Importance of Beta Cell Function for the Treatment of Type 2 Diabetes
Abstract
:1. Introduction
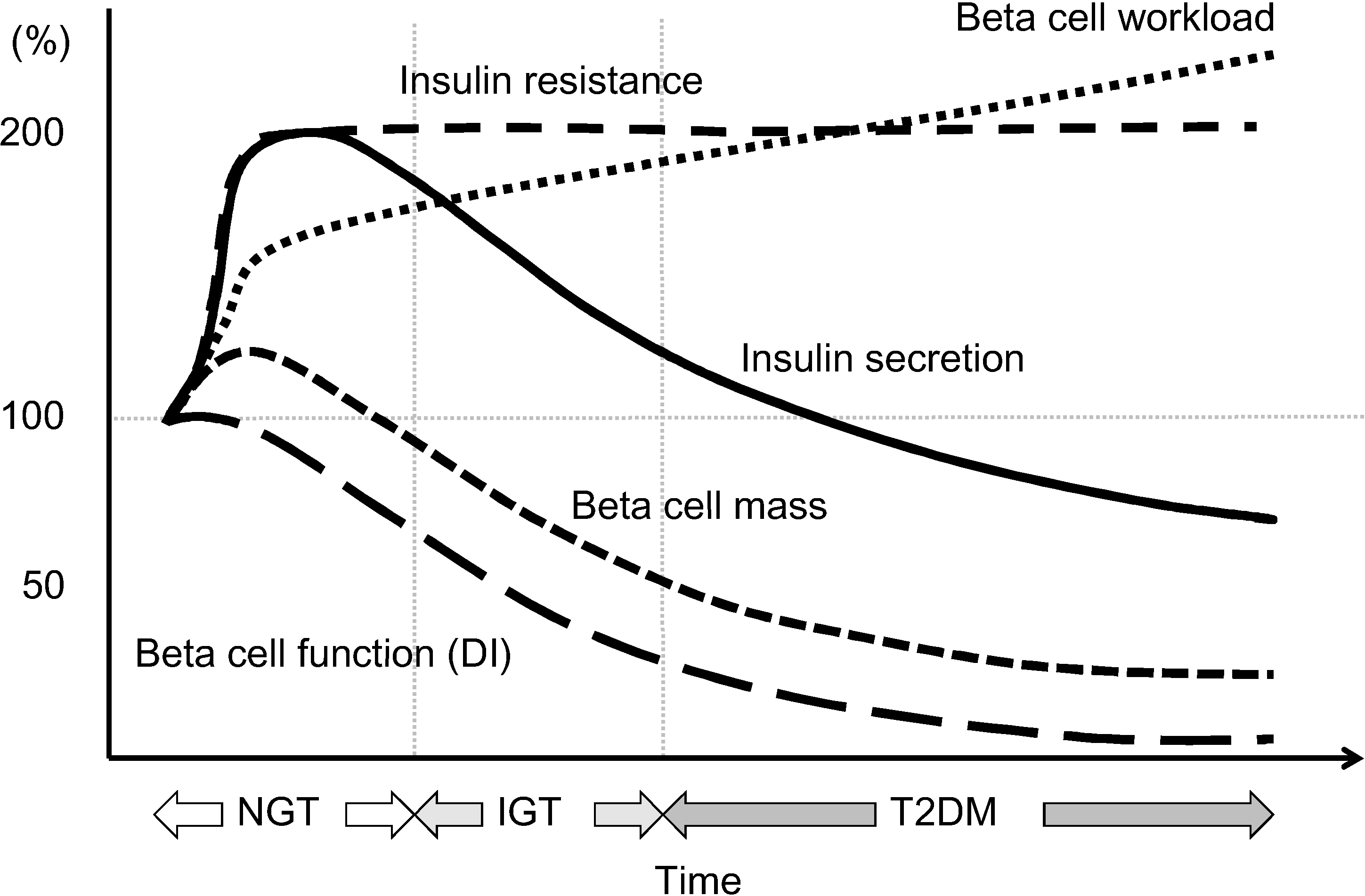
2. Deficit of Beta Cell Function and Mass in T2DM
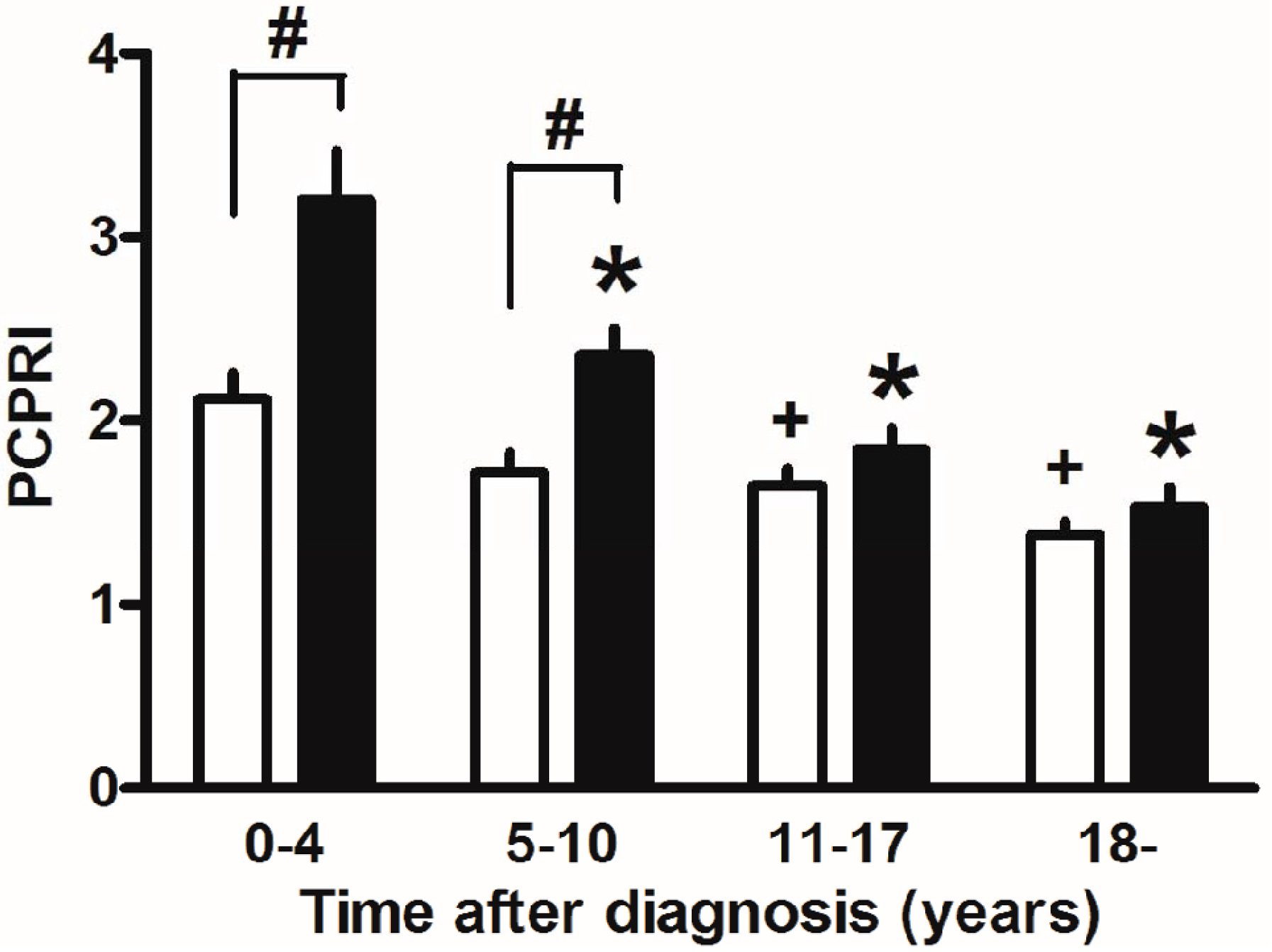
3. Beta Cell Function and Glycemic Control
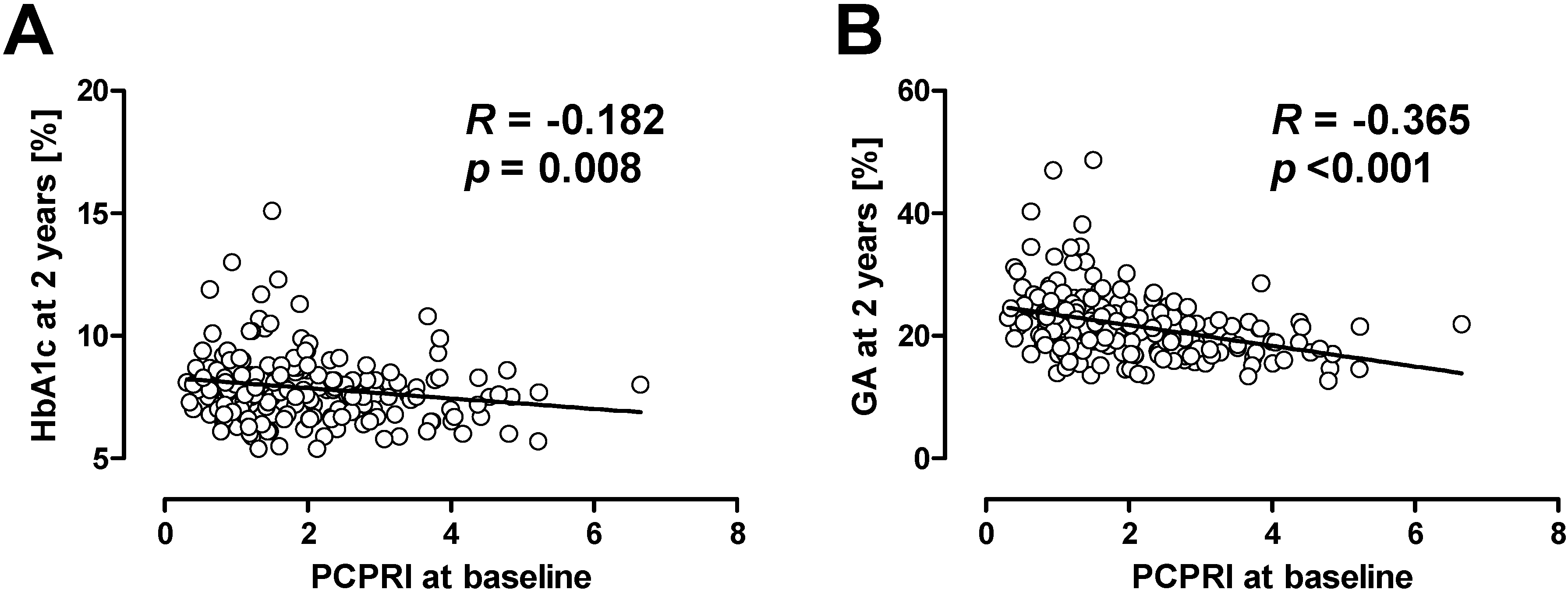
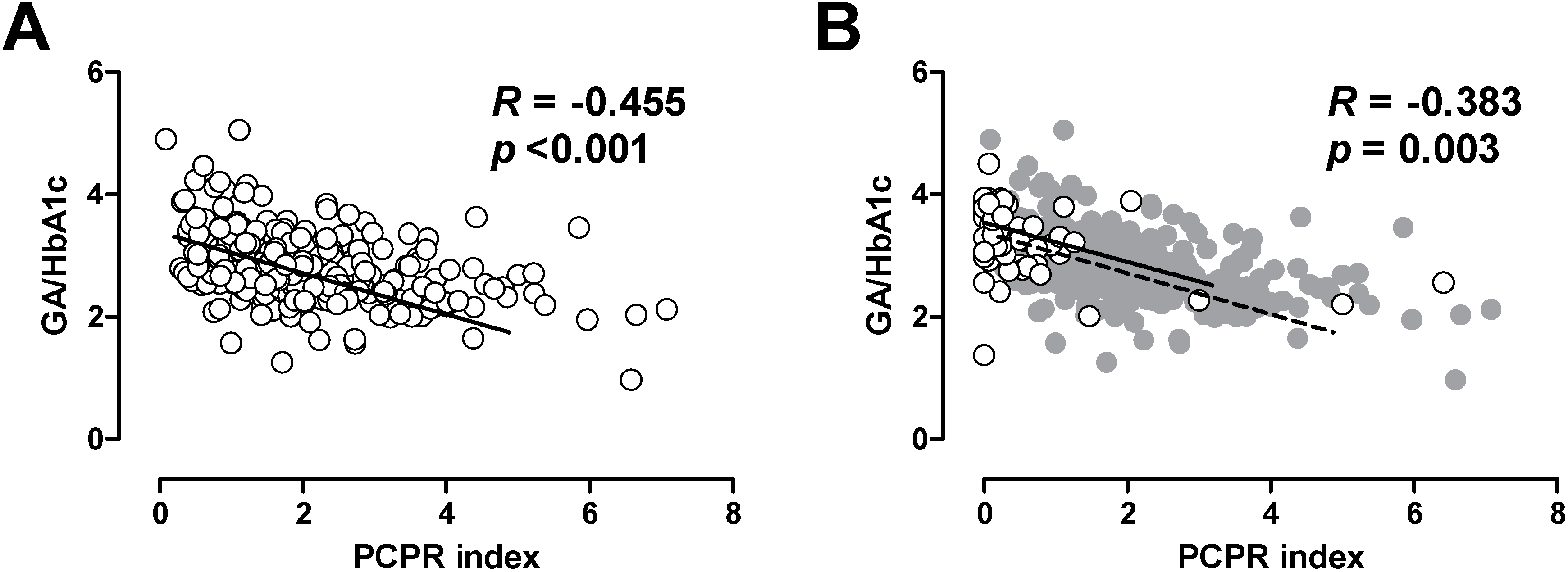
4. Current Issues in Treatment of Type 2 Diabetes: Inappropriate Insulin Supplementation
| Issue | Cause |
|---|---|
| Hypoglycemia | Excess insulin |
| Weight gain | Excess insulin |
| Concern of increased risk of malignancy and/or atherosclerosis | Excess insulin, especially peripheral hyperinsulinemia |
| Postprandial hyperglycemia | Insufficient insulin in postprandial state, especially in portal vein |
4.1. Hypoglycemia
4.2. Weight Gain
4.3. Peripheral Hyperinsulinemia
4.4. Postprandial Glycemic Excursion
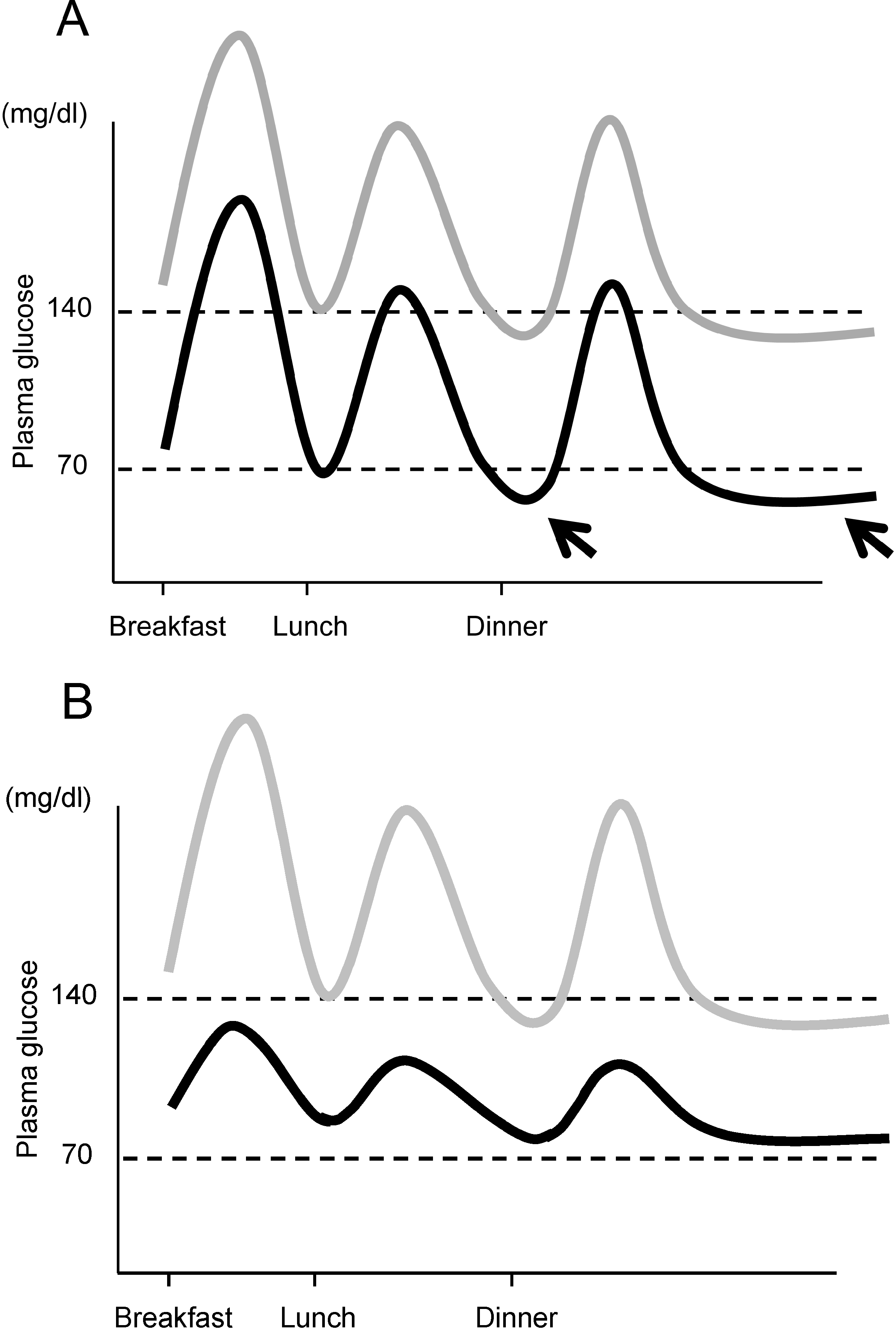
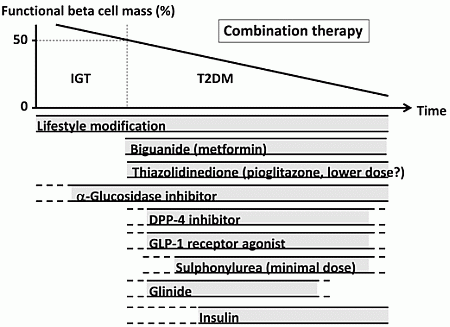
5. Therapeutic Strategy in Relation to Beta Cell Function
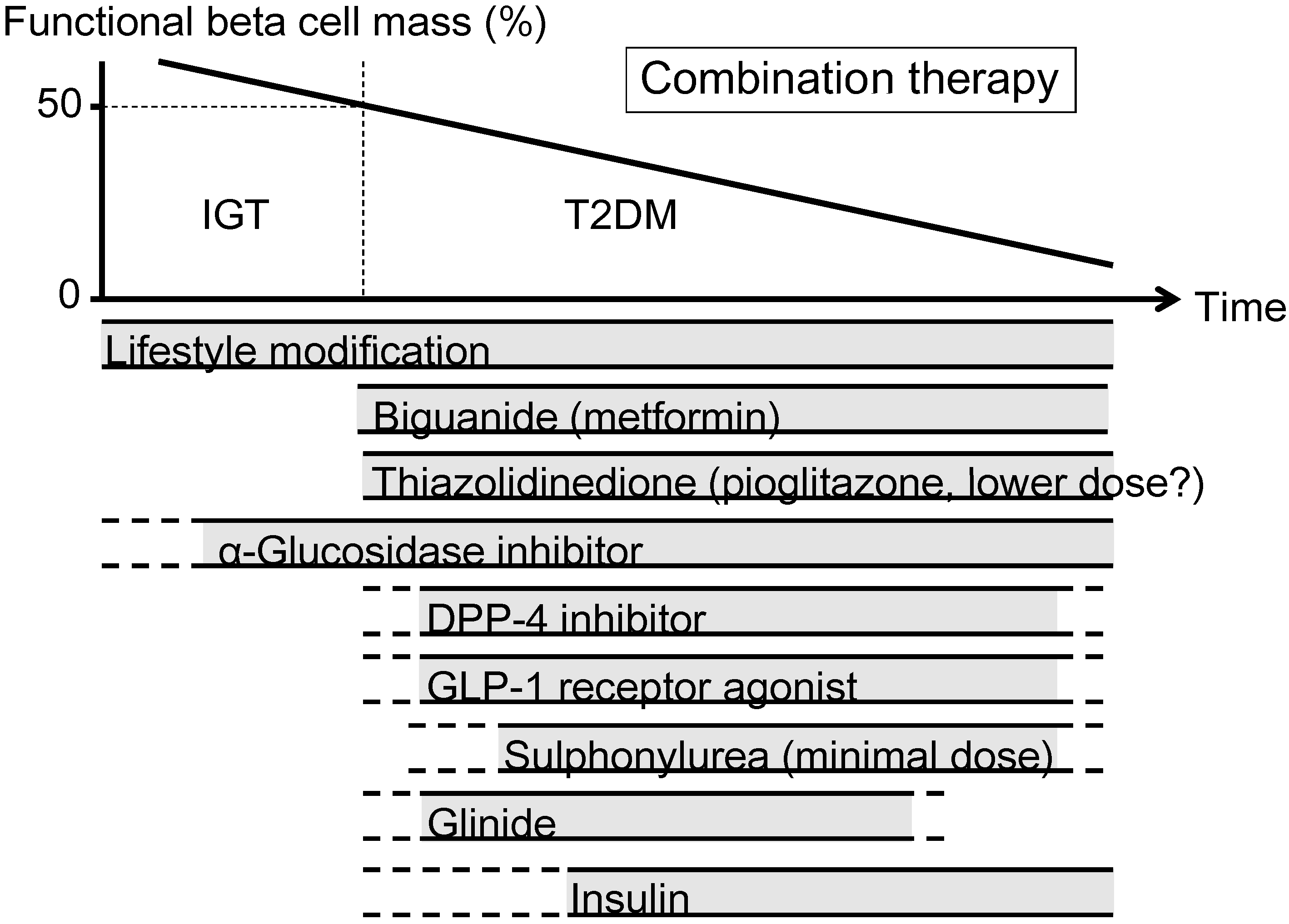
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (2013) IDF Diabetes Atlas, 6th ed. Available online: http://www.idf.org/diabetesatlas (accessed on 1 March 2014).
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef]
- Defronzo, R.A.; Eldor, R.; Abdul-Ghani, M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013, 36, S127–S138. [Google Scholar] [CrossRef]
- Defronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ader, M.; Huecking, K.; van Citters, G. Accurate assessment of beta-cell function: The hyperbolic correction. Diabetes 2002, 51, S212–S220. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10, 32–42. [Google Scholar] [CrossRef]
- Yoon, K.H.; Ko, S.H.; Cho, J.H.; Lee, J.M.; Ahn, Y.B.; Song, K.H.; Yoo, S.J.; Kang, M.I.; Cha, B.Y.; Lee, K.Y.; et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 2003, 88, 2300–2308. [Google Scholar] [CrossRef]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Abdul-Ghani, M.A. Preservation of beta-cell function: The key to diabetes prevention. J. Clin. Endocrinol. Metab. 2011, 96, 2354–2366. [Google Scholar] [CrossRef]
- U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16. Overview of 6 years therapy of type II diabetes: A progressive disease. Diabetes 1995, 44, 1249–1258. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Matthews, D.R.; Cull, C.A.; Stratton, I.M.; Holman, R.R.; Turner, R.C. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet. Med. 1998, 15, 297–303. [Google Scholar] [CrossRef]
- Kahn, S.E.; Lachin, J.M.; Zinman, B.; Haffner, S.M.; Aftring, R.P.; Paul, G.; Kravitz, B.G.; Herman, W.H.; Viberti, G.; Holman, R.R. Effects of rosiglitazone, glyburide, and metformin on beta-cell function and insulin sensitivity in ADOPT. Diabetes 2011, 60, 1552–1560. [Google Scholar] [CrossRef]
- TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care 2013, 36, 1749–1757. [Google Scholar] [CrossRef]
- Saisho, Y.; Kou, K.; Tanaka, K.; Abe, T.; Kurosawa, H.; Shimada, A.; Meguro, S.; Kawai, T.; Itoh, H. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr. J. 2011, 58, 315–322. [Google Scholar] [CrossRef]
- Saisho, Y.; Kou, K.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Association between beta cell function and future glycemic control in patients with type 2 diabetes. Endocr. J. 2013, 60, 517–523. [Google Scholar]
- Saisho, Y.; Kou, K.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Postprandial serum C-peptide to plasma glucose ratio predicts future insulin therapy in Japanese patients with type 2 diabetes. Acta Diabetol. 2013, 50, 987–988. [Google Scholar] [CrossRef]
- Saisho, Y.; Tanaka, K.; Abe, T.; Kawai, T.; Itoh, H. Lower beta cell function relates to sustained higher glycated albumin to glycated hemoglobin ratio in Japanese patients with type 2 diabetes. Endocr. J. 2014, 61, 149–157. [Google Scholar] [CrossRef]
- Cha, T.; Tahara, Y.; Ikegami, H.; Fukuda, M.; Yoneda, H.; Yamato, E.; Yamamoto, Y.; Noma, Y.; Shima, K.; Ogihara, T. Urinary C-peptide as an index of unstable glycemic control in insulin-dependent diabetes mellitus (IDDM). Diabetes Res. Clin. Pract. 1991, 13, 181–187. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kobayashi, T.; Inoko, H.; Tsuji, K.; Murase, T.; Kosaka, K. Residual beta-cell function and HLA-A24 in IDDM. Markers of glycemic control and subsequent development of diabetic retinopathy. Diabetes 1995, 44, 1334–1339. [Google Scholar] [CrossRef]
- Fukuda, M.; Tanaka, A.; Tahara, Y.; Ikegami, H.; Yamamoto, Y.; Kumahara, Y.; Shima, K. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes 1988, 37, 81–88. [Google Scholar] [CrossRef]
- Saisho, Y.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to beta cell function in both type 1 and type 2 diabetes. Diabetol. Int. 2011, 2, 146–153. [Google Scholar] [CrossRef]
- Tanaka, C.; Saisho, Y.; Tanaka, K.; Kou, K.; Tanaka, M.; Meguro, S.; Irie, J.; Jo, R.; Kawai, T.; Itoh, H. Factors associated with glycemic variability in Japanese patients with diabetes. Diabetol. Int. 2014, 5, 36–42. [Google Scholar] [CrossRef]
- Koga, M.; Kasayama, S. Clinical impact of glycated albumin as another glycemic control marker. Endocr. J. 2010, 57, 751–762. [Google Scholar] [CrossRef]
- Koga, M.; Murai, J.; Saito, H.; Kasayama, S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 2010, 33, 270–272. [Google Scholar] [CrossRef]
- Bo, S.; Gentile, L.; Castiglione, A.; Prandi, V.; Canil, S.; Ghigo, E.; Ciccone, G. C-Peptide and the risk for incident complications and mortality in type 2 diabetic patients: A retrospective cohort study after a 14-year follow-up. Eur. J. Endocrinol. 2012, 167, 173–180. [Google Scholar]
- Kim, B.Y.; Jung, C.H.; Mok, J.O.; Kang, S.K.; Kim, C.H. Association between serum C-peptide levels and chronic microvascular complications in Korean type 2 diabetic patients. Acta Diabetol. 2012, 49, 9–15. [Google Scholar] [CrossRef]
- Seshasai, S.R.; Kaptoge, S.; Thompson, A.; di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-Year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef]
- Frier, B.M.; Schernthaner, G.; Heller, S.R. Hypoglycemia and cardiovascular risks. Diabetes Care 2011, 34, S132–S137. [Google Scholar] [CrossRef]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar]
- Saenz, A.; Fernandez-Esteban, I.; Mataix, A.; Ausejo, M.; Roque, M.; Moher, D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005, 3. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Selph, S.; Ozpinar, A.; Foley, C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014, 168, 178–184. [Google Scholar] [CrossRef]
- Wang, H.; Ni, Y.; Yang, S.; Li, H.; Li, X.; Feng, B. The effects of gliclazide, metformin, and acarbose on body composition in patients with newly diagnosed type 2 diabetes mellitus. Curr. Ther. Res. Clin. Exp. 2013, 75, 88–92. [Google Scholar] [CrossRef]
- Kostev, K.; Rex, J.; Rockel, T.; Heilmaier, C. Effects of selected antidiabetics on weight loss—A retrospective database analysis. Prim. Care Diabetes 2014. [Google Scholar] [CrossRef]
- Karagiannis, T.; Paschos, P.; Paletas, K.; Matthews, D.R.; Tsapas, A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: Systematic review and meta-analysis. BMJ 2012, 344. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Cuddihy, R.M.; Hanefeld, M.; Kumar, A.; Gonzalez, J.G.; Chan, M.; Wolka, A.M.; Boardman, M.K. Efficacy and safety of exenatide once weekly vs. metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): A 26-week double-blind study. Diabetes Care 2012, 35, 252–258. [Google Scholar] [CrossRef]
- Apovian, C.M. The clinical and economic consequences of obesity. Am. J. Manag. Care 2013, 19, S219–S228. [Google Scholar]
- Hemkens, L.G.; Grouven, U.; Bender, R.; Gunster, C.; Gutschmidt, S.; Selke, G.W.; Sawicki, P.T. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: A cohort study. Diabetologia 2009, 52, 1732–1744. [Google Scholar] [CrossRef]
- Currie, C.J.; Poole, C.D.; Gale, E.A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009, 52, 1766–1777. [Google Scholar] [CrossRef]
- Jonasson, J.M.; Ljung, R.; Talback, M.; Haglund, B.; Gudbjornsdottir, S.; Steineck, G. Insulin glargine use and short-term incidence of malignancies—A population-based follow-up study in Sweden. Diabetologia 2009, 52, 1745–1754. [Google Scholar] [CrossRef]
- Colhoun, H.M. Use of insulin glargine and cancer incidence in Scotland: A study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009, 52, 1755–1765. [Google Scholar] [CrossRef]
- Rosenstock, J.; Fonseca, V.; McGill, J.B.; Riddle, M.; Halle, J.P.; Hramiak, I.; Johnston, P.; Davis, M. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: Findings from a 5 year randomised, open-label study. Diabetologia 2009, 52, 1971–1973. [Google Scholar] [CrossRef]
- Tang, X.; Yang, L.; He, Z.; Liu, J. Insulin glargine and cancer risk in patients with diabetes: A meta-analysis. PLoS One 2012, 7, e51814. [Google Scholar] [CrossRef]
- Sturmer, T.; Marquis, M.A.; Zhou, H.; Meigs, J.B.; Lim, S.; Blonde, L.; Macdonald, E.; Wang, R.; Lavange, L.M.; Pate, V.; et al. Cancer incidence among those initiating insulin therapy with glargine vs. human NPH insulin. Diabetes Care 2013, 36, 3517–3525. [Google Scholar] [CrossRef]
- Habel, L.A.; Danforth, K.N.; Quesenberry, C.P.; Capra, A.; van den Eeden, S.K.; Weiss, N.S.; Ferrara, A. Cohort study of insulin glargine and risk of breast, prostate, and colorectal cancer among patients with diabetes. Diabetes Care 2013, 36, 3953–3960. [Google Scholar] [CrossRef]
- Currie, C.J.; Peters, J.R.; Tynan, A.; Evans, M.; Heine, R.J.; Bracco, O.L.; Zagar, T.; Poole, C.D. Survival as a function of HbA(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet 2010, 375, 481–489. [Google Scholar] [CrossRef]
- Dandona, P.; Chaudhuri, A.; Ghanim, H.; Mohanty, P. Insulin as an anti-inflammatory and antiatherogenic modulator. J. Am. Coll. Cardiol. 2009, 53, S14–S20. [Google Scholar] [CrossRef]
- Smith, U.; Gale, E.A. Does diabetes therapy influence the risk of cancer? Diabetologia 2009, 52, 1699–1708. [Google Scholar] [CrossRef]
- Rensing, K.L.; von der Thusen, J.H.; Weijers, E.M.; Houttuijn Bloemendaal, F.M.; van Lammeren, G.W.; Vink, A.; van der Wal, A.C.; van Hinsbergh, V.W.; van der Loos, C.M.; Stroes, E.S.; et al. Endothelial insulin receptor expression in human atherosclerotic plaques: Linking micro- and macrovascular disease in diabetes? Atherosclerosis 2012, 222, 208–215. [Google Scholar] [CrossRef]
- Gamble, J.M.; Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Insulin use and increased risk of mortality in type 2 diabetes: A cohort study. Diabetes Obes. Metab. 2010, 12, 47–53. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Diaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef]
- Monnier, L.; Hanefeld, M.; Schnell, O.; Colette, C.; Owens, D. Insulin and atherosclerosis: How are they related? Diabetes Metab. 2013, 39, 111–117. [Google Scholar] [CrossRef]
- The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Nakagami, T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004, 47, 385–394. [Google Scholar] [CrossRef]
- Tominaga, M.; Eguchi, H.; Manaka, H.; Igarashi, K.; Kato, T.; Sekikawa, A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999, 22, 920–924. [Google Scholar] [CrossRef]
- Risso, A.; Mercuri, F.; Quagliaro, L.; Damante, G.; Ceriello, A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E924–E930. [Google Scholar]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef]
- Raz, I.; Wilson, P.W.; Strojek, K.; Kowalska, I.; Bozikov, V.; Gitt, A.K.; Jermendy, G.; Campaigne, B.N.; Kerr, L.; Milicevic, Z.; et al. Effects of prandial vs. fasting glycemia on cardiovascular outcomes in type 2 diabetes: The HEART2D trial. Diabetes Care 2009, 32, 381–386. [Google Scholar] [CrossRef]
- Raz, I.; Ceriello, A.; Wilson, P.W.; Battioui, C.; Su, E.W.; Kerr, L.; Jones, C.A.; Milicevic, Z.; Jacober, S.J. Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial vs. fasting/premeal glycemia. Diabetes Care 2011, 34, 1511–1513. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care 2009, 32, 1901–1903. [Google Scholar] [CrossRef]
- Cavalot, F.; Pagliarino, A.; Valle, M.; di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef]
- Munshi, M.N.; Pandya, N.; Umpierrez, G.E.; DiGenio, A.; Zhou, R.; Riddle, M.C. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J. Am. Geriatr. Soc. 2013, 61, 535–541. [Google Scholar] [CrossRef]
- Miller, M.E.; Williamson, J.D.; Gerstein, H.C.; Byington, R.P.; Cushman, W.C.; Ginsberg, H.N.; Ambrosius, W.T.; Lovato, L.; Applegate, W.B. Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older vs. younger adults in the ACCORD Trial. Diabetes Care 2014, 37, 634–643. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D.; Schwenke, D.C.; Banerji, M.; Bray, G.A.; Buchanan, T.A.; Clement, S.C.; Henry, R.R.; Hodis, H.N.; Kitabchi, A.E. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 2011, 364, 1104–1115. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Yusuf, S.; Bosch, J.; Pogue, J.; Sheridan, P.; Dinccag, N.; Hanefeld, M.; Hoogwerf, B.; Laakso, M.; Mohan, V.; et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef]
- Knowler, W.C.; Hamman, R.F.; Edelstein, S.L.; Barrett-Connor, E.; Ehrmann, D.A.; Walker, E.A.; Fowler, S.E.; Nathan, D.M.; Kahn, S.E. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005, 54, 1150–1156. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Xu, W.; Shi, L.; Zhang, Q.; Zhu, D.; Hu, Y.; Zhou, Z; Yan, X; Tian, H.; et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet 2008, 371, 1753–1760. [Google Scholar] [CrossRef]
- Pennartz, C.; Schenker, N.; Menge, B.A.; Schmidt, W.E.; Nauck, M.A.; Meier, J.J. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes Care 2011, 34, 2048–2053. [Google Scholar] [CrossRef]
- Holman, R.R.; Haffner, S.M.; McMurray, J.J.; Bethel, M.A.; Holzhauer, B.; Hua, T.A.; Belenkov, Y.; Boolell, M.; Buse, J.B.; Buckley, B.M.; et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 2010, 362, 1463–1476. [Google Scholar] [CrossRef]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Williamson, D.A.; Rejeski, J.; Lang, W.; van Dorsten, B.; Fabricatore, A.N.; Toledo, K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch. Intern. Med. 2009, 169, 163–171. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- Gregg, E.W.; Chen, H.; Wagenknecht, L.E.; Clark, J.M.; Delahanty, L.M.; Bantle, J.; Pownall, H.J.; Johnson, K.C.; Safford, M.M.; Kitabchi, A.E.; et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012, 308, 2489–2496. [Google Scholar] [CrossRef]
- Breyer, B.N.; Phelan, S.; Hogan, P.E.; Rosen, R.C.; Kitabchi, A.E.; Wing, R.R.; Brown, J.S. Intensive lifestyle intervention reduces urinary incontinence in overweight/obese men with type 2 diabetes: Results from the Look AHEAD trial. J. Urol. 2014. [Google Scholar] [CrossRef]
- Gerstein, H.C. Do lifestyle changes reduce serious outcomes in diabetes? N. Engl. J. Med. 2013, 369, 189–190. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Garvey, W.T.; et al. AACE comprehensive diabetes management algorithm 2013. Endocr. Pract. 2013, 19, 327–336. [Google Scholar]
- DeFronzo, R.A.; Barzilai, N.; Simonson, D.C. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J. Clin. Endocrinol. Metab. 1991, 73, 1294–1301. [Google Scholar] [CrossRef]
- Kim, C.H.; Han, K.A.; Oh, H.J.; Tan, K.E.; Sothiratnam, R.; Tjokroprawiro, A.; Klein, M. Safety, tolerability, and efficacy of metformin extended-release oral antidiabetic therapy in patients with type 2 diabetes: An observational trial in Asia. J. Diabetes 2012, 4, 395–406. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- FDA places greater restrictions on access to rosiglitazone. BMJ 2010, 341. [CrossRef]
- Blind, E.; Dunder, K.; de Graeff, P.A.; Abadie, E. Rosiglitazone: A European regulatory perspective. Diabetologia 2011, 54, 213–218. [Google Scholar] [CrossRef]
- McCarthy, M. US regulators relax restrictions on rosiglitazone. BMJ 2013, 347. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefebvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Molokhia, M.; Curcin, V.; Little, M.P.; Millett, C.J.; Ng, A.; Hughes, R.I.; Khunti, K.; Wilkins, M.R.; Majeed, A.; et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: Retrospective cohort study using UK general practice research database. BMJ 2009, 339. [Google Scholar] [CrossRef]
- Loke, Y.K.; Kwok, C.S.; Singh, S. Comparative cardiovascular effects of thiazolidinediones: Systematic review and meta-analysis of observational studies. BMJ 2011, 342. [Google Scholar] [CrossRef]
- Nissen, S.E.; Nicholls, S.J.; Wolski, K.; Nesto, R.; Kupfer, S.; Perez, A.; Jure, H.; De Larochelliere, R.; Staniloae, C.S.; Mavromatis, K.; et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: The PERISCOPE randomized controlled trial. JAMA 2008, 299, 1561–1573. [Google Scholar] [CrossRef]
- Meier, C.; Kraenzlin, M.E.; Bodmer, M.; Jick, S.S.; Jick, H.; Meier, C.R. Use of thiazolidinediones and fracture risk. Arch. Intern. Med. 2008, 168, 820–825. [Google Scholar] [CrossRef]
- Lewis, J.D.; Ferrara, A.; Peng, T.; Hedderson, M.; Bilker, W.B.; Quesenberry, C.P., Jr.; Vaughn, D.J.; Nessel, L.; Selby, J.; Strom, B.L. Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care 2011, 34, 916–922. [Google Scholar] [CrossRef]
- Neumann, A.; Weill, A.; Ricordeau, P.; Fagot, J.P.; Alla, F.; Allemand, H. Pioglitazone and risk of bladder cancer among diabetic patients in France: A population-based cohort study. Diabetologia 2012, 55, 1953–1962. [Google Scholar] [CrossRef]
- Colmers, I.N.; Bowker, S.L.; Majumdar, S.R.; Johnson, J.A. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: A meta-analysis. CMAJ 2012, 184. [Google Scholar] [CrossRef]
- Saisho, Y. Obesity, type 2 diabetes and beta cell failure: An Asian perspective. J. Mol. Genet. Med. 2014. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Kawamori, R.; Tajima, N.; Iwamoto, Y.; Kashiwagi, A.; Shimamoto, K.; Kaku, K. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 2009, 373, 1607–1614. [Google Scholar] [CrossRef]
- Chiasson, J.L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef]
- Hanefeld, M.; Chiasson, J.L.; Koehler, C.; Henkel, E.; Schaper, F.; Temelkova-Kurktschiev, T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 2004, 35, 1073–1078. [Google Scholar] [CrossRef]
- Hanefeld, M.; Cagatay, M.; Petrowitsch, T.; Neuser, D.; Petzinna, D.; Rupp, M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: Meta-analysis of seven long-term studies. Eur. Heart J. 2004, 25, 10–16. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Kendall, D.M.; Cuddihy, R.M.; Bergenstal, R.M. Clinical application of incretin-based therapy: Therapeutic potential, patient selection and clinical use. Am. J. Med. 2009, 122, S37–S50. [Google Scholar] [CrossRef]
- Aroda, V.R.; Henry, R.R.; Han, J.; Huang, W.; DeYoung, M.B.; Darsow, T.; Hoogwerf, B.J. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: Meta-analysis and systematic review. Clin. Ther. 2012, 34. [Google Scholar] [CrossRef]
- Rolin, B.; Larsen, M.O.; Gotfredsen, C.F.; Deacon, C.F.; Carr, R.D.; Wilken, M.; Knudsen, L.B. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E745–E752. [Google Scholar]
- Lamont, B.J.; Li, Y.; Kwan, E.; Brown, T.J.; Gaisano, H.; Drucker, D.J. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J. Clin. Investig. 2012, 122, 388–402. [Google Scholar] [CrossRef]
- Farilla, L.; Bulotta, A.; Hirshberg, B.; Li Calzi, S.; Khoury, N.; Noushmehr, H.; Bertolotto, C.; Di Mario, U.; Harlan, D.M.; Perfetti, R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003, 144, 5149–5158. [Google Scholar] [CrossRef]
- Bunck, M.C.; Corner, A.; Eliasson, B.; Heine, R.J.; Shaginian, R.M.; Taskinen, M.R.; Smith, U.; Yki-Jarvinen, H.; Diamant, M. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011, 34, 2041–2047. [Google Scholar] [CrossRef]
- Foley, J.E.; Bunck, M.C.; Moller-Goede, D.L.; Poelma, M.; Nijpels, G.; Eekhoff, E.M.; Schweizer, A.; Heine, R.J.; Diamant, M. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: A randomised controlled trial. Diabetologia 2011, 54, 1985–1991. [Google Scholar] [CrossRef]
- Kohro, T.; Yamazaki, T.; Sato, H.; Harada, K.; Ohe, K.; Komuro, I.; Nagai, R. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int. Heart J. 2013, 54, 93–97. [Google Scholar] [CrossRef]
- Kodani, N.; Saisho, Y.; Tanaka, K.; Kawai, T.; Itoh, H. Effects of mitiglinide, a short-acting insulin secretagogue, on daily glycemic variability and oxidative stress markers in Japanese patients with type 2 diabetes mellitus. Clin. Drug Investig. 2013, 33, 563–570. [Google Scholar]
- Del Prato, S.; Tiengo, A. The importance of first-phase insulin secretion: Implications for the therapy of type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2001, 17, 164–174. [Google Scholar] [CrossRef]
- Unger, R.H. Reinventing type 2 diabetes: Pathogenesis, treatment, and prevention. JAMA 2008, 299, 1185–1187. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013, 1, 140–151. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef]
- Riser Taylor, S.; Harris, K.B. The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy 2013, 33, 984–999. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery vs. non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347. [Google Scholar] [CrossRef]
- Saisho, Y.; Tanaka, K.; Abe, T.; Shimada, A.; Kawai, T.; Itoh, H. Effect of obesity on declining beta cell function after diagnosis of type 2 diabetes: A possible link suggested by cross-sectional analysis. Endocr. J. 2012, 59, 187–195. [Google Scholar] [CrossRef]
- Dixon, J.B.; Chuang, L.M.; Chong, K.; Chen, S.C.; Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Lee, W.J. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013, 36, 20–26. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, W.J.; Liew, P.L. Predictors of remission of type 2 diabetes mellitus in obese patients after gastrointestinal surgery. Obes. Res. Clin. Pract. 2013, 7. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saisho, Y. Importance of Beta Cell Function for the Treatment of Type 2 Diabetes. J. Clin. Med. 2014, 3, 923-943. https://doi.org/10.3390/jcm3030923
Saisho Y. Importance of Beta Cell Function for the Treatment of Type 2 Diabetes. Journal of Clinical Medicine. 2014; 3(3):923-943. https://doi.org/10.3390/jcm3030923
Chicago/Turabian StyleSaisho, Yoshifumi. 2014. "Importance of Beta Cell Function for the Treatment of Type 2 Diabetes" Journal of Clinical Medicine 3, no. 3: 923-943. https://doi.org/10.3390/jcm3030923