Modulation of Vascular ACE by Oxidative Stress in Young Syrian Cardiomyopathic Hamsters: Therapeutic Implications
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal Model
2.2. RNA Extraction and Real-Time RT-PCR
2.3. Protein Extraction and Western Blot
2.4. Evaluation of Vascular ACE Activity
2.5. Statistical Analysis
3. Results
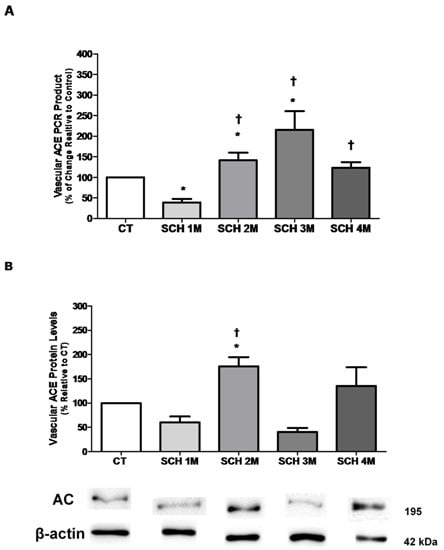
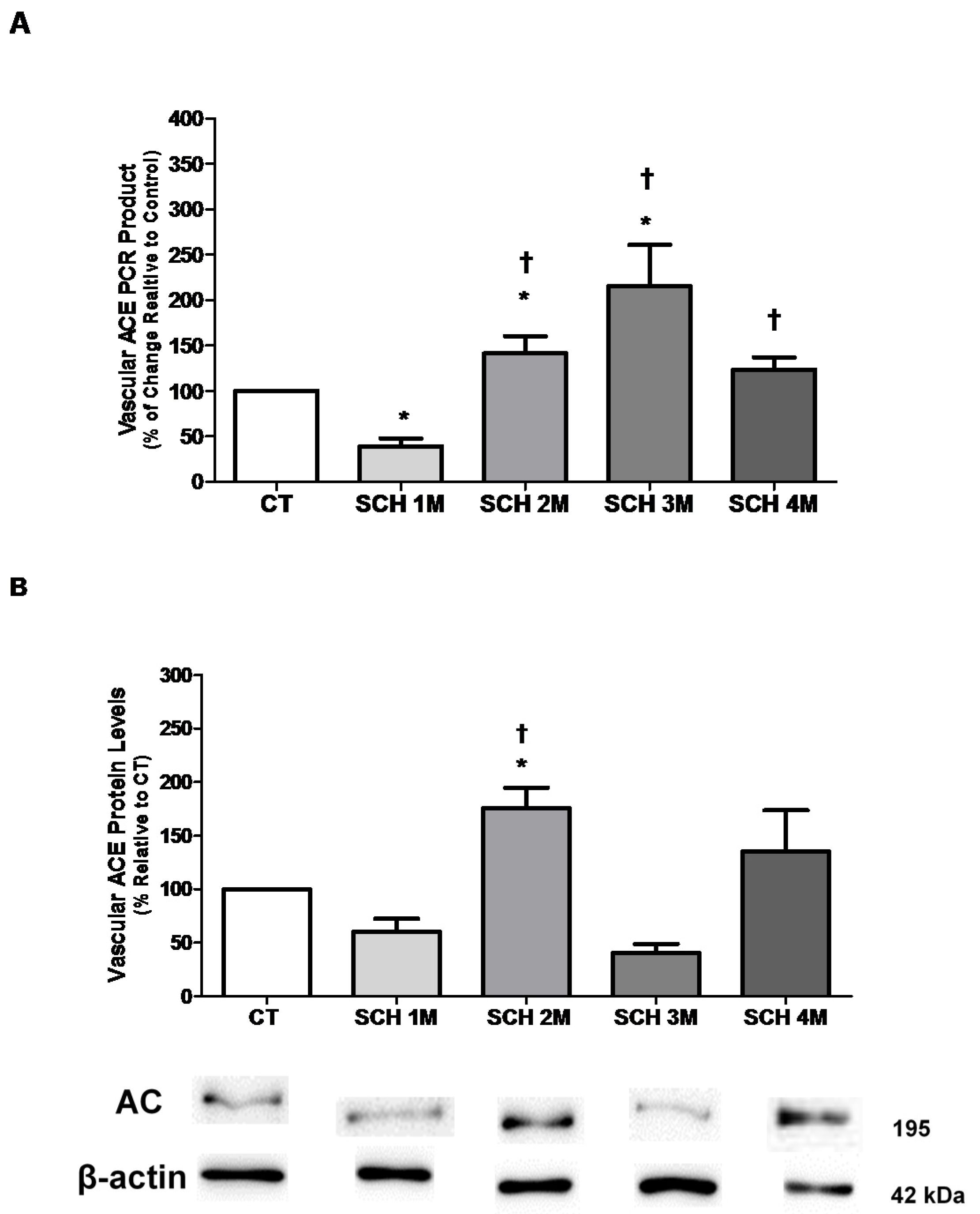
3.1. Vascular ACE mRNA Expression and Protein Levels
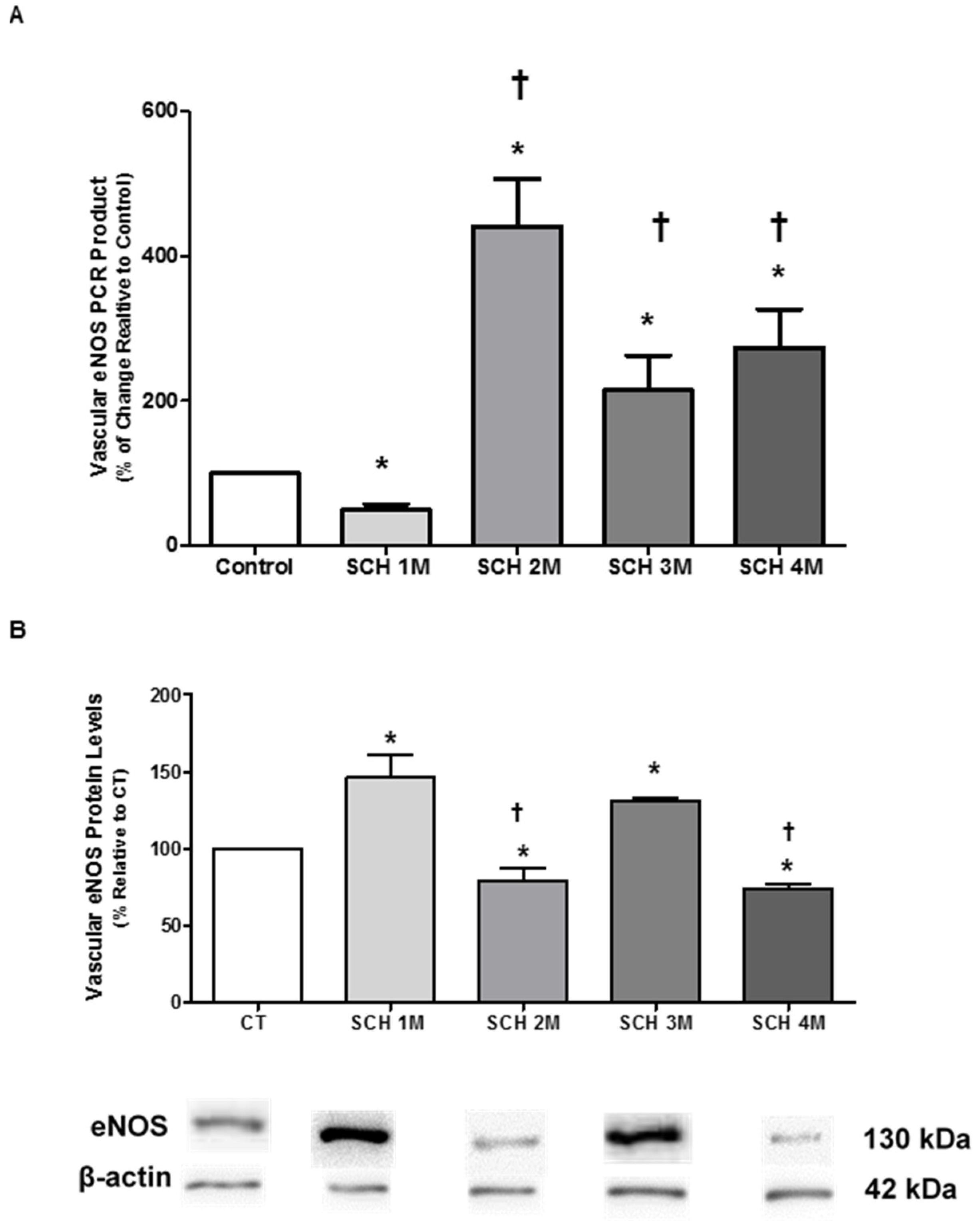
3.2. Vascular eNOS mRNA Expression and Protein Levels
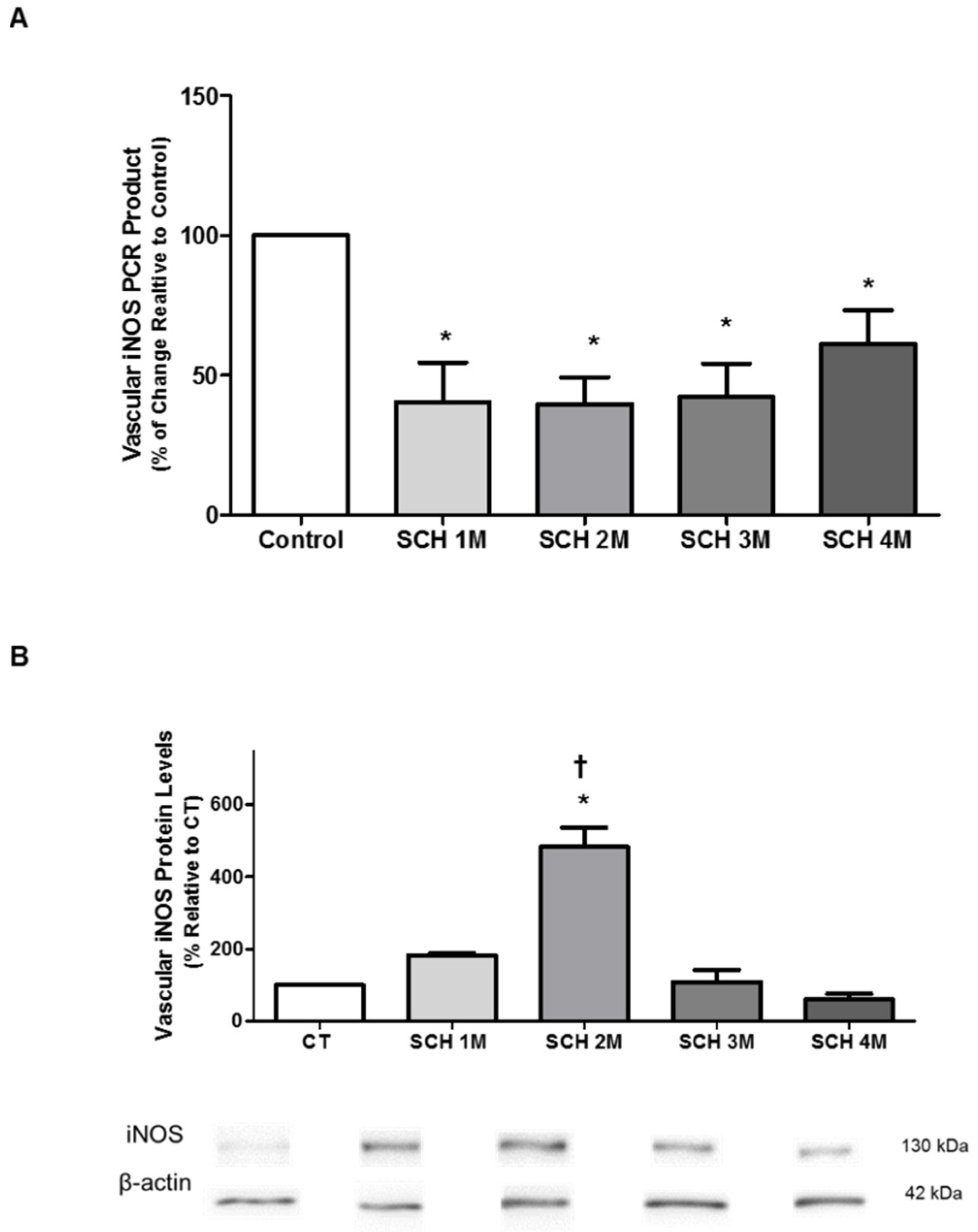
3.3. Vascular iNOS mRNA Expression and Protein Levels
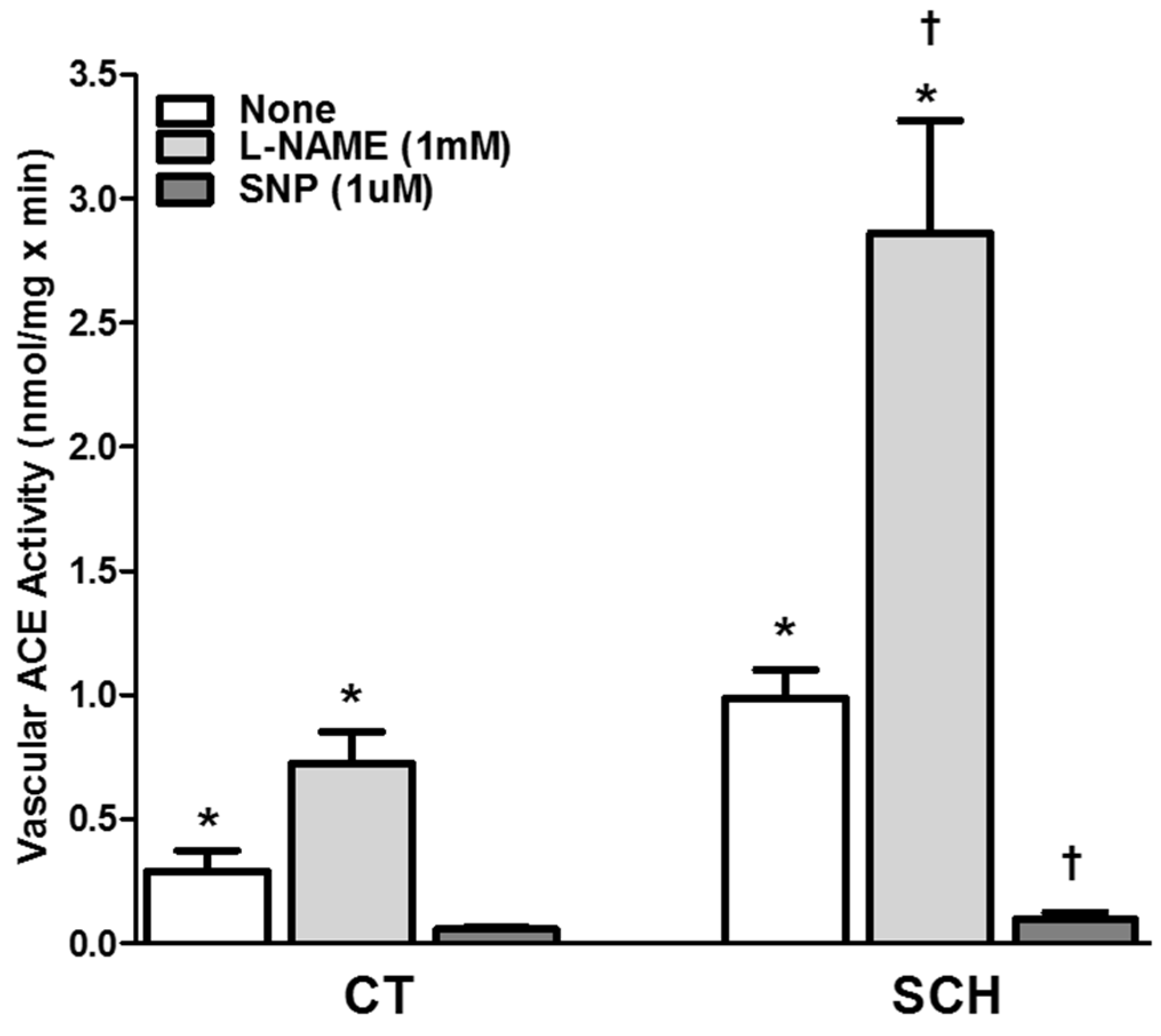
3.4. Effect of NO Bioavailability on Vascular ACE Activity
3.5. Effect of NO Bioavailability on Vascular ACE mRNA Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| ACEI | Angiotensin converting enzyme inhibitor |
| ARB | Angiotensin receptor blocker |
| cDNA | Complementary deoxyribonucleic acid |
| CT | Control hamster |
| DTT | dl-Dithiothreiol |
| eNOS | Endothelial nitric oxide synthase |
| HF | Heart failure |
| iNOS | Inducible nitric oxide synthase |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NO | Nitric Oxide |
| PMSF | Phenylmethanesulfonyl fluoride |
| RAS | Renin angiotensin system |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse Transcriptase-Polymerase chain reaction |
| SCH | Syrian Cardiomyopathic hamster |
References
- Francis, G.S. Neurohormonal control of heart failure. Clevel. Clin. J. Med. 2011, 78, S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Lüscher, T.F. Human endothelial dysfunction: EDRFs. Pflug. Arch. 2010, 459, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Schulz, R.; Liaudet, L.; Szabó, C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol. Sci. 2005, 26, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; van der Laarse, A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol. Cell. Biochem. 2010, 333, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Linz, W.; Wohlfart, P.; Schölkens, B.A.; Malinski, T.; Wiemer, G. Interactions among ACE, kinins and NO. Cardiovasc. Res. 1999, 43, 549–561. [Google Scholar] [CrossRef]
- Ackermann, A.; Fernández-Alfonso, M.S.; Sánchez de Rojas, R.; Ortega, T.; Paul, M.; González, C. Modulation of angiotensin-converting enzyme by nitric oxide. Br. J. Pharmacol. 1998, 124, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, C.A.; Struthers, A.D. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000, 101, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.J.; Altieri, P.I.; Escobales, N. Altered vascular function in early stages of heart failure in hamsters. J. Card. Fail. 1997, 3, 311–318. [Google Scholar] [CrossRef]
- Crespo, M.J. Vascular alterations during the development and progression of experimental heart failure. J. Card. Fail. 1999, 5, 55–63. [Google Scholar] [CrossRef]
- Escobales, N.; Crespo, M.J. Angiotensin II-dependent vascular alterations in young cardiomyopathic hamsters: Role for oxidative stress. Vasc. Pharmacol. 2006, 44, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.J.; Altieri, P.I.; Escobales, N. Increased vascular angiotensin II binding capacity and ET-1 release in young cardiomyopathic hamsters. Vasc. Pharmacol. 2006, 44, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Escobales, N.; Ramos, J.A.; Santacana, G.E.; Crespo, M.J. Hemodynamic Alterations in the Coronary Circulation of Cardiomyopathic Hamsters: Age and Ang II-dependent Mechanisms. J. Card. Fail. 2009, 15, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Oelze, M.; August, M.; Wendt, M.; Daiber, A.; Schulz, E.; Baldus, S.; Kleschyov, A.L.; Materne, A.; Wenzel, P.; et al. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.M.; Rosas, O.; Torrado, A.I.; González, M.M.; Kalyan-Masih, P.O.; Miranda, J.D. Molecular, anatomical, physiological, and behavioral studies of rats treated with buprenorphine after spinal cord injury. J. Neurotrauma 2009, 26, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.I.; Maldonado, H.M.; Velázquez, G.; Rubio-Dávila, M.; Miranda, J.D.; Aquino, E.; Mayol, N.; Cruz-Torres, A.; Jardón, J.; Salgado-Villanueva, I.K. Caveolin isoform expression during differentiation of C6 glioma cells. Int. J. Dev. Neurosci. 2005, 23, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Orengo, L.; Figueroa, J.D.; Torrado, A.; Puig, A.; Whittemore, S.R.; Miranda, J.D. Reduction of EphA4 receptor expression after spinal cord injury does not induce axonal regeneration or return of tcMMEP response. Neurosci. Lett. 2007, 418, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Crespo, M.J.; Cruz, N.; Altieri, P.I.; Escobales, N. Chronic Treatment with N-acetylcysteine (NAC) Improves Cardiac Function but Does Not Prevent Progression of Cardiomyopathy in Syrian Cardiomyopathic Hamsters. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.J.; Cruz, N.; Altieri, P.I.; Escobales, N. Enalapril and losartan are more effective than carvedilol in preventing dilated cardiomyopathy in the Syrian cardiomyopathic hamster. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Jessup, J.A.; Westwood, B.M.; Chappell, M.C.; Groban, L. Dual ACE-inhibition and AT1 receptor antagonism improves ventricular lusitropy without affecting cardiac fibrosis in the congenic mRen2.Lewis rat. Ther. Adv. Cardiovasc. Dis. 2009, 3, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Fox, K. Insight into the mode of action of ACE inhibition in coronary artery disease: The ultimate ‘EUROPA’ story. Drugs 2009, 69, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Guardigli, G.; Ceconi, C. Secondary prevention of CAD with ACE inhibitors: A struggle between life and death of the endothelium. Cardiovasc. Drugs Ther. 2010, 24, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Coral-Vazquez, R.; Cohn, R.D.; Moore, S.A.; Hill, J.A.; Weiss, R.M.; Davisson, R.L.; Straub, V.; Barresi, R.; Bansal, D.; Hrstka, R.F.; et al. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: A novel mechanism for cardiomyopathy and muscular dystrophy. Cell 1999, 98, 465–474. [Google Scholar] [CrossRef]
- Tian, Q.; Stepaniants, S.B.; Mao, M.; Weng, L.; Feetham, M.C.; Doyle, M.J.; Yi, E.C.; Dai, H.; Thorsson, V.; Eng, J.; et al. Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol. Cell. Proteom. 2004, 3, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Abreu Rda, S.; Ko, D.; Le, S.Y.; Shapiro, B.A.; Burns, S.C.; Sandhu, D.; Boutz, D.R.; Marcotte, E.M.; Penalva, L.O. Sequence signatures and mRNA concentration can explain two- thirds of protein abundance variation in a human cell line. Mol. Syst. Biol. 2010, 6, 400. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, E.; Fagerberg, L.; Klevebring, D.; Matic, I.; Geiger, T.; Cox, J.; Algenäs, C.; Lundeberg, J.; Mann, M.; Uhlen, M. Defining the transcriptome and proteome in three functionally different human cell lines. Mol. Syst. Biol. 2010, 6, 450. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Comini, L.; Bachetti, T.; Gaia, G.; Pasini, E.; Agnoletti, L.; Pepi, P.; Ceconi, C.; Curello, S.; Ferrari, R. Aorta and Skeletal Muscle NO Synthase expression in experimental heart failure. J. Mol. Cell. Cardiol. 1996, 28, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Lu, H.; Wang, X.Q.; Vaziri, N.D.; Zhou, X.J. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am. J. Hypertens. 2008, 21, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Egashira, K.; Kitamoto, S.; Koyanagi, M.; Katoh, M.; Kataoka, C.; Shimokawa, H.; Takeshita, A. Pathogenic role of oxidative stress in vascular angiotensin-converting enzyme activation in long-term blockade of nitric oxide synthesis in rats. Hypertension 1999, 34, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W.; Dzau, V.J. Vascular biology: The past 50 years. Circulation 2000, 102, IV112–IV116. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Elliott, P.; McKenna, W.J. Dilated cardiomyopathy: A genetically heterogeneous disease. Lancet 2002, 360, 654–655. [Google Scholar] [CrossRef]
- Jasmin, G.; Bajusz, E. Prevention of myocardial generation in hamsters with hereditary cardiomyopathy. Recent Adv. Stud. Cardiac Struct. Metab. 1975, 6, 219–229. [Google Scholar] [PubMed]
- Sole, M.J.; Liew, C.C. Catecholamines, calcium and cardiomyopathy. Am. J. Cardiol. 1988, 62, 20G–24G. [Google Scholar] [CrossRef]
| Primer | RNA Source | Sequence | Accession Number | Tm (°C) | Product Size (bp) |
|---|---|---|---|---|---|
| ACE sense | Hamster | 5′-CCACAGTCTCAACCTGCTCA-3′ | AB212958 | 55 | 181 |
| ACE anti-sense | Hamster | 5′-GCTCCACCACTCTTGGTTGT-3′ | |||
| eNOS sense | Hamster | 5′-GGTCAACTATTCCCTGTCC-3′ | AJ863053 | 57.5 | 287 |
| eNOS anti-sense | Hamster | 5′-ACACCACATCATACTCATCC-3′ | |||
| iNOS sense | Hamster | 5′-ATATACCTCCTGAGTGAAG-3′ | DQ355357 | 55 | 137 |
| iNOS anti-sense | Hamster | 5′-GGTCCTTGGTTGTAGATA-3′ | |||
| EF1-α sense | Rat | 5′-ATTGTTGCTGCTGGTGTTG-3′ | NM_175838 | 55 | 161 |
| EF1-α anti-sense | Rat | 5′-TCGTATCTCTTCTGACTGTATGG-3′ |
| Control | SCH | SCH + LNAME | SCH + SNP | |
|---|---|---|---|---|
| ACE mRNA | 100% | 141% ± 19% * | 442% ± 103% *,† | 32% ± 9% *,† |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, N.; Miranda, J.D.; Crespo, M.J. Modulation of Vascular ACE by Oxidative Stress in Young Syrian Cardiomyopathic Hamsters: Therapeutic Implications. J. Clin. Med. 2016, 5, 64. https://doi.org/10.3390/jcm5070064
Cruz N, Miranda JD, Crespo MJ. Modulation of Vascular ACE by Oxidative Stress in Young Syrian Cardiomyopathic Hamsters: Therapeutic Implications. Journal of Clinical Medicine. 2016; 5(7):64. https://doi.org/10.3390/jcm5070064
Chicago/Turabian StyleCruz, Nildris, Jorge D. Miranda, and Maria J. Crespo. 2016. "Modulation of Vascular ACE by Oxidative Stress in Young Syrian Cardiomyopathic Hamsters: Therapeutic Implications" Journal of Clinical Medicine 5, no. 7: 64. https://doi.org/10.3390/jcm5070064