Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids
Abstract
:1. Introduction
2. Why Study Fatty Acid Functions Using C. elegans?
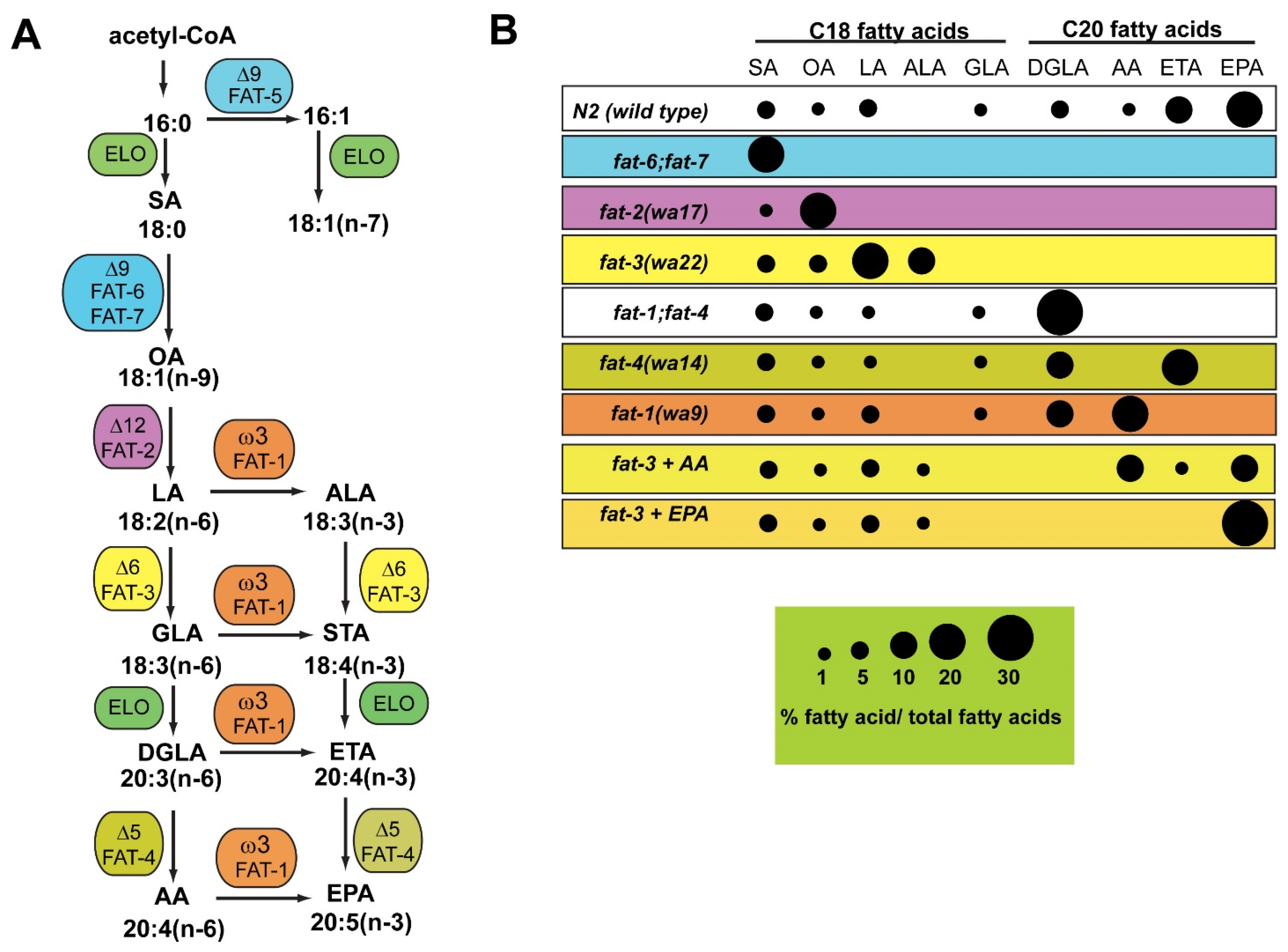
3. C. elegans Mutants Lacking Fatty Acid Desaturase Activity Are Useful for Studies of Conserved Functions of Omega-6 and Omega-3 Fatty Acids
4. Functions of Omega-6 and Omega-3 Fatty Acids in C. elegans Reproduction: Sperm Guidance and Germ Cell Maintenance
5. Functions of Omega-6 and Omega-3 Fatty Acids in Longevity: Critical Roles for ∆9 Desaturases
6. Functions of Omega-6 and Omega-3 Fatty Acids in C. elegans Neurons: Synaptic Vesicle Formation, Signal Transduction in Sensory Neurons, and Complex Behavioral Responses to Alcohol and Oxygen
7. Using the C. elegans Fat-1 Gene for Studies in Mammals: Endogenous Omega-3 Functions and Biotechnology Applications
8. Conclusions and Future Studies
Acknowledgments
Conflicts of Interest
References
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci. 2002, 27, 467. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Antonny, B.; Vanni, S.; Shindou, H.; Ferreira, T. From zero to six double bonds: Phospholipid unsaturation and organelle function. Trends Cell Biol. 2015, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bigay, J.; Antonny, B. Curvature, lipid packing, and electrostatics of membrane organelles: Defining cellular territories in determining specificity. Dev. Cell 2012, 23, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.L.; Mitchell, D.C.; Lim, S.Y.; Wen, Z.M.; Kim, H.Y.; Salem, N., Jr.; Litman, B.J. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J. Biol. Chem. 2004, 279, 31098–31104. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Kim, H.Y. Cytochrome p450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, C. Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist 2015, 21, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bab, R.; Biro, T.; Cabral, G.A.; Dey, S.K.; di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Olefsky, J.M. Omega 3 fatty acids and GPR120. Cell Metab. 2012, 15, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur. J. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.K.; Liang, B.; Watts, J.L. The influence of bacterial diet on fat storage in C. elegans. PLoS ONE 2009, 4, e7545. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L. Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends Endocrinol. Metab. 2009, 20, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Spychalla, J.P.; Kinney, A.J.; Browse, J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Peyou-Ndi, M.M.; Watts, J.L.; Browse, J. Identification and characterization of an animal delta(12) fatty acid desaturase gene by heterologous expression in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2000, 376, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. Isolation and characterization of a delta 5-fatty acid desaturase from Caenorhabditis elegans. Arch. Biochem. Biophys. 1999, 362, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 5854–5859. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Phillips, E.; Griffing, K.R.; Browse, J. Deficiencies in C20 polyunsaturated fatty acids cause behavioral and developmental defects in Caenorhabditis elegans fat-3 mutants. Genetics 2003, 163, 581–589. [Google Scholar] [PubMed]
- Deline, M.L.; Vrablik, T.L.; Watts, J.L. Dietary supplementation of polyunsaturated fatty acids in Caenorhabditis elegans. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kahn-Kirby, A.H.; Dantzker, J.L.; Apicella, A.J.; Schafer, W.R.; Browse, J.; Bargmann, C.I.; Watts, J.L. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 2004, 119, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. A palmitoyl-CoA-specific delta9 fatty acid desaturase from Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2000, 272, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.J.; Browse, J.; Watts, J.L. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics 2007, 176, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, T.L.; Watts, J.L. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol. Reprod. Dev. 2013, 80, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Kubagawa, H.M.; Watts, J.L.; Corrigan, C.; Edmonds, J.W.; Sztul, E.; Browse, J.; Miller, M.A. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 2006, 8, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J.W.; Prasain, J.K.; Dorand, D.; Yang, Y.; Hoang, H.D.; Vibbert, J.; Kubagawa, H.M.; Miller, M.A. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell 2010, 19, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.D.; Prasain, J.K.; Dorand, D.; Miller, M.A. A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 2013, 9, e1003271. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Wilson, L.; Hoang, H.D.; Moore, R.; Miller, M.A. Comparative lipidomics of Caenorhabditis elegans metabolic disease models by swath non-targeted tandem mass spectrometry. Metabolites 2015, 5, 677–696. [Google Scholar] [CrossRef] [PubMed]
- McKnight, K.; Hoang, H.D.; Prasain, J.K.; Brown, N.; Vibbert, J.; Hollister, K.A.; Moore, R.; Ragains, J.R.; Reese, J.; Miller, M.A. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science 2014, 344, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.L.; Browse, J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Dev. Biol. 2006, 292, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.M.; Deline, M.L.; Watts, J.L. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev. Biol. 2013, 373, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Deline, M.; Keller, J.; Rothe, M.; Schunck, W.H.; Menzel, R.; Watts, J.L. Epoxides derived from dietary dihomo-gamma-linolenic acid induce germ cell death in C. elegans. Sci. Rep. 2015, 5, 15417. [Google Scholar] [CrossRef] [PubMed]
- Amrit, F.R.; Ratnappan, R.; Keith, S.A.; Ghazi, A. The C. elegans lifespan assay toolkit. Methods 2014, 68, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.A.; Brunet, A. Lipid profiles and signals for long life. Trends Endocrinol. Metab. 2015, 26, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Kelly, M.A.; Abbott, S.K. Polyunsaturated fats, membrane lipids and animal longevity. J. Comp. Physiol. B Biochem.Syst. Environ. Physiol. 2014, 184, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Shmookler-Reis, R.J.; Xu, L.; Lee, H.; Chae, M.; Thaden, J.J.; Bharill, P.; Tazearslan, C.; Siegel, E.; Alla, R.; Zimniak, P.; et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging 2011, 3, 125–147. [Google Scholar] [PubMed]
- Sugawara, S.; Honma, T.; Ito, J.; Kijima, R.; Tsuduki, T. Fish oil changes the lifespan of Caenorhabditis elegans via lipid peroxidation. J. Clin. Biochem. Nutr. 2013, 52, 139–145. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Kuballa, P.; Xavier, R.; Ruvkun, G. Omega-6 polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013, 27, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Doonan, R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle 2009, 8, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Ishii, N.; Suzuki, K.; Matsuo, M. Oxygen-dependent perturbation of life span and aging rate in the nematode. J. Gerontol. 1993, 48, B57–B61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [PubMed]
- Van Raamsdonk, J.M.; Hekimi, S. Deletion of the mitochondrial superoxide dismutase SOD-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000361. [Google Scholar] [CrossRef] [PubMed]
- Cypser, J.R.; Johnson, T.E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B109–B114. [Google Scholar] [CrossRef] [PubMed]
- Cypser, J.R.; Tedesco, P.; Johnson, T.E. Hormesis and aging in Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Schaar, C.E.; Dues, D.J.; Spielbauer, K.K.; Machiela, E.; Cooper, J.F.; Senchuk, M.; Hekimi, S.; van Raamsdonk, J.M. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 2015, 11, e1004972. [Google Scholar] [CrossRef] [PubMed]
- Back, P.; Braeckman, B.P.; Matthijssens, F. ROS in aging Caenorhabditis elegans: Damage or signaling? Oxid. Med. Cell. Longev. 2012, 2012, 608478. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.; Yang, W.; Hekimi, S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 2014, 157, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ros signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Dancy, B.C.; Chen, S.W.; Drechsler, R.; Gafken, P.R.; Olsen, C.P. 13C- and 15N-Labeling strategies combined with mass spectrometry comprehensively quantify phospholipid dynamics in C. elegans. PLoS ONE 2015, 10, e0141850. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2010, 1204, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Flatt, T.; Aguilaniu, H. Reproduction, fat metabolism, and life span: What is the connection? Cell Metab. 2013, 17, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.J.; Browse, J.; Watts, J.L. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006, 2, e108. [Google Scholar] [CrossRef] [PubMed]
- Van Gilst, M.R.; Hadjivassiliou, H.; Jolly, A.; Yamamoto, K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005, 3, e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnappan, R.; Amrit, F.R.; Chen, S.W.; Gill, H.; Holden, K.; Ward, J.; Yamamoto, K.R.; Olsen, C.P.; Ghazi, A. Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet. 2014, 10, e1004829. [Google Scholar] [CrossRef] [PubMed]
- Goudeau, J.; Bellemin, S.; Toselli-Mollereau, E.; Shamalnasab, M.; Chen, Y.; Aguilaniu, H. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011, 9, e1000599. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.R.; Gelino, S.; Melendez, A.; Hansen, M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol. 2011, 21, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; O’Rourke, E.J.; Ruvkun, G. Fat metabolism links germline stem cells and longevity in C. elegans. Science 2008, 322, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Folick, A.; Oakley, H.D.; Yu, Y.; Armstrong, E.H.; Kumari, M.; Sanor, L.; Moore, D.D.; Ortlund, E.A.; Zechner, R.; Wang, M.C. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 2015, 347, 83–86. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kolderup, A.; Svihus, B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J. Nutr. Metab. 2015, 2015, 823081. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Murphy, C.T.; Kenyon, C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009, 10, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jeong, D.E.; Son, H.G.; Yamaoka, Y.; Kim, H.; Seo, K.; Khan, A.A.; Roh, T.Y.; Moon, D.W.; Lee, Y.; et al. SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat. Genes Dev. 2015, 29, 2490–2503. [Google Scholar] [CrossRef] [PubMed]
- Taubert, S.; van Gilst, M.R.; Hansen, M.; Yamamoto, K.R. A mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006, 20, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Jacobs, R.L.; Watts, J.L.; Rottiers, V.; Jiang, K.; Finnegan, D.M.; Shioda, T.; Hansen, M.; Yang, F.; Niebergall, L.J.; et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 2011, 147, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Vought, B.W.; Satterlee, J.S.; Walker, A.K.; Jim Sun, Z.Y.; Watts, J.L.; DeBeaumont, R.; Saito, R.M.; Hyberts, S.G.; Yang, S.; et al. An ARC/mediator subunit required for SREBPP control of cholesterol and lipid homeostasis. Nature 2006, 442, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Steinbaugh, M.J.; Narasimhan, S.D.; Robida-Stubbs, S.; Moronetti Mazzeo, L.E.; Dreyfuss, J.M.; Hourihan, J.M.; Raghavan, P.; Operana, T.N.; Esmaillie, R.; Blackwell, T.K. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.A.; Dalton, H.M.; Sowa, J.N.; Wang, M.C.; Soukas, A.A.; Curran, S.P. Omega-3 and -6 fatty acids allocate somatic and germline lipids to ensure fitness during nutrient and oxidative stress in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 15378–15383. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Begg, D.; Mathai, M.; Weisinger, R.S. Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pac. J. Clin.Nutr. 2007, 16, 391–397. [Google Scholar] [PubMed]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [CrossRef]
- Lesa, G.M.; Palfreyman, M.; Hall, D.H.; Clandinin, M.T.; Rudolph, C.; Jorgensen, E.M.; Schiavo, G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 2003, 116, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, D.M.; Altshuler-Keylin, S.; Lee, J.I.; L’Etoile, N.D. Regulators of AWC-mediated olfactory plasticity in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000761. [Google Scholar] [CrossRef] [PubMed]
- Pastuhov, S.I.; Fujiki, K.; Nix, P.; Kanao, S.; Bastiani, M.; Matsumoto, K.; Hisamoto, N. Endocannabinoid-goalpha signalling inhibits axon regeneration in Caenorhabditis elegans by antagonizing gqalpha-PKC-JNK signalling. Nat. Commun. 2012, 3, 1136. [Google Scholar] [CrossRef] [PubMed]
- Lucanic, M.; Held, J.M.; Vantipalli, M.C.; Klang, I.M.; Graham, J.B.; Gibson, B.W.; Lithgow, G.J.; Gill, M.S. N-Acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature 2011, 473, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Raabe, R.C.; Mathies, L.D.; Davies, A.G.; Bettinger, J.C. The omega-3 fatty acid eicosapentaenoic acid is required for normal alcohol response behaviors in C. elegans. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Davies, A.G.; Bettinger, J.C.; Thiele, T.R.; Judy, M.E.; McIntire, S.L. Natural variation in the NPR-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron 2004, 42, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, V.; Krieg, M.; Lockhead, D.; Goodman, M.B. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Rep. 2014, 6, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kosel, M.; Wild, W.; Bell, A.; Rothe, M.; Lindschau, C.; Steinberg, C.E.; Schunck, W.H.; Menzel, R. Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem. J. 2011, 435, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kulas, J.; Schmidt, C.; Rothe, M.; Schunck, W.H.; Menzel, R. Cytochrome P450-dependent metabolism of eicosapentaenoic acid in the nematode Caenorhabditis elegans. Arch. Biochem. Biophys. 2008, 472, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Falck, J.R.; Rothe, M.; Schunck, W.H.; Menzel, R. Role of CYP eicosanoids in the regulation of pharyngeal pumping and food uptake in Caenorhabditis elegans. J. Lipid Res. 2015, 56, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Rothe, M.; Zheng, S.; Bhatla, N.; Pender, C.L.; Menzel, R.; Horvitz, H.R. Cytochrome P450 drives a HIF-regulated behavioral response to reoxygenation by C. elegans. Science 2013, 341, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Ellieva, A.; Ma, D.K.; Ju, J.J.; Nehk, E.; Konkel, A.; Falck, J.R.; Schunck, W.H.; Menzel, R. CYP-13A12 of the nematode Caenorhabditis elegans is a PUFA-epoxygenase involved in behavioural response to reoxygenation. Biochem. J. 2014, 464, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Wang, J.; Wu, L.; Kang, Z.B. Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004, 427, 504. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Kendall, A.C.; Dennis, E.A.; Nicolaou, A. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim. Biophys. Acta 2015, 1851, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. From fat to fat-1: A tale of omega-3 fatty acids. J. Membr. Biol. 2005, 206, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. Fat-1 transgenic mice: A new model for omega-3 research. Prostaglandins Leukot Essent Fat. Acids 2007, 77, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. The omega-6/omega-3 fatty acid ratio in chronic diseases: Animal models and molecular aspects. World Rev. Nutr. Diet. 2011, 102, 22–29. [Google Scholar] [PubMed]
- Kang, J.X.; Liu, A. The role of the tissue omega-6/omega-3 fatty acid ratio in regulating tumor angiogenesis. Cancer Metastasis Rev. 2013, 32, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Weylandt, K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. Sub-Cell. Biochem. 2008, 49, 133–143. [Google Scholar]
- Bak, D.H.; Zhang, E.; Yi, M.H.; Kim, D.K.; Lim, K.; Kim, J.J.; Kim, D.W. High omega3-polyunsaturated fatty acids in fat-1 mice prevent streptozotocin-induced purkinje cell degeneration through bdnf-mediated autophagy. Sci. Rep. 2015, 5, 15465. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, B.; Li, X.; Kang, J.X. Endogenously elevated n-3 polyunsaturated fatty acids alleviate acute ethanol-induced liver steatosis. BioFactors 2015, 41, 453–462. [Google Scholar] [PubMed]
- Hwang, W.M.; Bak, D.H.; Kim, D.H.; Hong, J.Y.; Han, S.Y.; Park, K.Y.; Lim, K.; Lim, D.M. Attenuation of streptozotocin-induced pancreatic beta cell death in transgenic fat-1 mice via autophagy activation. Endocrinol. Metab. 2015. Available online: http://e-enm.org/DOIx.php?id=10.3803/EnM.2015.30.e24 (accessed on 2 February 2016).
- Li, X.; Ballantyne, L.L.; Che, X.; Mewburn, J.D.; Kang, J.X.; Barkley, R.M.; Murphy, R.C.; Yu, Y.; Funk, C.D. Endogenously generated omega-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J. Am. Heart Assoc. 2015, 4, e001856. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Human requirement for n-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Kang, J.X.; Li, R.; Wang, J.; Witt, W.T.; Yong, H.Y.; Hao, Y.; Wax, D.M.; Murphy, C.N.; Rieke, A.; et al. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006, 24, 435–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, Y.; Dou, H.; Yin, J.; Chen, Y.; Pang, X.; Vajta, G.; Bolund, L.; Du, Y.; Ma, R.Z. Handmade cloned transgenic piglets expressing the nematode fat-1 gene. Cell. Reprogram. 2012, 14, 258–266. [Google Scholar] [PubMed]
- Zhou, Y.; Lin, Y.; Wu, X.; Feng, C.; Long, C.; Xiong, F.; Wang, N.; Pan, D.; Chen, H. The high-level accumulation of n-3 polyunsaturated fatty acids in transgenic pigs harboring the n-3 fatty acid desaturase gene from caenorhabditis briggsae. Transgenic Res. 2014, 23, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liu, X.F.; Ding, X.B.; Yang, F.F.; Nie, Y.W.; An, Y.J.; Guo, H. Fat-1 transgenic cattle as a model to study the function of omega-3 fatty acids. Lipids Health Dis. 2011, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ouyang, H.; Duan, B.; Pang, D.; Zhang, L.; Yuan, T.; Xue, L.; Ni, D.; Cheng, L.; Dong, S.; et al. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res. 2012, 21, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Pohlmeier, W.E.; Hovey, R.C.; Van Eenennaam, A.L. Reproductive abnormalities in mice expressing omega-3 fatty acid desaturase in their mammary glands. Transgenic Res. 2011, 20, 283–292. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watts, J.L. Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. J. Clin. Med. 2016, 5, 19. https://doi.org/10.3390/jcm5020019
Watts JL. Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. Journal of Clinical Medicine. 2016; 5(2):19. https://doi.org/10.3390/jcm5020019
Chicago/Turabian StyleWatts, Jennifer L. 2016. "Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids" Journal of Clinical Medicine 5, no. 2: 19. https://doi.org/10.3390/jcm5020019




