The Impact of Remote Ischemic Preconditioning on Arterial Stiffness and Heart Rate Variability in Patients with Angina Pectoris
Abstract
:1. Introduction
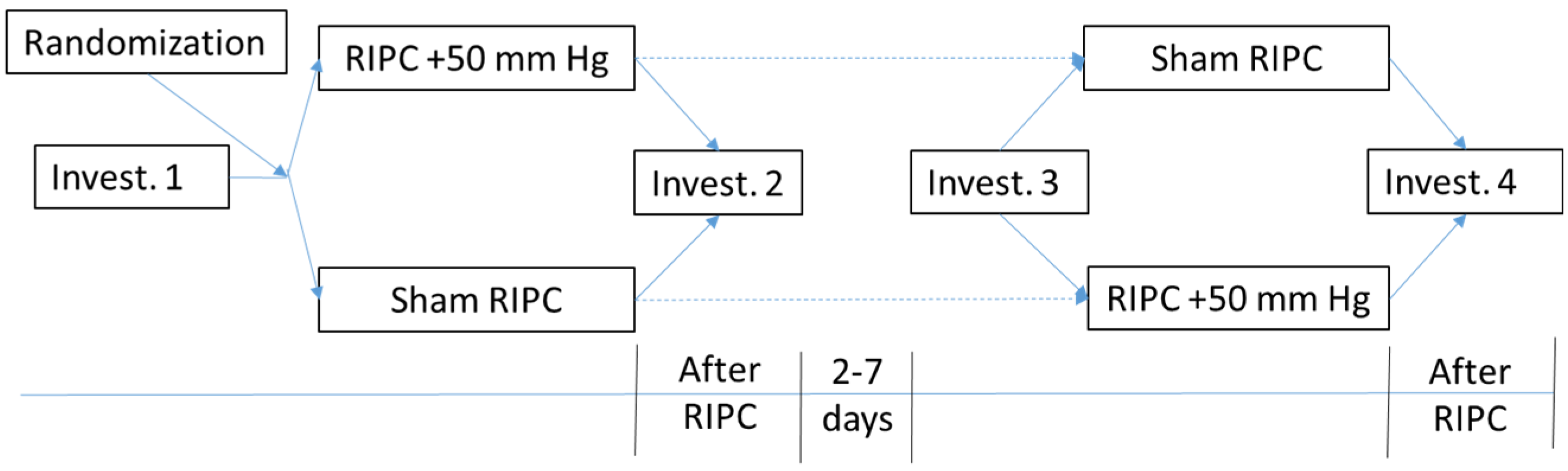
2. Experimental Section
- CHD, stable angina NYHA II or III.
- Signed consent form.
- Significant arrhythmias (atrial fibrillation, atrial flutter, frequent premature atrial and ventricular beats, AV block, etc.),
- Electric cardiostimulator,
- Obesity, body mass index >30 kg/m2,
- Peripheral arterial disease,
- Acute coronary syndrome and myocardial infarction within 3 months,
- Severe heart failure.
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AP | pulse augmentation |
| CHD | coronary heart disease |
| DBP | diastolic blood pressure |
| HF | high frequency low frequency (domain) |
| HR | heart rate |
| HRV | heart rate variability |
| RIPC | ischemic preconditioning |
| LF | low frequency low frequency (domain) |
| NYHA | New York Heart Association |
| PCI | percutaneous coronary interventions |
| Pp | pulse pressure |
| PWV | pulse wave velocity |
| SBP | systolic blood pressure |
| sRIPC | sham ischemic preconditioning |
| SPo2 | oxygen saturation |
| STEMI | ST-elevated myocardial infarction |
| nSTEMI | non ST-elevated myocardial infarction |
| TP | total power |
References
- Reimer, K.A.; Murry, C.E.; Yamasawa, I.; Hill, M.L.; Jennings, R.B. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am. J. Physiol. Heart Circ. Physiol. 1986, 251, H1306–H1315. [Google Scholar]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Sherbakova, E.S.; Zagidullin, N.S.; Plechev, V.V. Ischemic preconditioning in internal medicine and vascular surgery. Med. Vestnik Bashkortostana. 2014, 9, 118–123. [Google Scholar]
- Pickard, J.M.; Bøtker, H.E.; Crimi, G.; Davidson, B.; Davidson, S.M.; Dutka, D.; Ferdinandy, P.; Ganske, R.; Garcia-Dorado, D.; Giricz, Z.; et al. Remote ischemic conditioning: From experimental observation to clinical application: Report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res. Cardiol. 2015, 110, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrett, E.; Bhaskaran, K.; Timmis, A.; Denaxas, S.; Hemingway, H.; Smeeth, L. Association between clinical presentations before myocardial infarction and coronary mortality: A prospective population−based study using linked electronic records. Eur. Heart J. 2014, 35, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Horváth-Puhó, E.; Pedersen, L.; Sørensen, H.T.; Bøtker, H.E. Time-dependent effect of preinfarction angina pectoris and intermittent claudication on mortality following myocardial infarction: A Danish nationwide cohort study. Int. J. Cardiol. 2015, 187, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Wu, Y.; Wei, Y.; Yang, Y; Teng, S; Zhang, H. Remote Ischemic Preconditioning Reduces Perioperative Cardiac and Renal Events in Patients Undergoing Elective Coronary Intervention: A Meta−Analysis of 11 Randomized Trials. PLoS ONE 2014, 9, e115500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lang, X.B.; Zhang, P.; Lv, R.; Wang, Y.F.; Chen, J.H. Remote ischemic preconditioning for prevention of acute kidney injury: A meta−analysis of randomized controlled trials. Am. J. Kidney Dis. 2014, 64, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Ackbarkhan, A.K.; Balgobin, V.; Bulluck, H.; Deelchand, A.; Dhuny, M.R.; Domah, N.; Gaoneadry, D.; Jagessur, R.K.; Joonas, N.; et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J. Am. Coll. Cardiol. 2015, 65, 2764–2765. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Kawai, Y.; Miyoshi, T.; Mima, T.; Takagaki, K.; Tsukuda, S.; Kazatani, Y.; Nakamura, K.; Ito, H. Remote ischemic preconditioning reduces contrast−induced acute kidney injury in patients with ST−elevation myocardial infarction: A randomized controlled trial. Int. J. Cardiol. 2015, 178, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.A.; Boyle, E.; McCartan, D.; Bourke, M.; Medani, M.; Ferguson, J.; Yagoub, H.; Bashar, K.; O’Donnell, M.; Newell, J. A MultiCenter Pilot Randomized Controlled Trial of Remote Ischemic Preconditioning in Major Vascular Surgery. Vasc. Endovasc. Surg. 2015, 49, 220–227. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Moretti, C.; Omedè, P.; Cerrato, E.; Cavallero, E.; Er, F.; Presutti, D.G.; Colombo, F.; Crimi, G.; Conrotto, F. Cardiac remote ischaemic preconditioning reduces periprocedural myocardial infarction for patients undergoing percutaneous coronary interventions: A meta−analysis of randomised clinical trials. Euro Intervention 2014, 9, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Alreja, G.; Bugano, D.; Lotfi, A. Effect of remote ischemic preconditioning on myocardial and renal injury: Meta−analysis of randomized controlled trials. J. Invasive Cardiol. 2012, 24, 42–48. [Google Scholar] [PubMed]
- Er, F.; Nia, A.M.; Dopp, H.; Hellmich, M.; Dahlem, K.M.; Caglayan, E.; Kubacki, T.; Benzing, T.; Erdmann, E.; Burst, V.; Gassanov, N. Randomized Pilot Ren Pro Trial (Renal Protection Trial). Ischemic Preconditioning for Prevention of Contrast Medium−Induced Nephropathy. Circulation 2012, 126, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ghaemian, A.; Nouraei, S.; Abdollahian, F.; Naghshvar, F.; Giussani, D.A.; Nouraei, S.A. Remote ischemic preconditioning in percutaneous coronary revascularization: A double−blind randomized controlled clinical trial. Asian Cardiovasc. Thorac. Ann. 2012, 20, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Mwamure, P.K.; Venugopal, V.; Harris, J.; Barnard, M.; Grundy, E.; Ashley, E.; Vichare, S.; Di Salvo, C.; Kolvekar, S.; et al. Effect of remote ischemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet 2007, 370, 575–579. [Google Scholar] [CrossRef]
- Davel, A.P.; Wenceslau, C.F.; Akamine, E.H.; Xavier, F.E.; Couto, G.K.; Oliveira, H.T.; Rossoni, L.V. Endothelial dysfunction in cardiovascular and endocrine−metabolic diseases: An update. Braz. J. Med. Biol. Res. 2011, 44, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Della-Morte, D.; Dave, K.R.; Sacco, R.L.; Perez-Pinzon, M.A. Biomarkers for ischemic preconditioning: Finding the responders. J. Cereb. Blood Flow Metab. 2014, 34, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.P.; He, F.; Liu, X.Q.; Zhang, J.Y. Long−term, regular remote ischemic preconditioning improves endothelial function in patients with coronary heart disease. Braz. J. Med. Biol. Res. 2015, 48, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.B.; Yang, W.X.; Fu, X.H.; Zhao, L.F.; Gao, J.L. Remote ischemic precondition prevents radial artery endothelial dysfunction induced by ischemia and reperfusion based on a cyclooxygenase−2−dependent mechanism. Int. J. Clin. Exp. Med. 2015, 8, 20946–20952. [Google Scholar] [PubMed]
- Heusch, G.; Bøtker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote Ischemic Conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.; Buchholz, B.; Rodríguez, M.; Pérez, V.; Inserte, J.; García-Dorado, D.; Gelpi, R.J. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischemic preconditioning. Exp. Physiol. 2012, 98, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Mastitskaya, S.; Marina, N.; Gourine, A.; Gilbey, M.P.; Spyer, K.M.; Teschemacher, A.G.; Kasparov, S.; Trapp, S.; Ackland, G.L.; Gourine, A.V. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre−ganglionic neurones. Cardiovasc. Res. 2012, 95, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Remote ischaemic preconditioning: Underlying mechanisms and clinical application. Cardiovasc. Res. 2008, 79, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Manchurov, V.; Ryazankina, N.; Khmara, T.; Skrypnik, D.; Reztsov, R.; Vasilieva, E.; Shpektor, A. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am. J. Med. 2014, 127, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Huveneers, S.; Daemen, M.J.; Hordijk, P.L. Between Rho(k) and a hard place: The relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ. Res. 2015, 116, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Rucka, D.; Marek, J.; Rucklova, Z.; Lubanda, J.C.; Havranek, S.; Skvaril, J.; Varejka, P.; Chochola, M.; Karetova, D.; Korinek, J.; et al. Arterial Stiffening Contributes to Impairment of Cerebrovascular Reactivity in Patients With Coronary Artery Disease Without Carotid Stenosis. Physiol. Res. 2015, 64, 335–343. [Google Scholar] [PubMed]
- Veerasamy, M.; Ford, G.A.; Neely, D.; Bagnall, A.; MacGowan, G.; Das, R.; Kunadian, V. Association of aging, arterial stiffness, and cardiovascular disease: A review. Cardiol. Rev. 2014, 22, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Mortensen, U.M.; White, P.A.; Kristiansen, S.B.; Schmidt, M.R.; Hoschtitzky, J.A.; Vogel, M.; Sorensen, K.; Redington, A.N.; MacAllister, R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002, 106, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, M.; Kottenberg, E.; Kleinbongard, P.; Wendt, D.; Gedik, N.; Pasa, S.; Price, V.; Tsagakis, K.; Neuhäuser, M.; Peters, J.; et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: A single−centre randomised, doubleblind, controlled trial. Lancet 2013, 382, 597–604. [Google Scholar] [CrossRef]
- Hoole, S.P.; Heck, P.M.; Sharples, L.; Khan, S.N.; Duehmke, R.; Densem, C.G.; Clarke, S.C.; Shapiro, L.M.; Schofield, P.M.; O’Sullivan, M.; Dutka, D.P. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: A prospective, randomized control trial. Circulation 2009, 119, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Konstantinov, I.E.; Kharbanda, R.K.; Cheung, M.H.; Redington, A.N. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol. (Oxf.) 2007, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Enko, K.; Nakamura, K.; Yunoki, K.; Miyoshi, T.; Akagi, S.; Yoshida, M.; Toh, N.; Sangawa, M.; Nishii, N.; Nagase, S.; et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J. Physiol. Sci. 2011, 61, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Loukogeorgakis, S.P.; Panagiotidou, A.T.; Broadhead, M.W.; Donald, A.; Deanfield, J.E.; MacAllister, R.J. Remote ischemic preconditioning provides early and late protection against endothelial ischemia−reperfusion injury in humans: Role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005, 46, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, E.-M.; Zhou, P.; Frys, K.; Ross, M.E.; Iadecola, C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J. Cereb. Blood Flow Metab. 2005, 25, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xing, B.; Liu, X.; Zhan, B.; Zhou, J.; Zhu, H.; Chen, Z. Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl. Int. 2008, 21, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.; Nishimura, H.; Iwakura, A.; Wecker, A.; Eaton, E.; Asahara, T.; Losordo, D.W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 2005, 111, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Kawata, H.; Yoshida, K.; Kawamoto, A.; Kurioka, H.; Takase, E.; Sasaki, Y.; Hatanaka, K.; Kobayashi, M.; Ueyama, T.; Hashimoto, T.; Dohi, K. Ischemic preconditioning upregulates vascular endothelial growth factor mRNA expression and neovascularization via nuclear translocation of protein kinase C epsilon in the rat ischemic myocardium. Circ. Res. 2001, 88, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Caru, M.; Lalonde, F.; Gravel, H.; Daigle, C.; Tournoux, F.; Jacquemet, V.; Curnier, D. Remote preconditioning shortens QT intervals during exercise in healthy subjects. Eur. J. Sport Sci. 2016, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.R.; Hsu, A.K.; Urban, T.J.; Mochly-Rosen, D.; Gross, G.J. Nociceptive−induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res. Cardiol. 2013, 108, 381. [Google Scholar] [CrossRef] [PubMed]
- Steensrud, T.; Li, J.; Dai, X.; Manlhiot, C.; Kharbanda, R.K.; Tropak, M.; Redington, A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra−arterial adenosine. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1598–H1603. [Google Scholar] [CrossRef] [PubMed]
- Merlocco, A.C.; Redington, K.L.; Disenhouse, T.; Strantzas, S.C.; Gladstone, R.; Wei, C.; Tropak, M.B.; Manlhiot, C.; Li, J.; Redington, A.N. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res. Cardiol. 2014, 109, 406. [Google Scholar] [CrossRef] [PubMed]
| Parameters | CHD, n = 30 | Control, n = 20 |
|---|---|---|
| Age, (in years) | 63.9 ± 1.6 | 58.2 ± 2.49 |
| Male (n)/Female (n) | 21/9 | 16/4 |
| Height, cm | 169.7 ± 1.7 | 173.45 ± 1.5 |
| Weight, kg | 81.4 ± 2.2 | 82.45 ± 3.4 |
| Body mass index, kg/m2 | 28.27 ± 1.6 | 27.4 ± 1.1 |
| NYHA (n) | ||
| II | 26 (86.7%) | 0 |
| III | 4 (13.3%) | 0 |
| Arterial hypertension, n | 23 (76.7%) | 6 (30.0%) |
| Parameter | Sham Ischemic Preconditioning | Ischemic Preconditioning | ||||
|---|---|---|---|---|---|---|
| Baseline | After sRIPC | Delta | Baseline | After RIPC | Delta | |
| SBP, mm Hg | 118.6 ± 2.8 | 115.6 ± 3.7 | 3.0 ± 1.8 | 123.1 ± 2.6 | 120.1 ± 2.9 | 3.0 ± 2.8 |
| DBP, mm Hg | 74.6 ± 1.4 | 75.5 ± 1.8 | −0.9 ± 0.8 | 77.6 ± 2.2 | 77.1 ± 1.9 | 0.5 ± 2.2 |
| Central SBP, mm Hg | 108.3 ± 3.5 | 106.5 ± 2.5 | 1.8 ± 1.9 | 111.2 ± 2.5 | 110.2 ± 2.9 | 1.0 ± 2.9 |
| Central DBP, mm Hg | 75.9 ± 1.7 | 78.6 ± 1.8 | −2,7 ± 2.1 | 78.6 ± 1.8 | 77.5 ± 1.8 | 1.1 ± 2.1 |
| Рр, mm Hg | 33.9 ± 2.1 | 30.6 ± 3.1 | −3.3 ± 1.0 | 32.6 ± 2.2 | 32.7 ± 2.4 | −0.1 ± 1.5 |
| SpO2, % | 97.9 ± 0.2 | 97.3 ± 0.28 | 0.6 ± 1.1 | 97.7 ± 0.3 | 97.7 ± 0.3 | 0.07 ± 1.4 |
| АP (augmentation pressure), % | 9.7 ± 1.8 | 9.0 ± 2.3 | 0.7 ± 1.2 | 6.5 ± 1.3 | 8.4 ± 1.5 | −1.9 ± 1.4 |
| Pp amplification, % | 133.2 ± 4.5 | 132.7 ± 4.1 | 0.5 ± 3.1 | 140.9 ± 4.7 | 133.7 ± 3.8 | 7.2 ± 3.1 |
| PWV, m/s | 7.22 ± 0.59 | 7.2 ± 0.49 | −0.02 ± 0.19 | 7.33 ± 0.63 | 7.35 ± 0.6 | −0.02 ± 0.29 |
| Heart rate, min | 68.1 ± 2.8 | 65.5 ± 2.7 | 2.6 ± 0.77 | 68.1 ± 2.8 | 65.5 ± 2.7 | 2.6 ± 0.87 |
| Triangular index | 6.85 ± 0.51 | 7.1 ± 0.51 | −0.25 ± 0.5 | 5.75 ± 0.28 | 6.4 ± 0.6 | −0.65 ± 0.6 |
| LF norm | 59.4 ± 5.1 | 66.6 ± 4.4 | −7.2 ± 4.2 | 58.9 ± 8.5 | 68.9 ± 8.4 | −10.0 ± 11.1 |
| HF norm | 40.6 ± 5.1 | 33.4 ± 4.4 | 7.2 ± 4.5 | 41.1 ± 8.5 | 31.0 ± 4.8 | 10.1 ± 10.5 |
| LF:HF ratio | 2.0 ± 0.39 | 3.0 ± 0.74 | −1.0 ± 0.7 | 1.43 ± 0.9 | 2.22 ± 0.9 | −0.79 ± 1.0 |
| TP | 753.1 ± 300.2 | 661.9 ± 151.6 | 91.2 ± 91.1 | 1096.3 ± 431.5 | 627.1 ± 238.5 | 469.2 ± 91.1 |
| Parameter | Sham Ischemic Preconditioning | Ischemic Preconditioning | ||||
|---|---|---|---|---|---|---|
| Base | After sRIPC | ∆ | Base | After RIPC | ∆ | |
| SBP, mm Hg | 137.0 ± 3.3 | 120.1 ± 3.2 * | 16.9 ± 2.4 | 134.7 ± 5.3 | 119.4 ± 4.0 * | 15.3 ± 3.5 |
| DBP, mm Hg | 84.7 ± 1.8 | 77.7 ± 1.8 | 7.0 ± 1.4 | 79.2 ± 2.7 | 75.2 ± 2.3 | 4.0 ± 2.8 |
| Central SBP, mm Hg | 134.9 ± 3.7 | 122.1 ± 3.8 * | 12.8 ± 3.1 | 132.9 ± 5.8 | 118.5 ± 4.0 * | 14.4 ± 3.9 |
| Central DBP, mm Hg | 84.0 ± 1.9 | 78.4 ± 2.0 | 5.6 ± 1.6 | 78.7 ± 2.8 | 76.1 ± 2.3 | 2.6 ± 1.8 |
| Pp, mm Hg | 50.7 ± 2.8 | 44.1 ± 2.9 | 6.6 ± 2.1 | 54.3 ± 4.2 | 42.25 ± 2.3 * | 12.0 ± 3.9 |
| SpO2, % | 97.2 ± 0.13 | 97.93 ± 0.2 | −0.73 ± 0.25 | 97.2 ± 0.4 | 97.3 ± 0.3 | −0.1 ± 0.3 |
| АP, % | 127.2 ± 1.8 | 125.8 ± 2.7 | 1.4 ± 2.05 | 131.4 ± 2.6 | 127.2 ± 1.8 * | 4.2 ± 2.1 |
| Pp, amplification, % | 12.3 ± 1 | 11.68 ± 1.2 | 0.62 ± 1.39 | 12.3 ± 1.0 | 11.7 ± 1.2 | 0.5 ± 1.5 |
| PWV, m/sec | 5.5 ± 0.6 | 6.0 ± 0.63 | −0.5 ± 0.3 | 5.37 ± 0.8 | 4.95 ± 0.49 | 0.42 ± 0.53 |
| Heart rate, min | 62.5 ± 2.2 | 61.2 ± 2.4 | 1.3 ± 0.9 | 68.6 ± 3.1 | 63.9 ± 3.0 | 4.7 ± 1.16 |
| Triangular index | 5.78 ± 0.46 | 6.7 ± 0.59 | −0.92 ± 0.7 | 6.0 ± 0.46 | 9.0 ± 1.1 * | −3.0 ± 1.04 |
| LF norm | 26.4 ± 3.6 | 31.8 ± 4.2 | −5.4 ± 3.8 | 42.7 ± 5.5 | 48.1 ± 7.2 | −5.4 ± 2.2 |
| HF norm | 73.6 ± 3.6 | 68.2 ± 4.2 | 5.4 ± 3.8 | 57.3 ± 5.5 | 55.5 ± 7.2 | 1.8 ± 4.27 |
| LF:HF ratio | 0.38 ± 0.07 | 0.51 ± 0.12 | −0.13 ± 0.11 | 0.75 ± 0.3 | 0.87 ± 0.25 | −0.12 ± 0.2 |
| TP | 2767.5 ± 1912.6 | 1736.1 ± 544.9 | 1031.4 ± 747.7 | 1077.4 ± 451.5 | 1156.1 ± 223.5 | −78.7 ± 84.2 |
| Parameters | ∆ RIPC, Control Group, n = 20 | ∆ RIPC, CHD Group, n = 30 | p |
|---|---|---|---|
| SBP, mm Hg | 3.0 ± 2.8 | 15.3 ± 3.5 * | 0.028 |
| DBP, mm Hg | 0.5 ± 2.2 | 4.0 ± 2.8 | 0.19 |
| Central SBP, mm Hg | 1.0 ± 2.9 | 14.4 ± 3.9 * | 0.009 |
| Central DBP, mm Hg | 1.1 ± 2.1 | 2.6 ± 1.8 | 0.31 |
| Pp, mm Hg | −0.1 ± 1.5 | 12.0 ± 3.9 ** | 0.008 |
| SpO2, % | 0.07 ± 1.4 | −0.1 ± 0.3 | 0.26 |
| АP, % | −1.9 ± 1.4 | 4.2 ± 2.1 * | 0.041 |
| Pp, amplification,% | 7.2 ± 3.1 | 0.5 ± 1.5 | 0.086 |
| PWV, m/s | −0.02 ± 0.29 | 0.42 ± 0.53 | 0.101 |
| HR, beat/min | 2.6 ± 0.87 | 4.7 ± 1.16 | 0.066 |
| Triangular index | −0.65 ± 0.6 | −3.0 ± 1.04 | 0.057 |
| LF norm | −10.0 ± 11.1 | −5.4 ± 2.2 | 0.47 |
| HF norm | 10.1 ± 10.5 | 1.8 ± 4.27 | 0.067 |
| LF:HF ratio | −0.79 ± 1.0 | −0.12 ± 0.2 | 0.076 |
| TP | 469.2 ± 91.1 | −78.7 ± 84.2 ** | 0.006 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagidullin, N.; Scherbakova, E.; Safina, Y.; Zulkarneev, R.; Zagidullin, S. The Impact of Remote Ischemic Preconditioning on Arterial Stiffness and Heart Rate Variability in Patients with Angina Pectoris. J. Clin. Med. 2016, 5, 60. https://doi.org/10.3390/jcm5070060
Zagidullin N, Scherbakova E, Safina Y, Zulkarneev R, Zagidullin S. The Impact of Remote Ischemic Preconditioning on Arterial Stiffness and Heart Rate Variability in Patients with Angina Pectoris. Journal of Clinical Medicine. 2016; 5(7):60. https://doi.org/10.3390/jcm5070060
Chicago/Turabian StyleZagidullin, Naufal, Elena Scherbakova, Yuliana Safina, Rustem Zulkarneev, and Shamil Zagidullin. 2016. "The Impact of Remote Ischemic Preconditioning on Arterial Stiffness and Heart Rate Variability in Patients with Angina Pectoris" Journal of Clinical Medicine 5, no. 7: 60. https://doi.org/10.3390/jcm5070060






