; Magkos, F. Lean, but not healthy: The ‘metabolically obese, normal-weight’ phenotype. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Chawla, A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013, 339, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Way, S.S. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009, 126, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D.; Brenchley, J.M.; Laurence, A.; Freeman, A.F.; Hill, B.J.; Elias, K.M.; Kanno, Y.; Spalding, C.; Elloumi, H.Z.; Paulson, M.L.; et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008, 452, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12β1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor rorgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; De Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Flavell, R.A.; Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 2006, 7, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007, 8, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector Th17 cells and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L.; et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Eljaafari, A.; Robert, M.; Chehimi, M.; Chanon, S.; Durand, C.; Vial, G.; Bendridi, N.; Madec, A.M.; Disse, E.; Laville, M.; et al. Adipose tissue-derived stem cells from obese subjects contribute to inflammation and reduced insulin response in adipocytes through differential regulation of the Th1/Th17 cell balance and monocyte activation. Diabetes 2015, 64, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic Th17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Aphale, A.; Vatan, L.; Szeliga, W.; Wang, Y.; Liu, Y.; Welling, T.H.; et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008, 181, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Yosef, N. Understanding Th17 cells through systematic genomic analyses. Curr. Opin. Immunol. 2014, 28, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, C.S.; Kaiser, A.; Paulos, C.M.; Cassard, L.; Sanchez-Perez, L.; Heemskerk, B.; Wrzesinski, C.; Borman, Z.A.; Muranski, P.; Restifo, N.P. Type 17 CD8+ Tcells display enhanced antitumor immunity. Blood 2009, 114, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, D.H.; Park, H.J. IL-18 and cutaneous inflammatory diseases. Int. J. Mol. Sci. 2015, 16, 29357–29369. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Duarte, J.H.; Veldhoen, M.; Hornsby, E.; Li, Y.; Cua, D.J.; Ahlfors, H.; Wilhelm, C.; Tolaini, M.; Menzel, U.; et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011, 12, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Hirota, K.; Cua, D.J.; Stockinger, B.; Veldhoen, M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009, 31, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Petermann, F. Development and function of interleukin 17-producing gammadelta T cells. Ann. N. Y. Acad. Sci. 2012, 1247, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Raifer, H.; Mahiny, A.J.; Bollig, N.; Petermann, F.; Hellhund, A.; Kellner, K.; Guralnik, A.; Reinhard, K.; Bothur, E.; Huber, M.; et al. Unlike αβ T cells, gammadelta T cells, LTi cells and NKT cells do not require IRF4 for the production of IL-17A and IL-22. Eur. J. Immunol. 2012, 42, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Poggi, A.; Catellani, S.; Battaglia, F.; Ferrera, A.; Setti, M.; Murdaca, G.; Zocchi, M.R. Vdelta1 T lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood 2009, 113, 6611–6618. [Google Scholar] [CrossRef] [PubMed]
- Roark, C.L.; French, J.D.; Taylor, M.A.; Bendele, A.M.; Born, W.K.; O’Brien, R.L. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J. Immunol. 2007, 179, 5576–5583. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 cell responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.; Resende, M.; Coffre, M.; Devergne, O.; Herbeuval, J.P.; Hermine, O.; Schneider, E.; Rogge, L.; Ruemmele, F.M.; Dy, M.; et al. Proinflammatory environment dictates the IL-17-producing capacity of human invariant NKT cells. J. Immunol. 2011, 186, 5758–5765. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Kim, H.O.; Kyparissoudis, K.; Corpuz, T.M.; Pinget, G.V.; Uldrich, A.P.; Brink, R.; Belz, G.T.; Cho, J.H.; Godfrey, D.I.; et al. IL-17-producing nkt cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014, 7, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Almeida, C.F.; Agua-Doce, A.; Graca, L. Induced IL-17-producing invariant NKT cells require activation in presence of TGF-β and IL-1β. J. Immunol. 2013, 190, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Gautron, A.S.; Beaudoin, L.; Bui, L.C.; Michel, M.L.; Coumoul, X.; Eberl, G.; Leite-de-Moraes, M.; Lehuen, A. Nod mice contain an elevated frequency of iNKT17 cells that exacerbate diabetes. Eur. J. Immunol. 2011, 41, 3574–3585. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Di Santo, J.P. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, M.R.; Fung, T.C.; Masur, S.H.; Kelsen, J.R.; McConnell, F.M.; Dubrot, J.; Withers, D.R.; Hugues, S.; Farrar, M.A.; Reith, W.; et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 2015, 348, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Goc, J.; Hepworth, M.R.; Sonnenberg, G.F. Group 3 innate lymphoid cells: Regulating host-commensal bacteria interactions in inflammation and cancer. Int. Immunol. 2016, 28, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.; Flutter, B.; Tosi, I.; Grys, K.; Sreeneebus, H.; Perera, G.K.; Chapman, A.; Smith, C.H.; Di Meglio, P.; Nestle, F.O. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Investig. Dermatol. 2014, 134, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, Y.J.; Subramanian, S.; Lee, H.H.; Albacker, L.A.; Matangkasombut, P.; Savage, P.B.; McKenzie, A.N.; Smith, D.E.; Rottman, J.B.; et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012, 129, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.S.; Han, S.; Xu, Q.; Herman, M.L.; Kennedy, L.B.; Csako, G.; Bielekova, B. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci. Transl. Med. 2012, 4, 145ra106. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Liu, L.F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 cell bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Sumarac-Dumanovic, M.; Stevanovic, D.; Ljubic, A.; Jorga, J.; Simic, M.; Stamenkovic-Pejkovic, D.; Starcevic, V.; Trajkovic, V.; Micic, D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. 2009, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, Y.; Wang, H.; Du, M.; Hess, B.W.; Ford, S.P.; Nathanielsz, P.W.; Zhu, M.J. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm. Bowel Dis. 2011, 17, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Cella, M.; McCartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374.e3. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, E.; Venteclef, N.; Caer, C.; Poitou, C.; Cremer, I.; Aron-Wisnewsky, J.; Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V.; Clement, K.; et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: Relevance to obesity and type 2 diabetes. Diabetes 2014, 63, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bian, Z.; Zhao, L.; Liu, Y.; Liang, S.; Wang, Q.; Han, X.; Peng, Y.; Chen, X.; Shen, L.; et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin. Exp. Immunol. 2011, 166, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Bourlier, V.; Zakaroff-Girard, A.; Miranville, A.; De Barros, S.; Maumus, M.; Sengenes, C.; Galitzky, J.; Lafontan, M.; Karpe, F.; Frayn, K.N.; et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008, 117, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and Th1-Th17 cellsresponses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, E.; Toubal, A.; Alzaid, F.; Blazek, K.; Eames, H.L.; Lebozec, K.; Pini, M.; Hainault, I.; Montastier, E.; Denis, R.G.; et al. IRF5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat. Med. 2015, 21, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Anderson, D.E.; Baecher-Allan, C.; Hastings, W.D.; Bettelli, E.; Oukka, M.; Kuchroo, V.K.; Hafler, D.A. IL-21 and TGF-β are required for differentiation of human T(H)17 cells. Nature 2008, 454, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human t helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Iwai, S.; Tsujiyama, K.; Kurahashi, C.; Takeshita, K.; Naoe, M.; Masunaga, A.; Ogawa, Y.; Oguchi, K.; Miyazaki, A. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 cell responses. J. Immunol. 2007, 179, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Tatano, Y.; Shimizu, T.; Tomioka, H. Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 cell polarization. Sci. Rep. 2014, 4, 4146. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Robert, M.; Bechwaty, M.E.; Vial, G.; Rieusset, J.; Vidal, H.; Pirola, L.; Eljaafari, A. Adipocytes, like their progenitors, contribute to inflammation of adipose tissues through promotion of Th-17 cells and activation of monocytes, in obese subjects. Adipocyte 2016, 5, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Gaffen, S.L. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Kruppel-like factors. Cytokine 2013, 61, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Shin, D.W.; Noh, M. Interleukin-17a inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem. Pharmacol. 2009, 77, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Binger, K.J.; Corte-Real, B.F.; Kleinewietfeld, M. Immunometabolic regulation of interleukin-17-producing T helper cells: Uncoupling new targets for autoimmunity. Front. Immunol. 2017, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Yokote, K.; Nakayama, T. The obesity-related pathology and Th17 cells. Cell. Mol. Life Sci. 2017, 74, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Solt, L.A. Metabolism of murine Th 17 cells: Impact on cell fate and function. Eur. J. Immunol. 2016, 46, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 cells and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.V.; Barbi, J.; Yang, H.Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.R.; et al. Control of T(H)17/T(REG) balance by hypoxia-inducible factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chi, H. mTOR and metabolic pathways in T cell quiescence and functional activation. Semin. Immunol. 2012, 24, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Kishton, R.J.; Nichols, A.G.; Macintyre, A.N.; Inoue, M.; Ilkayeva, O.; Winter, P.S.; Liu, X.; Priyadharshini, B.; Slawinska, M.E.; et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015, 125, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mtor kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bahre, H.; et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, acc1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols are agonist ligands of rorgammat and drive Th17 cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Chen, K.; Zhou, Q.; Wei, W.; Wang, Y.; Wang, Y. The role of oxidized low-density lipoprotein in breaking peripheral Th17 cell/Treg balance in patients with acute coronary syndrome. Biochem. Biophys. Res. Commun. 2010, 394, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, Y.U.; Sun, H.; Lee, J.H.; Reynolds, J.M.; Hanabuchi, S.; Wu, H.; Teng, B.B.; Chung, Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 2014, 40, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, Y.; Miyabe, C.; Iwai, Y.; Takayasu, A.; Fukuda, S.; Yokoyama, W.; Nagai, J.; Jona, M.; Tokuhara, Y.; Ohkawa, R.; et al. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheumatol. 2013, 65, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Koutedakis, Y.; Kitas, G.D. Obesity in rheumatoid arthritis. Rheumatology 2011, 50, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.Y.; Yoon, B.Y.; Park, M.K.; Oh, H.J.; Byun, J.K.; Lee, S.Y.; Min, J.K.; Park, S.H.; Kim, H.Y.; Cho, M.L. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 cell differentiation. Exp. Mol. Med. 2012, 44, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Derdemezis, C.S.; Voulgari, P.V.; Drosos, A.A.; Kiortsis, D.N. Obesity, adipose tissue and rheumatoid arthritis: Coincidence or more complex relationship? Clin. Exp. Rheumatol. 2011, 29, 712–727. [Google Scholar] [PubMed]
- Lu, M.C.; Yan, S.T.; Yin, W.Y.; Koo, M.; Lai, N.S. Risk of rheumatoid arthritis in patients with type 2 diabetes: A nationwide population-based case-control study. PLoS ONE 2014, 9, e101528. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Barcellos, L.F. Obesity and multiple sclerosis susceptibility: A review. J. Neurol. Neuromed. 2016, 1, 1–5. [Google Scholar]
- Guerrero-Garcia, J.J.; Carrera-Quintanar, L.; Lopez-Roa, R.I.; Marquez-Aguirre, A.L.; Rojas-Mayorquin, A.E.; Ortuno-Sahagun, D. Multiple sclerosis and obesity: Possible roles of adipokines. Mediat. Inflamm. 2016, 2016, 4036232. [Google Scholar] [CrossRef] [PubMed]
- Galgani, M.; Procaccini, C.; De Rosa, V.; Carbone, F.; Chieffi, P.; La Cava, A.; Matarese, G. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J. Immunol. 2010, 185, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Cantoni, C.; Henderson, J.G.; Hawiger, D.; Ramsbottom, M.; Mikesell, R.; Ryu, J.; Hsieh, C.S.; Cremasco, V.; Haynes, W.; et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur. J. Immunol. 2013, 43, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Zabel, B.A.; Loghavi, S.; Zuniga, L.A.; Ho, P.P.; Sobel, R.A.; Butcher, E.C. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 2009, 183, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Tomalka-Kochanowska, J.; Baranowska, B.; Wolinska-Witort, E.; Uchman, D.; Litwiniuk, A.; Martynska, L.; Kalisz, M.; Bik, W.; Kochanowski, J. Plasma chemerin levels in patients with multiple sclerosis. Neuro Endocrinol. Lett. 2014, 35, 218–223. [Google Scholar] [PubMed]
- Piccio, L.; Stark, J.L.; Cross, A.H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 2008, 84, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Bowles, A.C.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; Gimble, J.M.; Bunnell, B.A. Human adipose stromal/stem cells from obese donors show reduced efficacy in halting disease progression in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Love, T.J.; Canning, C.; Schneeweiss, S. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann. Rheum. Dis. 2010, 69, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, K.; Herbert, D.; Popkova, Y.; Lorz, A.; Schiller, J.; Gericke, M.; Kloting, N.; Bluher, M.; Franz, S.; Simon, J.C.; et al. Free fatty acids sensitize dendritic cells to amplify Th1/Th17 cell-immune responses. Eur. J. Immunol. 2016, 46, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Matsuyuki, A.; Nakamura, Y.; Fukami, K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp. Dermatol. 2015, 24, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Kanda, N.; Tada, Y.; Shibata, S.; Uozaki, H.; Fukusato, T.; Sato, S.; Watanabe, S. Lipocalin-2 exacerbates psoriasiform skin inflammation by augmenting T-helper 17 response. J. Dermatol. 2016, 43, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Vainio, H.; Kaaks, R.; Bianchini, F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur. J. Cancer Prev. 2002, 11, S94–S100. [Google Scholar] [PubMed]
- Vansaun, M.N. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kitayama, J.; Nagawa, H. Enhanced expression of leptin and leptin receptor (ob-r) in human breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 4325–4331. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Delort, L.; Lequeux, C.; Dubois, V.; Dubouloz, A.; Billard, H.; Mojallal, A.; Damour, O.; Vasson, M.P.; Caldefie-Chezet, F. Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3d adipose equivalent model. PLoS ONE 2013, 8, e66284. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonne, A.; Delorme, B.; Herault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bellows, C.F.; Kolonin, M.G. Adipose tissue-derived progenitor cells and cancer. World J. Stem Cells 2010, 2, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting edge: Th17 cells and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol. 2007, 178, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Odunsi, K.; Chen, W.; Peng, G.; Matsuzaki, J.; Wang, R.F. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 15505–15510. [Google Scholar] [CrossRef] [PubMed]
- Numasaki, M.; Fukushi, J.; Ono, M.; Narula, S.K.; Zavodny, P.J.; Kudo, T.; Robbins, P.D.; Tahara, H.; Lotze, M.T. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003, 101, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic analysis of prostate-infiltrating lymphocytes reveals Th17 cell and treg skewing. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Rong, G.; Wei, H.; Zhang, M.; Bi, J.; Ma, L.; Xue, X.; Wei, G.; Liu, X.; Fang, G. The prevalence of Th17 cells in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2008, 374, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautes-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002, 99, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Moisan, J.; Wang, Y.; Lesch, C.A.; Spooner, C.; Morgan, R.W.; Zawidzka, E.M.; Mertz, D.; Bousley, D.; et al. Synthetic rorgamma agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology 2016, 5, e1254854. [Google Scholar] [CrossRef] [PubMed]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Boni, A.; Antony, P.A.; Cassard, L.; Irvine, K.R.; Kaiser, A.; Paulos, C.M.; Palmer, D.C.; Touloukian, C.E.; Ptak, K.; et al. Tumor-specific Th17 cell-polarized cells eradicate large established melanoma. Blood 2008, 112, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qian, L.; Shen, G.; Bian, G.; Xu, T.; Xu, W.; Shen, G.; Hu, S. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res. 2014, 74, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.; Teijeiro, A.; Buren, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.P.; Perna, C.; Djouder, N. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Ryan, C.; Nolan, B.; Tosetto, M.; Geraghty, R.; Winter, D.C.; O’Connell, P.R.; Hyland, J.M.; Doherty, G.A.; Sheahan, K.; et al. Enrichment of inflammatory IL-17 and TNF-α secreting CD4+ T cells within colorectal tumors despite the presence of elevated CD39+ T regulatory cells and increased expression of the immune checkpoint molecule, PD-1. Front. Oncol. 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Ge, D.; Cunningham, D.M.; Huang, F.; Ma, L.; Burris, T.P.; You, Z. Targeting Th17 cell-IL-17 pathway in prevention of micro-invasive prostate cancer in a mouse model. Prostate 2017, 77, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Cheng, Q.; Cai, Y.; Gong, H.; Wu, Y.; Yu, X.; Shi, L.; Wu, D.; Dong, C.; Liu, H. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014, 74, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, J.; Liu, L.; Li, Q.; Rong, W.; Wang, L.; Wang, Y.; Zang, M.; Wu, Z.; Zhang, Y.; et al. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS ONE 2012, 7, e50035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.S. TNFalpha and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Mol. Cancer 2013, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Restifo, N.P. T(h)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010, 10, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6-STAT3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Dave, M.A.; Bliss, S.A.; Giec-Ujda, A.B.; Bryan, M.; Pliner, L.F.; Rameshwar, P. Treg/Th17 cell polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. J. Cancer. Stem. Cell. Res. 2014, 2014, e1003. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.P.; Park, A.; Yang, B.G.; Yun, C.H.; Kwak, M.J.; Lee, G.W.; Kim, J.H.; Jang, M.S.; Lee, E.J.; Jeun, E.J.; et al. Gut-specific delivery of T-helper 17 cells reduces obesity and insulin resistance in mice. Gastroenterology 2017, 152, 1998–2010. [Google Scholar] [CrossRef] [PubMed]

| IL-17 Effects | IL-17 Secreting Cells | Cancer Type | Experimental Model | Key Findings | Ref. |
|---|---|---|---|---|---|
| ANTI-TUMORAL EFFECTS OF IL-17 | Th17 Tc17 | MC38 colorectal tumor cells | in vitro assays; cells were SC injected in C57BL/6 mice followed by adoptive transfer of polarized-Tc17 treated or not with LYC-54143 | Activation of RORγt with an agonist (LYC-54143) enhanced Th17 (IL-17+-CD4+) and Tc17 (IL-17+-CD8+) effector activity and reduced Treg immunosuppressive afunction, allowing potent anti-tumor response in vitro. Adoptive transfer of Tc17 cells treated with RORγt agonists inhibited tumor growth. | [121] |
| pTh17 Th1-Th17 γδT17 | MCA205 fibrosarcoma, MC38-OVAdim colorectal cells | SC injection of tumoral cells into ATBs-treated C57BL/6 mice followed with E. hirae or B. intestinihominis bacteria inoculation | Hirae markedly increased pTh17 cells (a population of double positive IFN-γ+IL-17+ T cells), cytotoxic IFN-γ+CD8+ T cells, but decreased regulatory Foxp3+CD25+CD4+ TILs. B. intestinihominis-inoculated mice increased the numbers of IL-17+γδT cells and IFN-γ+ γδT cells infiltrating tumor. These 2 bacteria species enhanced anticancer immune responses. | [122] | |
| pTh17 | MCA205 fibrosarcoma, B16 melanoma | SC injection of tumoral cells into cyclophosphamide-treated SPF mice | The anti-tumor efficacy of cyclophosphamide depended on the translocation of Gram+ bacteria in secondary lymphoid oragans, which stimulated pathogenic Th17 (gut pTh17) generation and memory Th1 immune responses. These results suggest that the gut microbiota help shape the anti-cancer immune response. | [137] | |
| Th17 | B16 lung melanoma | IL-17 deficient mice bearing B16/F10 melanoma | Adoptive transfer of tumor-specific CCL20+ -secreting Th17 cells inhibited tumor growth through improvement of tumor antigen presentation to dendritic cells (CCR6+CD11c+CD11b+ and CCR6+CD11c+CD8α+ DC), and amplification of tumor-specific CD8+ T cell killing activity. Th17 (75% fewer tumor colonies) had a greater potency to control tumor growth than Th1 (40% fewer tumor colonies) when transferred into mice harboring melanoma. | [123] | |
| Th17 | B16 melanoma | SC injection of tumoral cells into TRP-1 TCR transgenic mice | Among the Th0, Th1, and Th17 subtypes, it was found that Th17-polarized cells better mediated destruction of advanced B16 melanoma. However, their therapeutic effect was critically dependent on interferon-gamma (IFNγ) production. | [124] | |
| γδT17 | B16/F10 melanoma and lung Lewis carcinoma | IV injection of tumoral cells in ATBs-treated C57BL/6 mice after adoptive transfer of γδT cells | Antibiotics increased susceptibility to develop engrafted B16/F10 melanoma and Lewis lung carcinoma in mice. Adding normal γδT cells or supplementing IL-17 restored the impaired immune surveillance phenotype, which demonstrated the importance of commensal bacteria in supporting the host immune response against cancer. | [125] | |
| Th17 | Ovarian cancer | Human | Levels of tumor-infiltrating Th17 cells are negatively correlated with tumor-infiltrating regulatory T cells. Th17 cells contributed to protective human tumor immunity by inducing Th1-type chemokines, CXCL9 and CXCL10, and recruiting effector cells to the tumor microenvironment. | [135] | |
| PRO-TUMORAL EFFECTS OF IL-17 | γδT17 | Hepa1–6 hepatocellular carcinoma cell line | SC injection of tumoral cells in C57BL/6 or IL-17−/−C57BL/6 | The tumor-promoting effect of IL-17A, mainly produced by γδT17 cells, was mediated through suppression of antitumor responses, especially CD8+ T cell responses. IL-17A induced CXCL5 production by tumor cells to enhance the infiltration of myeloid-derived suppressor cells (MDSC) to tumor sites in a CXCL5/CXCR2-dependent manner | [130] |
| Th17 Tc17 | Hepatocellular carcinoma (HCC) | Human | A cohort of 105 patients with liver cirrhosis and an established HCC revealed higher serum levels of IL-17 and a massive hepatic infiltration of CD4+ and CD8+ IL-17 secreting cells | [131] | |
| Th17 | HCC | High Fat Diet fed C57BL/6 mice | Hepatic unconventional prefoldin RPB5 interactor (URI) induced enriched diet to cause liver DNA damages, recruitment of Th17 cells in liver, development of a non-alcoholic steatohepatitis (NASH) and of HCC. pharmacological suppression of Th17 cell differentiation, IL-17A blocking antibodies, and genetic ablation of the IL-17A receptor in myeloid cells, prevented diet induced Th17 and subsequent HCC. | [126] | |
| Th17 | CRC (Colorectal Cancer) | Human | Elevated numbers of CD4+ Th17 in 54 CRC patients were observed inside tumors compared with non-tumor regions, together with a higher microvessel density. Colorectal cell lines stimulated IL-17 produced VEGFα in a dose dependent manner | [127] | |
| IL-17 and TNF-α secreting Th17 | CRC (HCT116) and prostate (LNCaP) cancer cell lines | Human | IL-17 and TNF-α individually rather than cooperatively, up-regulated PD-L1 expression in human prostate and colon cancer cell lines. PD-L1 expression acts on PD-1 ligands (PD-L1 and PD-L2) to suppress activation of cytotoxic T lymphocytes. | [132] | |
| IL-17 and TNF-α secreting Th17 | CRC | human | An increased frequency of Th17 cells was observed inside 22 CRC tumor tissue, as compared to adjacent uninvolved tissue. These Th17 cells mostly coproduced TNF-α, but not IFN-γ. There was a negative correlation between expression of PD-1 and IFN-γ, but not IL-17, in CRC, and an enrichment in Tregs. Thus PD-1 expressing T cells and Treg cells within the tumor may have a suppressive effect on T cells secreting IFN-γ, IL-2, or TNF-α, but not Th17 cells | [128] | |
| IL-17 and TNF-α cytokines | CRC cell lines (Caco-2, HCT116) | Human | IL-17 and TNF-α were shown to enhance glycolysis in several colorectal cancer cell lines, through increased expression of HIF-1α and c-myc. TNFα and IL-17 also synergistically stimulated production by HT-29 cells of a growth factor that simulated proliferation/survival of NIL8 fibroblastic cells, promoting thus colorectal tumorigenesis | [133] | |
| γδT17 | CRC | Human | γδT17 were found predominant in CRC tissues of 154 patients. γδT17 cells promoted migration, survival and proliferation of MDSC via production of IL-8, GM-CSF and TNF-α in vitro | [134] | |
| RORγt+ Th17 | Prostate cancer | Pten-null mice | The treatment of Pten-null mice with SR1001, Th17 cell inhibitor, or an anti-IL17 monoclonal antibody during 6 weeks was sufficient to reduce prostate tumor progression, angiogenesis and tumor cell infiltration. | [129] | |
| Th17 | B16 melanoma MB49 bladder carcinoma | SC injection of tumoral cells into WT or IL17−/−mice | IL-17 promotes tumor growth through an IL-6/Stat3 pathway | [138] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017, 6, 68. https://doi.org/10.3390/jcm6070068
Chehimi M, Vidal H, Eljaafari A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. Journal of Clinical Medicine. 2017; 6(7):68. https://doi.org/10.3390/jcm6070068
Chicago/Turabian StyleChehimi, Marwa, Hubert Vidal, and Assia Eljaafari. 2017. "Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases" Journal of Clinical Medicine 6, no. 7: 68. https://doi.org/10.3390/jcm6070068




