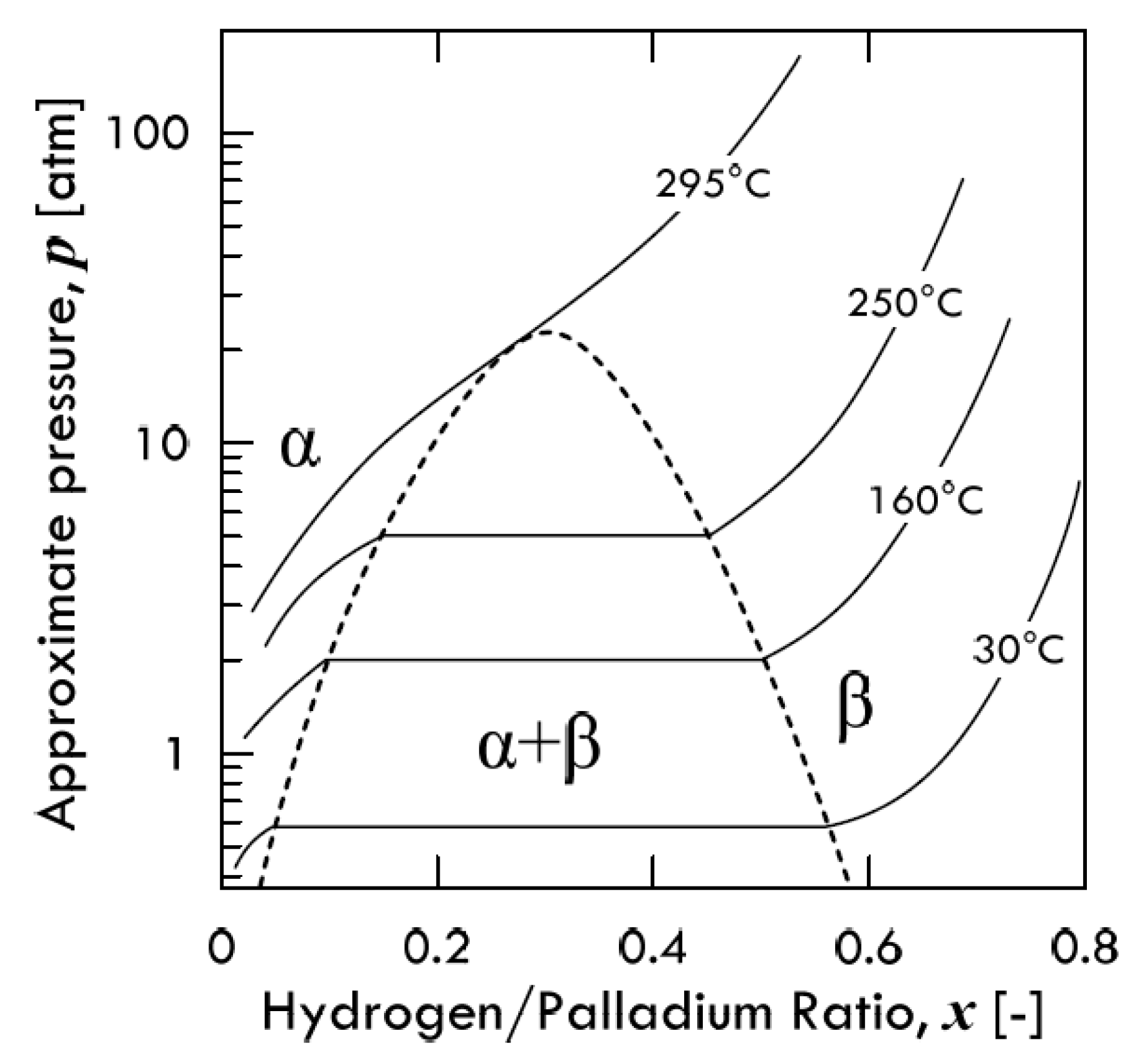
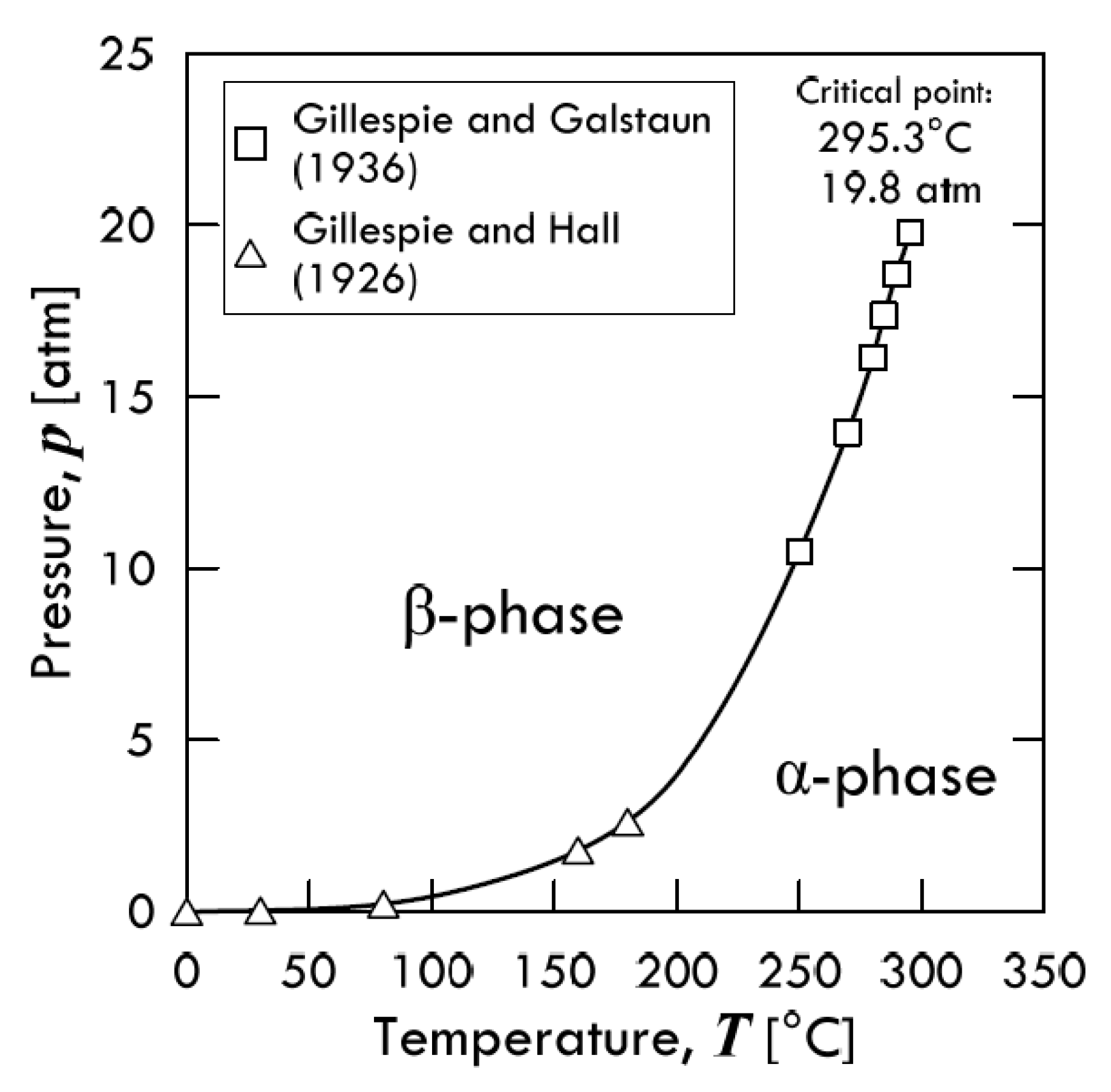
2.1. Hydrogen Dissolution in Metal Lattices
Hydrogen absorbed in metals occupies interstitial positions in metal lattices, as demonstrated by neutron diffraction [
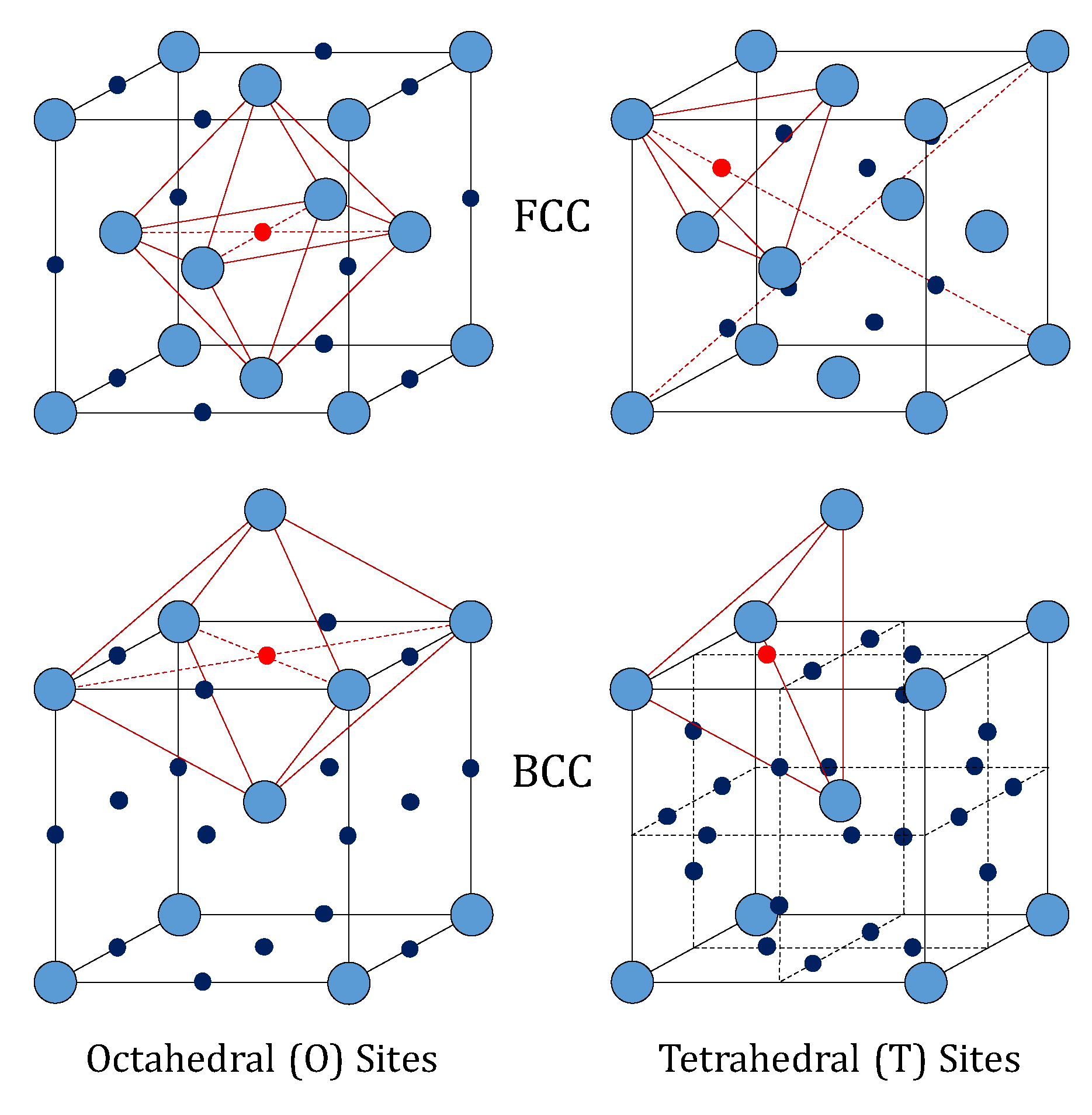
9]. The FCC lattice (like that of palladium) features an atom at each corner of the cubic cell, and one atom at the center of each face. It shows one octahedral (O) and two tetrahedral (T) interstitial sites per metal atom (14 sites and 14 metal atoms in a single cell).
Differently, the BCC lattice (like that of vanadium, niobium and tantalum) features an atom at each corner of the cubic cell and one at the center. Such a configuration has three octahedral and six tetrahedral interstitial sites per metal atom. The structures of these two lattices are depicted in
Figure 1. In particular, the octahedral sites of the FCC lattice have the largest free volume, whereas the tetrahedral sites are the largest in the BCC lattice [
10]. This means that the occupation of tetrahedral interstices by hydrogen in BCC lattices of the groups IV and V is favored over the octahedral ones, whereas the opposite behavior is found for hydrogen in palladium [
11].
In fact, Cser et al. [
12] found by neutron holographic study that the H
2 molecule dissociates in H-atom in palladium and these atoms preferentially occupy the octahedral sites of the FCC lattice.
As for the dissolution of hydrogen in vanadium, the V-H system presents a phase diagram consisting of several different phases: (i) the solid solution (
α-phase), observed at high temperature, in which the H-atoms are randomly distributed in the tetrahedral sites of its BCC structure; (ii) the hydride V
2H
β1-phase, showing a monoclinic structure where the H-atoms are located in the octahedral sites; (iii) the hydride V
2H or VH
β2-phase, showing a monoclinic body-centered tetragonal (BCT) structure; (iv) the V
3H
2 phase with a monoclinic structure; (v) the VH
2 δ-phase, showing a CaF
2-like structure with the H-atoms occupying the tetrahedral sites; (vi) the
γ-phase VH
x, observed at high H-concentration and temperature below 375 K ca. [
13,
14,
15,
16,
17,
18,
19]. The overall result of such a complex situation is that neutron diffraction on vanadium-deuterium system at 50% of V-D atomic ratio showed that about 90% of the dissolved deuterium atoms occupies the tetrahedral BCC sites, with the rest being placed in the octahedral ones [
20]. More recently, using a Density Functional Theory (DFT) analysis applied to pure vanadium, Lu et al. [
21] estimated that the H
2 solution energy into the T-sites is −0.332 eV, which is higher than the value calculated into the O-ones (−0.149 eV). Since a more favorable solution energy has a more negative value, this result demonstrates that hydrogen is preferentially absorbed into the BCC T-sites with respect to the BCC O-ones.
In general, in a given circumstance, hydrogen prefers one type of interstitial site with respect to all the other available ones. In case of palladium, the preferred site are the octahedral ones. Understanding why a certain type of site is preferred over another one would allow getting crucial information on the diffusion mechanism of hydrogen into metals [
13].
The interstitial sites represent a finite population, whose occupancy by the dissolved hydrogen can be described by means of a thermodynamic approach in terms of Fermi-Dirac statistics. Smirnov and Pronchenko provided a detailed expression of chemical potential of the Pd-H system, taking into account three different contributions: (i) conformational, (ii) oscillatory and (iii) electronic. Then, chemical potential can be obtained as the derivative of the Helmholtz free energy (
F) with respect to the hydrogen molar fraction
ξ in the metal lattice (Equation (1)), which is defined as the hydrogen/interstices concentration ratio [
22].
The conformational chemical potential can be written as described in Equation (2):
where
and
are the bond energy of atom H with the metal lattice and the H-H interaction energy within the interstices. The oscillatory contribution to the Helmholtz free energy is reported in Equation (3) [
23]:
from which the corresponding expression of chemical potential can be obtained as follows (Equation (4)):
The parameter
is the frequency of local oscillations of H-atoms, which depends on H-concentration. Smirnov et al. suggested the following linear approximation for
(Equation (5)) [
22]:
As for the electronic contribution to chemical potential, Smirnov and Pronchenko proposed the following two-parameter expression valid in the entire H-content range within an approximation of 5% (Equation (6)) [
22]:
From the previous expressions, the following complete expression of chemical potential is obtained (Equation (7)):
Once chosen an appropriate reference chemical potential
μ0, the corresponding activity of atomic hydrogen in the metal lattice is defined (Equation (8)):
In the present paper, to obtain a complete expression of hydrogen activity in the metal bulk, the chemical potential in the infinite dilution conditions (i.e., hydrogen content tending to zero) is chosen as the reference chemical potential (Equation (9)):
From Equations (8) and (9), an explicit expression of the H-activity is finally obtained (Equation (10)):
In the limit of infinite dilution system (ξ → 0), the activity a coincides with the hydrogen composition ξ. Based on Equation (7), as ξ tends to the limit value of 1, the chemical potential μ tends to infinity. From a physical point of view, as the available sites are gradually more occupied, it becomes progressively more difficult to fill the remaining sites, which corresponds to a situation of a gradually higher chemical potential.
From the energetic point of view, the hydrogen atoms progressively occupy the interstitial sites from the lowest-energy class to the highest-energy one [
20].
Indeed, despite a former theory, which expected the occupancy of just one class of sites, i.e., the octahedral ones in a Pd-H system, McLennan et al. confirmed through diffraction evidence that, for the palladium-deuterium system, the tetrahedral sites, which are the highest-energy class of sites, are partially occupied when the deuterides PdD
x are formed above the thermodynamic critical point. Moreover, the same authors found via Rietveld profile analysis that the maximum tetrahedral occupancy occurs at a D/Pd atomic ratio of around 0.6, at which one third of all D-atoms are placed in tetrahedral sites [
24].
An explanation for these observations can be provided if considering that the interactions between the H-atoms dissolved in the metal bulk induce an energetic difference between occupied and empty sites belonging to the same class. In particular, it is proposed that the hydrogen atoms occupies tetrahedral sites on alternate planes, forming in this way a symmetric, ordered and more energetically favorable sub-lattice, as occurs in tantalum–hydrogen system [
20].
The occupation of the interstitial sites induces a stress state [
20,
25,
26]. In fact, the metal lattice expands upon H
2 absorption. To intuitively understand how the occupation of the interstices occurs, it is useful to consider the
ball-in-hole model in continuum elasticity. In this model, the hydrogen atom is assumed to be an incompressible ball, while the metal cavity in which the ball fits is thought as an elastic, spherical
hole. When the ball enters the hole, the hole size increases because of the ball-hole interactions, inducing an additional volume metal matrix. If Δ
V1 is the volume of the intruding ball, the Δ
V2 volume of the cavity is given by:
where
ν is the Poisson ratio of the matrix. For a typical value of
nH/
nPd of 0.3, it results that Δ
V2 is greater than Δ
V1 by 50%. Thus, the volume of the body increases by an extent larger than that required to accommodate an H-atom: this is the origin of the increase of the lattice parameters caused by the insertion of dissolved H-atoms [
20].
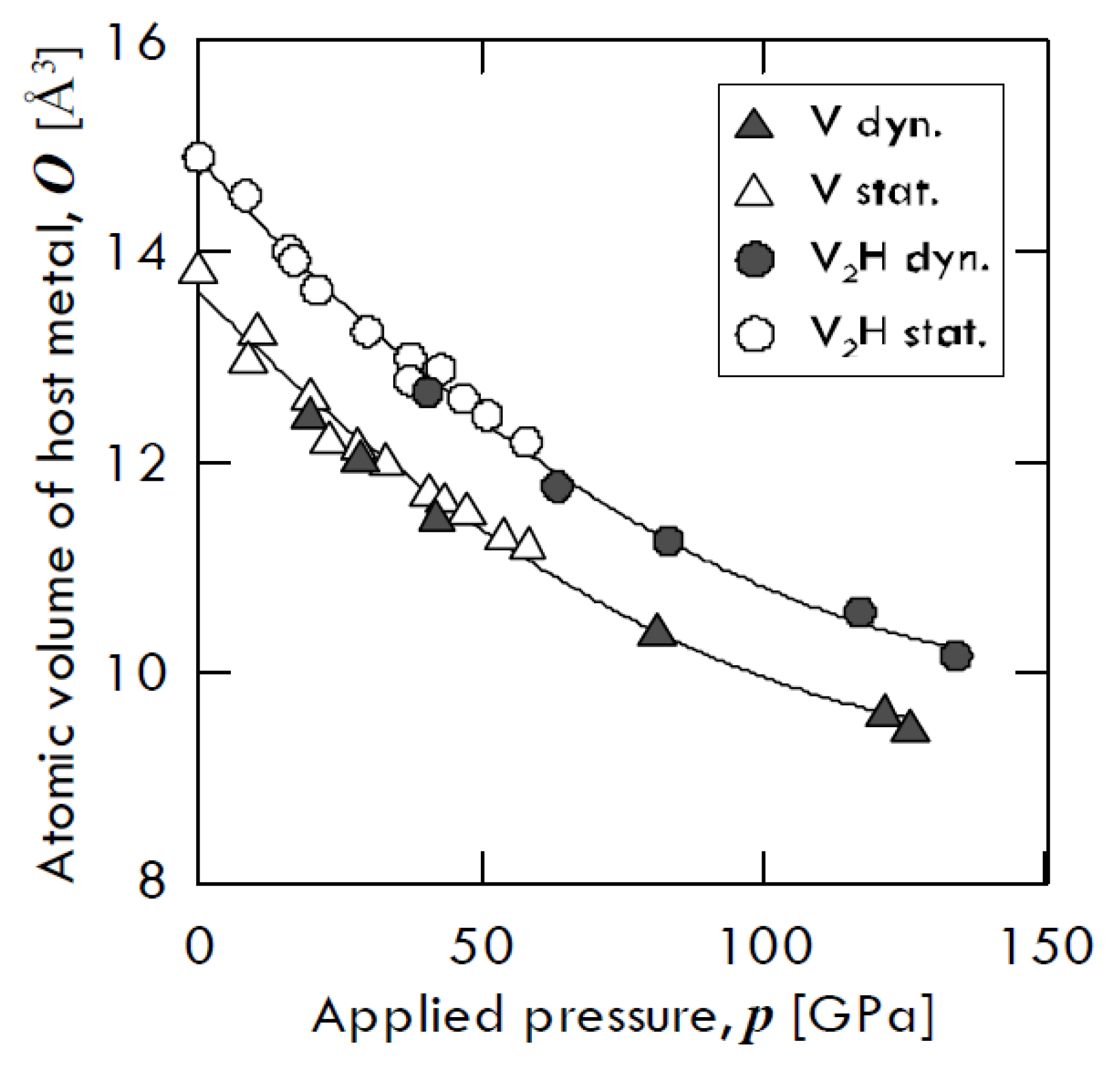
Besides, Fukai [
13] found that hydrogen-induced augmented volume is basically incompressible. The compression curves of pure vanadium and that of
β-V
2H hydride, obtained by means of two different compression ways—i.e., through diamond anvil cell (open symbols) and through shock wave (closed symbols)—are shown in
Figure 2. From this figure, one can notice that both vanadium and V
2H trend keep almost the same atomic volume gap. In particular, pure V and V
2H volume decrease of about 69% and 74% of its original value at 130 GPa, respectively, with the two values being relatively close to each other.
It must be remarked that, although the same amount of hydrogen could be included into the metal lattice, the method used to inject hydrogen could affect the lattice distortion and, thus, the internal thermodynamic state of the metal-solute system. Indeed, inserting hydrogen simply by applying a pressure difference contrasts the lattice expansion, whereas the expansion effect is not hindered when metal is subject, for example, to cathodic charging [
20].
2.2. Elastic and Electronic Effects of Alloying
The lattice constants are different for different systems as well as the volumes of the tetrahedrons. The larger the volume, the wider the free space available for the dissolution of the interstitial atoms, which thus results facilitated.
When an alloying element is inserted in the metal lattice, the lattice constants could change, affecting the solubility of hydrogen. Lu et al. [
21] simulated the behavior of hydrogen in a V-based 2 × 2 × 2 supercell made by 8 unit cells, in which an alloying M-atom (Al, Ti, Cr, Fe, Ni and Nb) replaces the central V-atom of such a supercell, thus forming a V-based alloy. This alloy is indicated as V
15M, because 15 V-atoms and a single M-atom form the unit cell lattice.
When an alloying element is added to vanadium, its atomic radius does not match the vanadium original matrix, leading to lattice distortions and change in the volume of tetrahedrons. These distortions produce an elastic stress field, which affects the H-atoms dissolution, resulting in the variation of the solution energies. Such variations were found to depend significantly on the lattice constants of V
15M, which in their turn depend on the size of alloyed atoms [
21].
As the M-atom size increases, the cubic lattice tends to swell to a certain extent, thus causing the available space for the dissolution of an additional H-atom to increase as well. In this way, the M-atoms having a size smaller than the V-atom one (like Cr, Ni and Fe) reduce the solubility of hydrogen in vanadium by making the solution energy values less negative. The opposite occurrence is found for M-atoms with a bigger size (Al, Ti, Nb) [
21]. Results are illustrated in
Figure 3.
A remarkable exception to the previously explained general cases is found by observing the behavior of niobium. In fact, since it is the biggest atom in the analyzed series, it is expected to form an alloy with a solution energy more negative than that of titanium, which has a smaller atomic size. However, such an expectation is not confirmed by simulation. Those authors explain this apparently anomalous behavior by considering that the effective available space in the interstices of the V-based lattice is reduced by the size of the Nb-atom, which is too large compared with the V-atom one [
21]. The addition of an alloying atom into the vanadium lattice affects the interstitial H-diffusion not only by producing elastic distortion, but also by modifying the original electronic structure around the interstitial solute. Investigating the local density of states, however, Lu and his co-workers found that the intensity values of the resonance peaks of the hydrogen in the pure vanadium system are similar to those of the hydrogen in V
15M system. From this observation, it is withdrawn that the electronic effect due to the M-atom presence may be not the main influencing factor of hydrogen dissolution [
21].
2.3. Effects of Dissolved Atoms on Global Electronic Structure
The enthalpy of hydrogen dissolution
from the molecular state to the atomic one inside metal is given by the contribution of two terms. The first is related to the dissociation energy of the molecule and the second is related to the interaction energy between dissolved atoms as well as between atoms and metal. In particular, the metal–atoms interaction determines the metals classification into two classes of occluders: endothermic and exothermic ones. The former has a solubility increasing with increasing temperature, whereas the latter shows the opposite behavior [
20].
Endothermic occluders, such as Mn, Fe, Co and Ni, form a metal–hydrogen solid solution in which the H-atom is located at random interstices [
11]. However, the most interesting transition metals for hydrogen separation, e.g., Pd, Ta, V, Nb, are exothermic occluders, which show a tendency to form ordered hydrides at high H-concentrations [
27].
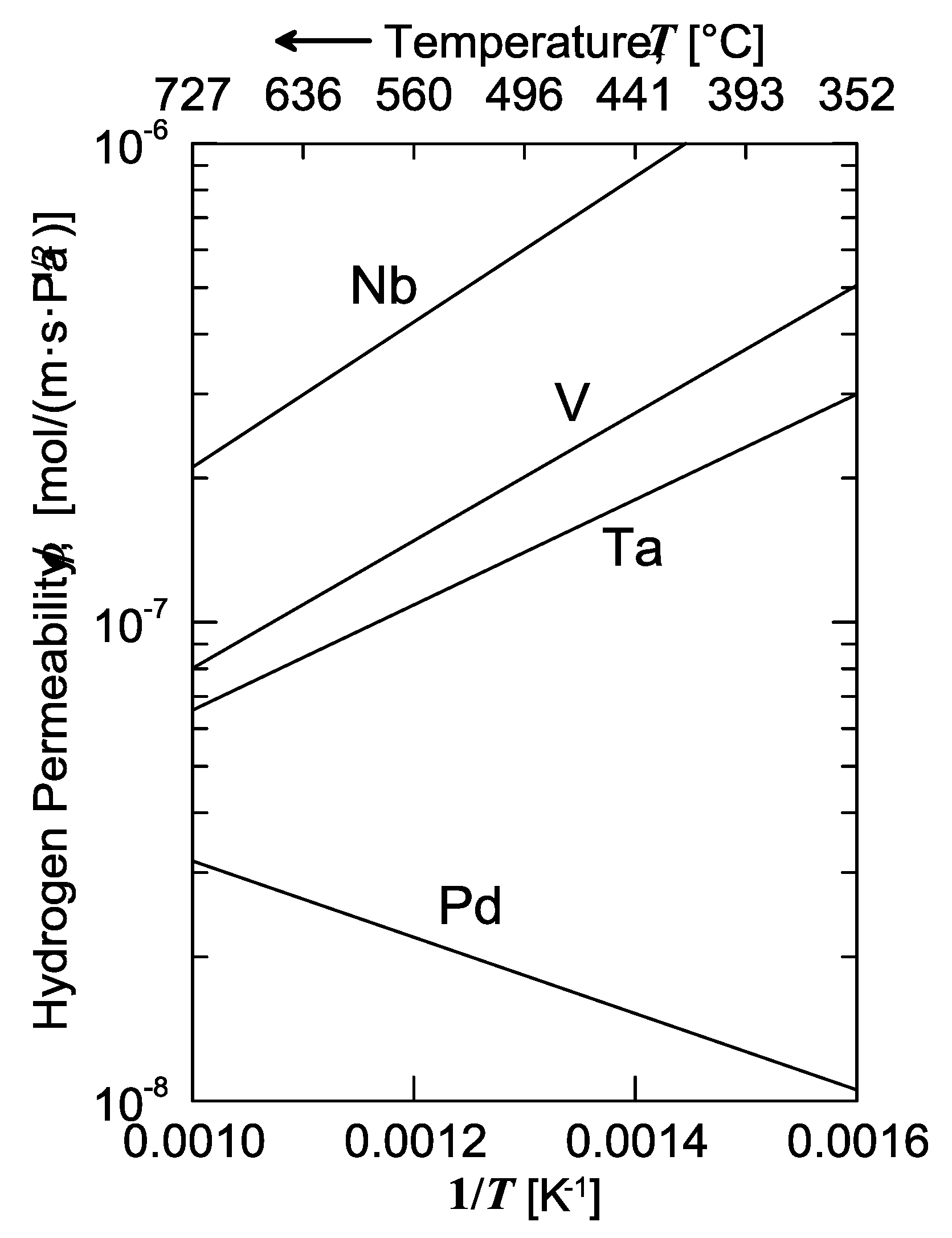
The solubility isotherms of some metals and a V-alloy (vanadium, tantalum and V
85Ni
15 alloy at 400 °C, and palladium at 340 °C) are shown in
Figure 4. As is possible to notice, vanadium clearly absorbs much more hydrogen than palladium. Under a hydrogen partial pressure of 7 bar, the H/V ratio at 400 °C would be around 0.6, i.e., 6 hydrogen atoms per 10 vanadium atoms, while, at the same pressure, the H/Pd ratio at 340 °C would settle at about 0.02 (ca. 2 ÷ 98). Since exothermic occluders show a solubility decreasing with increasing temperature, the difference between these two metals would become even more pronounced with palladium at 400 °C. Therefore, it is possible to state that the dominant contributor to permeability is solubility for BCC metals, whereas for FCC metals (Pd in particular), the dominant factor is represented by diffusivity [
11].
Consequently, permeability of BCC metals decreases with increasing temperature, because this behavior is driven by solubility. On the contrary, permeability of FCC metals increases with increasing temperature, because it mainly driven by diffusivity. It is interesting to underline how alloying vanadium with a small percentage of nickel drastically reduces hydrogen solubility. At the same condition of pressure and temperature, the hydrogen/metal ratio would pass from 0.6 to 0.09. By reducing the hydrogen solubility, the embrittlement tendency reduces as well [
11].
The H-content in the metal lattice affects also other physical properties, such as electrical resistance, magnetic susceptibility and thermoelectric power. For their extent, these variations cannot be simply explained by lattice expansion induced by solute atoms. Differently, it is clear that hydrogen consistently perturbs the electronic structure of the host metal [
20]. Indeed, the
1s electrons of the hydrogen atoms enters the
s- and
d- bands of the host metal, causing shifts in the energy bands. As a result, the Fermi electrons of the host metal start surrounding the positive H
+ nuclei, leading to the formation of a sort of
screened entity, which could be thought as neutral atoms even if the electrons are not bound to the nuclei. Accordingly, it is possible to distinguish two kinds of effects. The first consists in the shift of metal bands, which has a
long-range effect, as the induced interactions involve the entire metal domain. The second is related to the heaping of Fermi electrons placed on the hydrogen nuclei and is a
short-range effect. An attractive interaction is observed between two hydrogens caused by the
d-vacancies of the Pd-atom, which is the common neighbor of both H-atoms. This interaction could explain the elastic interactions as well as the hydrogen cluster formation in Pd-H systems. Since hydrogen remarkably affects the global electronic structure and, more importantly, the metal atoms inter-distance, it is not surprising that the cohesive forces of the host metal are unavoidably reduced. Such an occurrence is explained through molecular dynamic simulations, which show that hydrogen fills antibonding states in
4d band, reducing the bond strength between Pd-atoms. The decrease of the electron density between metal atoms, resulting in the Pd-Pd bonds impairment in several transition metals, is confirmed through cluster calculation and embedded atom method [
20].
2.4. Hydrogen-Lattice Defects Interactions
Hydrogen can also interact with structural defects. Metals usually exhibit defects of various size, which can be distinguished into three classes, so-called zero-dimensional, one-dimensional, and two-dimensional one.
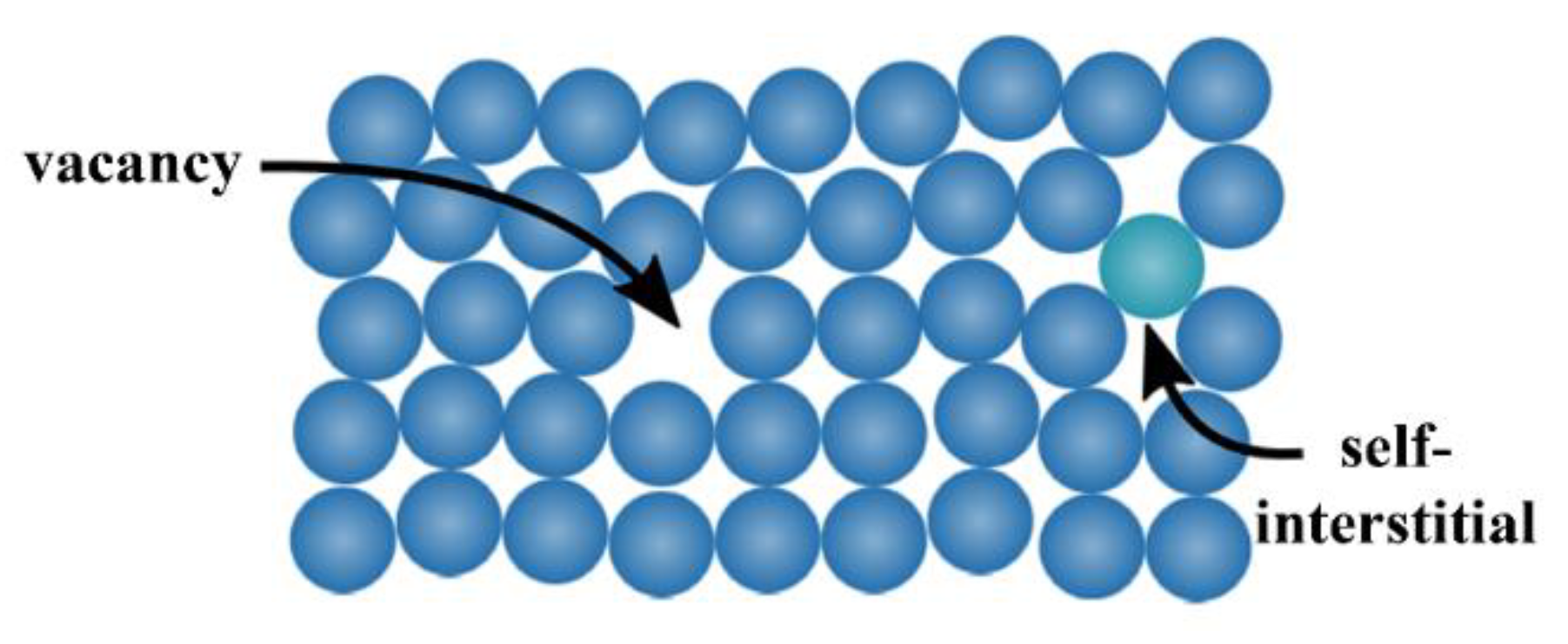
The zero-dimensional defects, also known as
point defects, are local defects, i.e., locations where an atom is missing (vacancy) or is placed into an interstitial void of the lattice structure (self-interstitial), as illustrated in
Figure 5. The latter occurs only at low concentration because of the strain field induced in the tightly packed metal structure, whereas the former can be found more often, with a probability increasing with increasing temperature. In fact, the higher the temperature, the more frequent and random the position changes of the atom in the lattice. It can also happen that the vacancies population is in excess with respect to the equilibrium value, for example after a quenching from high temperature. Anyway, both defect types can also occur after plastic deformations.
Regarding the interaction between these defects and H-atoms, a curious aspect was observed by Kirchheim [
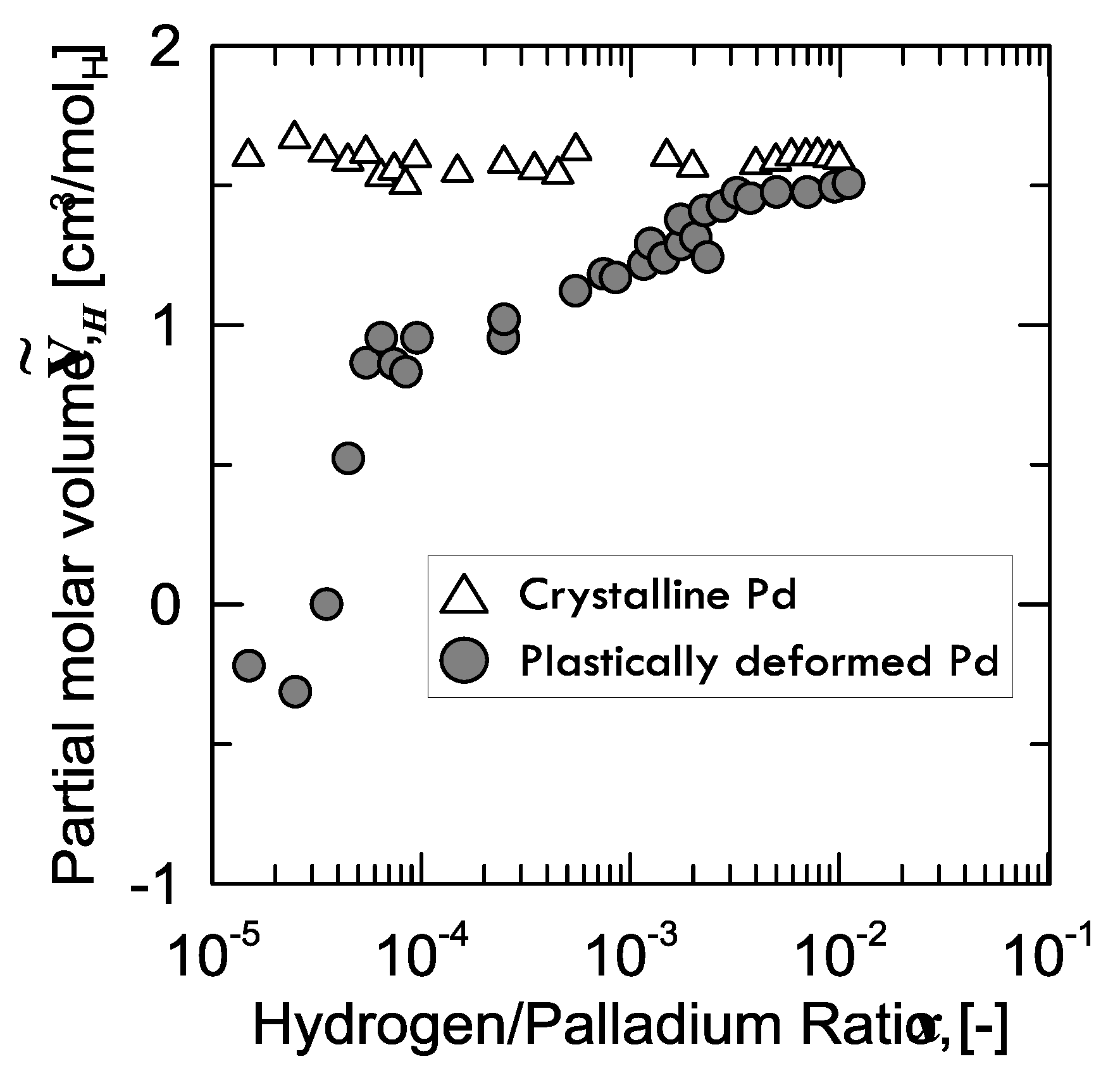
28], who prepared two samples of palladium: the first one, which was structurally defect-free, and the second one plastically stretched (up to 50%) through cold drawing and subsequent rolling, in order to enhance the number of defects. Afterwards, Kirchheim measured the partial molar volume
of hydrogen in both samples (
Figure 6).
It can be observed that, for the virtually defect-free Pd-sample, is constant within the whole range of H/Pd ratio, whereas the plastically deformed one shows a continuously increasing value tending to the same level as that of the former.
A peculiar aspect to be observed is that, for the defected sample,
is even negative below a certain value of hydrogen concentration (40 ppm). In other words, instead of expanding because of the hydrogen absorption, the lattice shrinks. Kirchheim explained this weird behavior considering the phenomena occurring when hydrogen fills an empty site or vacancy. In fact, if hydrogen is close enough to the nearest palladium atom at low concentration, attractive forces Pd-H come into play and, thus, the lattice contracts. From around 40 ppm on, the lattice starts growing in the positive values, as the effect of hydrogen in the substitutional vacancies is overcome by the expansion of the normal interstitial sites [
28].
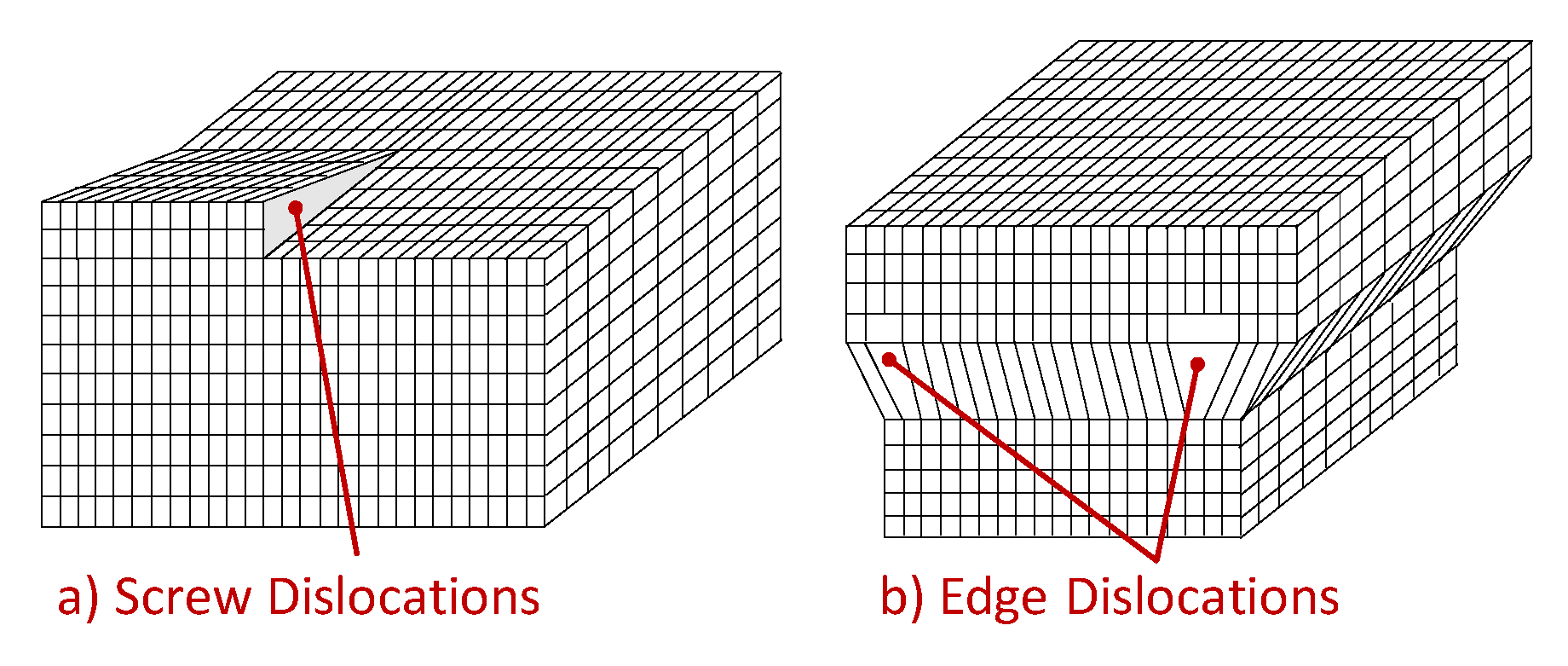
The one-dimensional defects, also known as linear defects, consist in lattice local dislocations, produced in the metal by plastic deformations and many other processes. One can basically distinguish two types of dislocations: the edge dislocation and the screw dislocation, even if most dislocations are probably a hybrid of these two limits.
The shear stress causes screw defects, which propagates along a direction normal to the shear stress, as depicted in
Figure 7a. As well, the edge dislocations can be visualized as an extra half-planes inserted in the crystal homogeneous lattice, as sketched in
Figure 7b. The interatomic bonds are significantly distorted but only in the immediate neighborhood. When a shear stress is applied, dislocations can not only originate but also move. It is important to go deeper into this phenomenon, to understand the hydrogen dragging induced by dislocation motion. To make an analogy, this type of movement can be compared to the motion of a caterpillar: first, it moves the rear part creating a hump, and then moves the front part, similar to how the defect slips one cell per time. In this way, only a small amount of bonds is broken at a given time, thus requiring a much smaller energy than that needed to break all the bonds simultaneously. For the same reason, dislocations move easily over dense planes, because the stress required to move the dislocations increases with increasing spacing between planes. As FCC and BCC metals have several dense planes, the dislocations move relatively easily, resulting in materials with a relatively high ductility.
Kirchheim [
29] evaluated the interaction energy of hydrogen in palladium edge dislocations. He found that the closer the core of the edge defect, the larger the interaction energy, which includes a self H-H attractive interaction energy. Far from the cores, the H-H interactions do not provide an appreciable contribution and, in general, the attractive interaction energy decreases with the reciprocal of the distance. Anyway, the additional population of hydrogen can be measured only at very small quantities of dissolved hydrogen due to the attractive interaction with edge dislocations. When carbon embeds in
α-Fe, it leads to tetrahedral distortions causing attractive interactions towards screw dislocations. On the contrary, the octahedral positions occupied by the H-atoms in palladium make it interact with this type of dislocation at negligible or null levels [
29].
The two-dimensional or planar defects include several forms of dislocations. Overall, stacking faults, grain boundaries and internal surfaces of micro-voids and micro-cracks are mentioned. To explain the stacking fault phenomenon, one can consider the case of the crystalline structure of two common lattices, the FCC and the HCP structures, for which the former can be oriented in two different planes, i.e., square or triangular, whereas the latter can be seen in the triangular orientation. In particular, one can imagine the crystal structure as a sequence of stacks. If A indicates the triangle, B the hexagon and C the reverse triangle, one can describe the HCP structure as an AB sequence, whereas the FCC structure as an ABC sequence. With this in mind, a stacking fault occurs, for example, when an unpredicted C-layer is present in the AB sequence of the HCP structure or when a C-layer is missing, or ABC default sequence randomly changes in the FCC structure.
In their study, Whiteman and Troiano showed a hydrogen-charging operation in a stainless-steel sample (25%Cr–20%Ni) conducted through electrolytic way. In those conditions, they found that the activation energy required to create a stacking fault lowers. Such an energy variation can be associated with an attractive interaction between hydrogen dissolved in metal and stacking faults [
30]. Another type of planar defect is represented by the grain boundary.
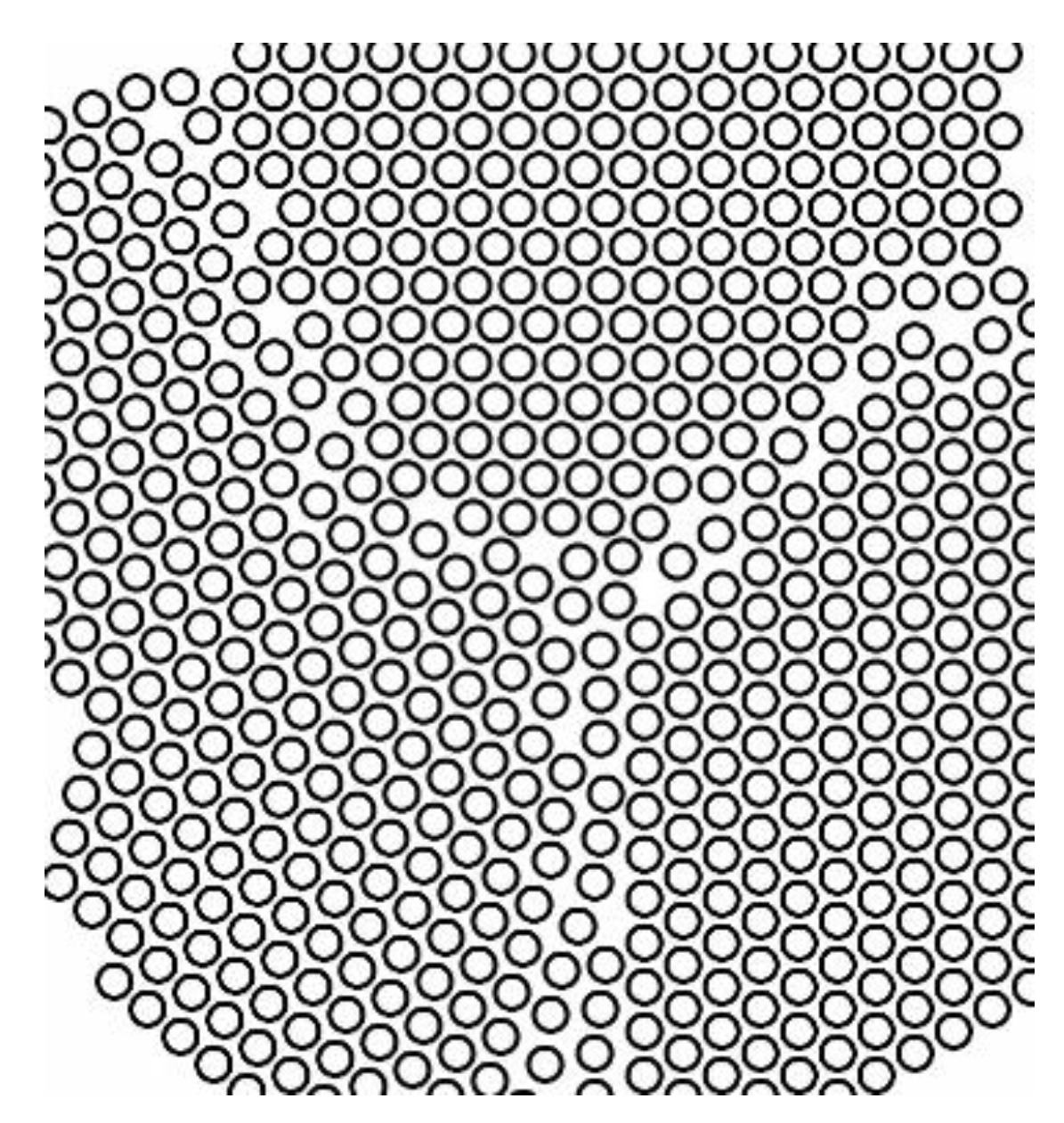
Generally, a solid consists of several crystallites or grains, whose size varies from nanometers to millimeters. Each grain has usually a different orientation with respect to neighboring grains. The border separating one grain from another one is known as grain boundary. A representation of the grain boundaries present in crystal is sketched in
Figure 8.
Grain boundaries have been demonstrated to absorb an excess of H-atoms population in several metals other than palladium. Mütschele and Kirchheim [
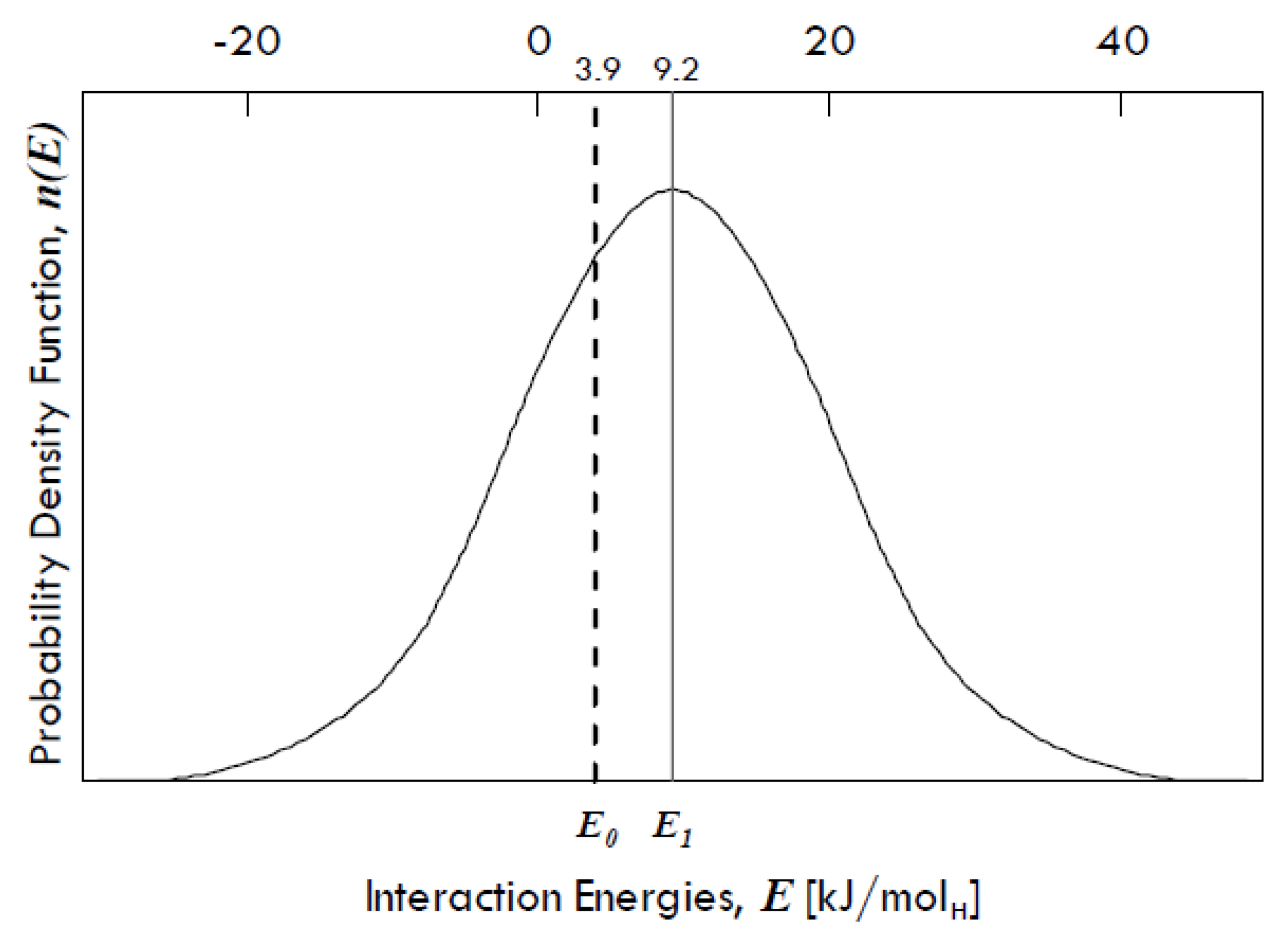
31] found that there is a Gaussian distribution of interaction energies between dissolved hydrogen and grain boundaries in palladium (
Figure 9), denoting a variety of binding sites in grain boundaries.
The energy E1 is equal to 9.2 kJ/molH and represents the expected value (µ) of the interaction energies distribution around which site energies for the grain boundaries might vary, whereas E0 is equal to 3.9 kJ/molH and represents the interaction energy of the sites within the grains, which has the same value as that of the single crystals of Pd. The difference between these two energies (equal to 5.3 kJ/molH) gives the spectrum of the segregation energies, i.e., the energies of molecular partitioning from metal bulk to defects.
The standard deviation of the distribution (
σ) is equal to 15 kJ/mol, so that it is possible that some grain boundary sites show even a negative interaction energy, which means that there can be also repulsive forces. However, grain boundaries in any metal exhibit in general attractive interactions towards dissolved hydrogen [
31].
In addition to stacking faults and grain boundaries, a third class of defects is represented by micro-voids, which can occur when a ductile material is pulled during a tensile strain. If the stress continues, a coalescence of some voids can occur, leading to the formation of micro-cracks. Hydrogen is absorbed upon these cracks in the same way as if it would be absorbed upon a generic external surface. In addition, the volume of the internal cavity (which can be referred to as a
three-dimensional defect) would acquire a concentration of molecular hydrogen consistent with the input pressure of external environment at equilibrium [
20]. It is possible to divide the interactions between dissolved hydrogen and lattice imperfections into two categories: chemical and elastic one. The former refers to positions where the atomic displacements are remarkable, e.g., at dislocation cores and internal interfaces, whereas the latter refers to positions where atomic displacements are small, which means sufficiently far from the imperfections. In this case, an approximated linear elasticity model can be applied [
20].