Chemical Crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for Improved CO2/CH4 Separation
Abstract
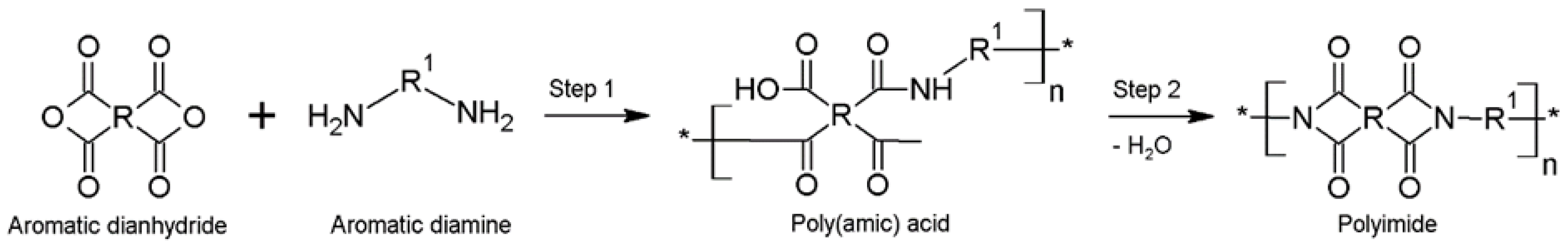
:1. Introduction
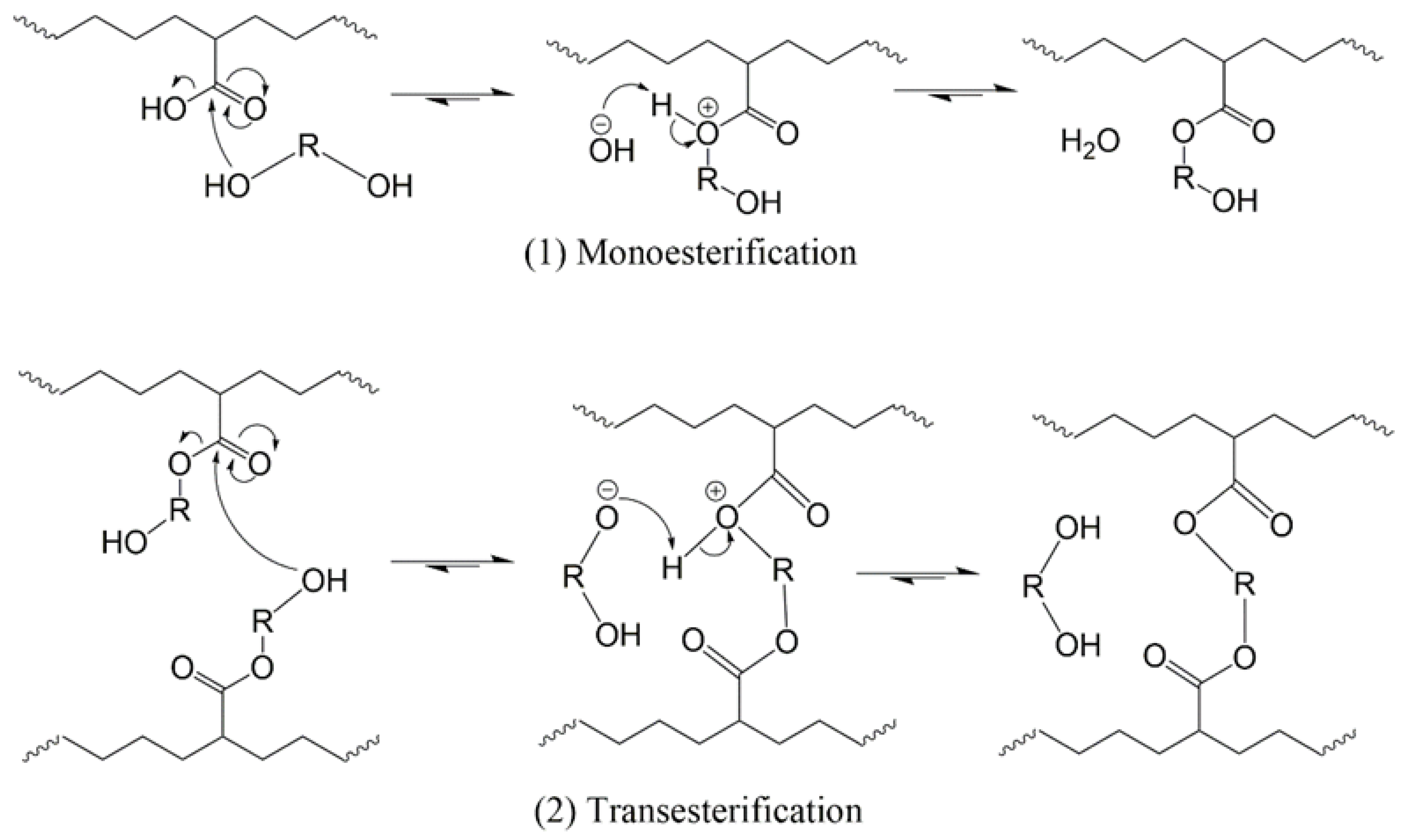
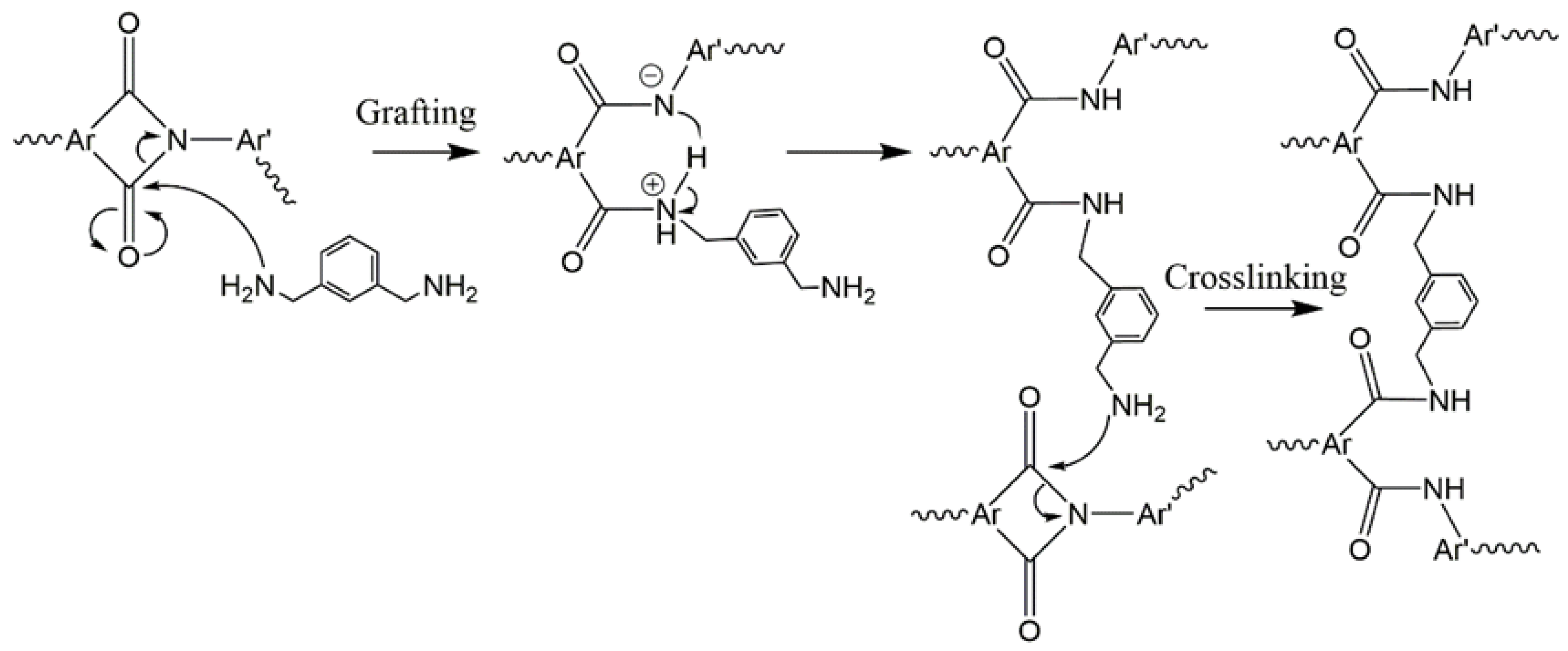
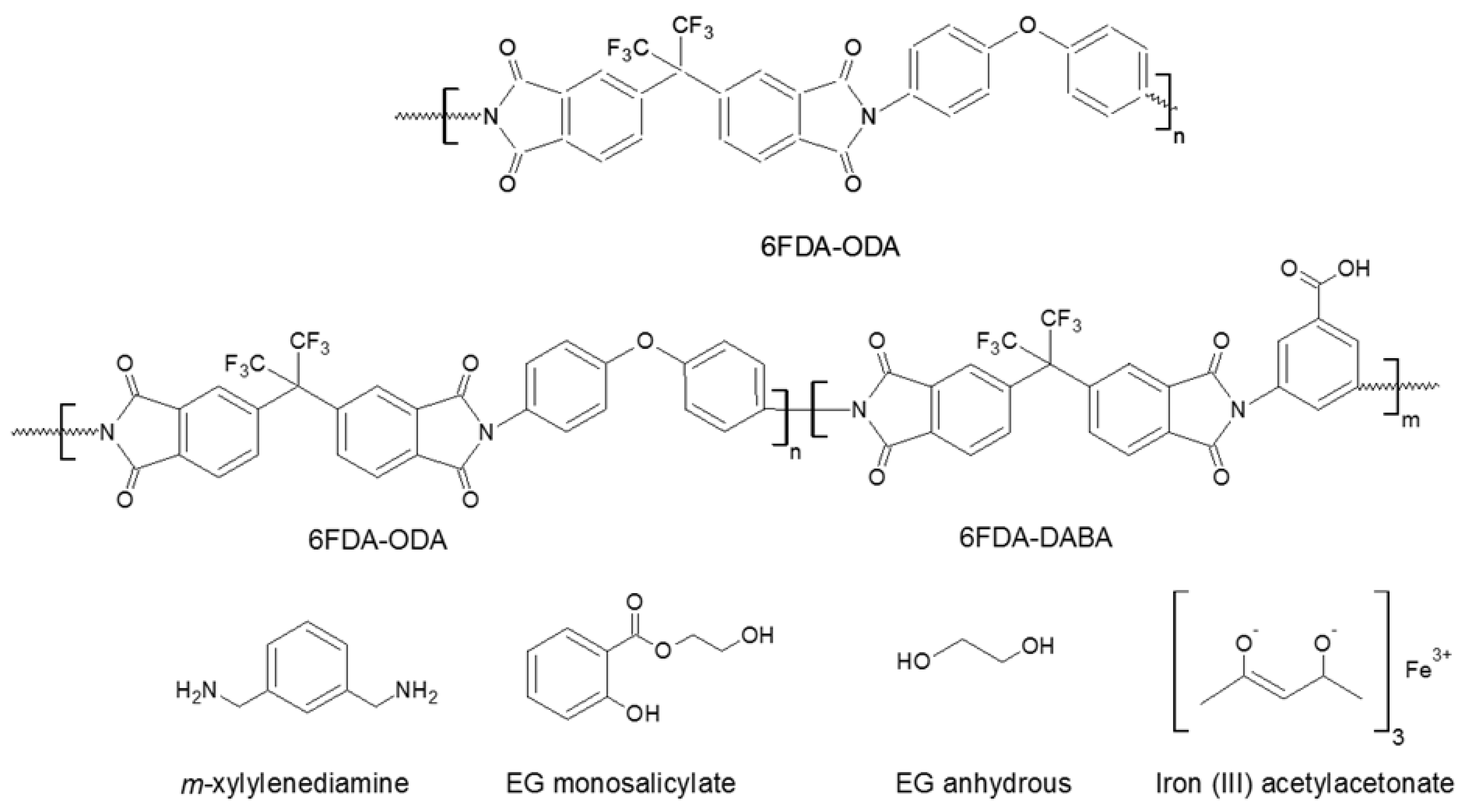
2. Materials and Methods
3. Results and Discussion
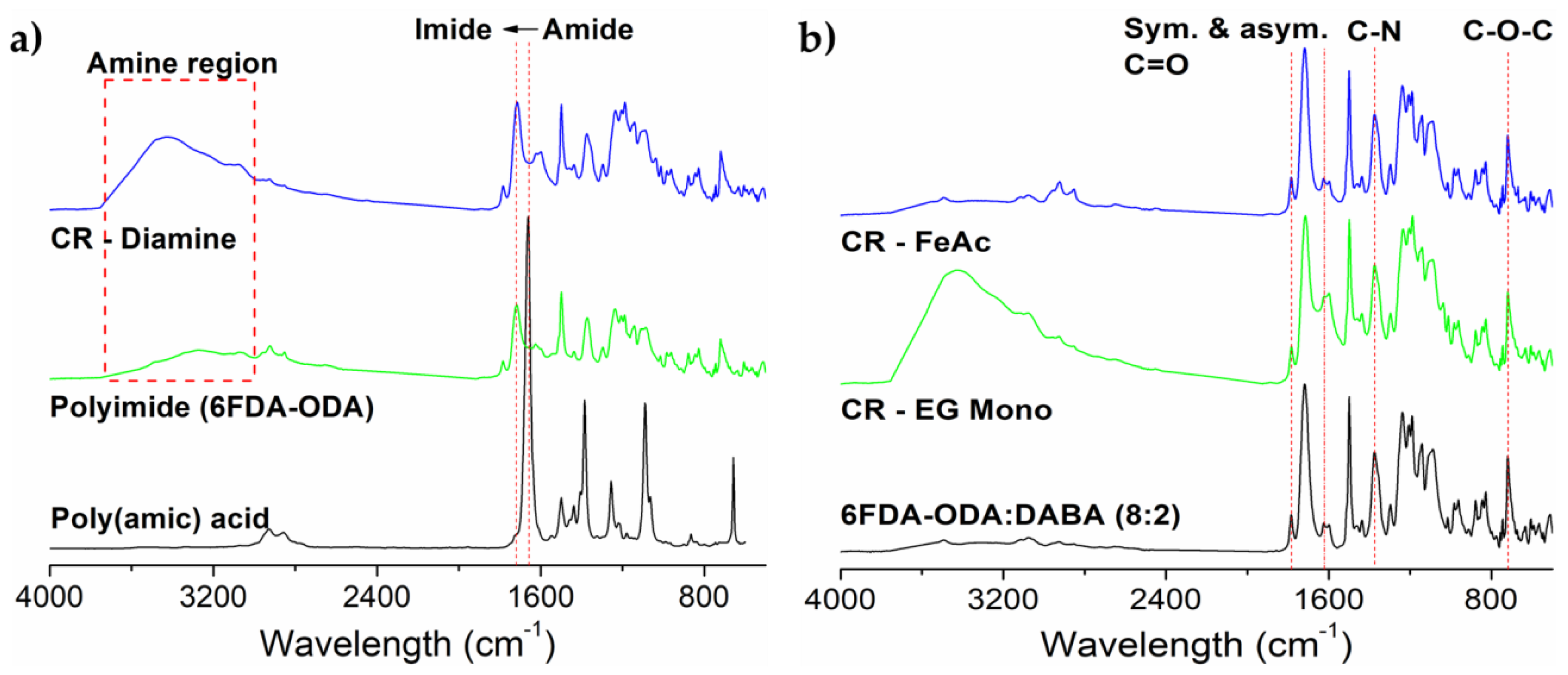
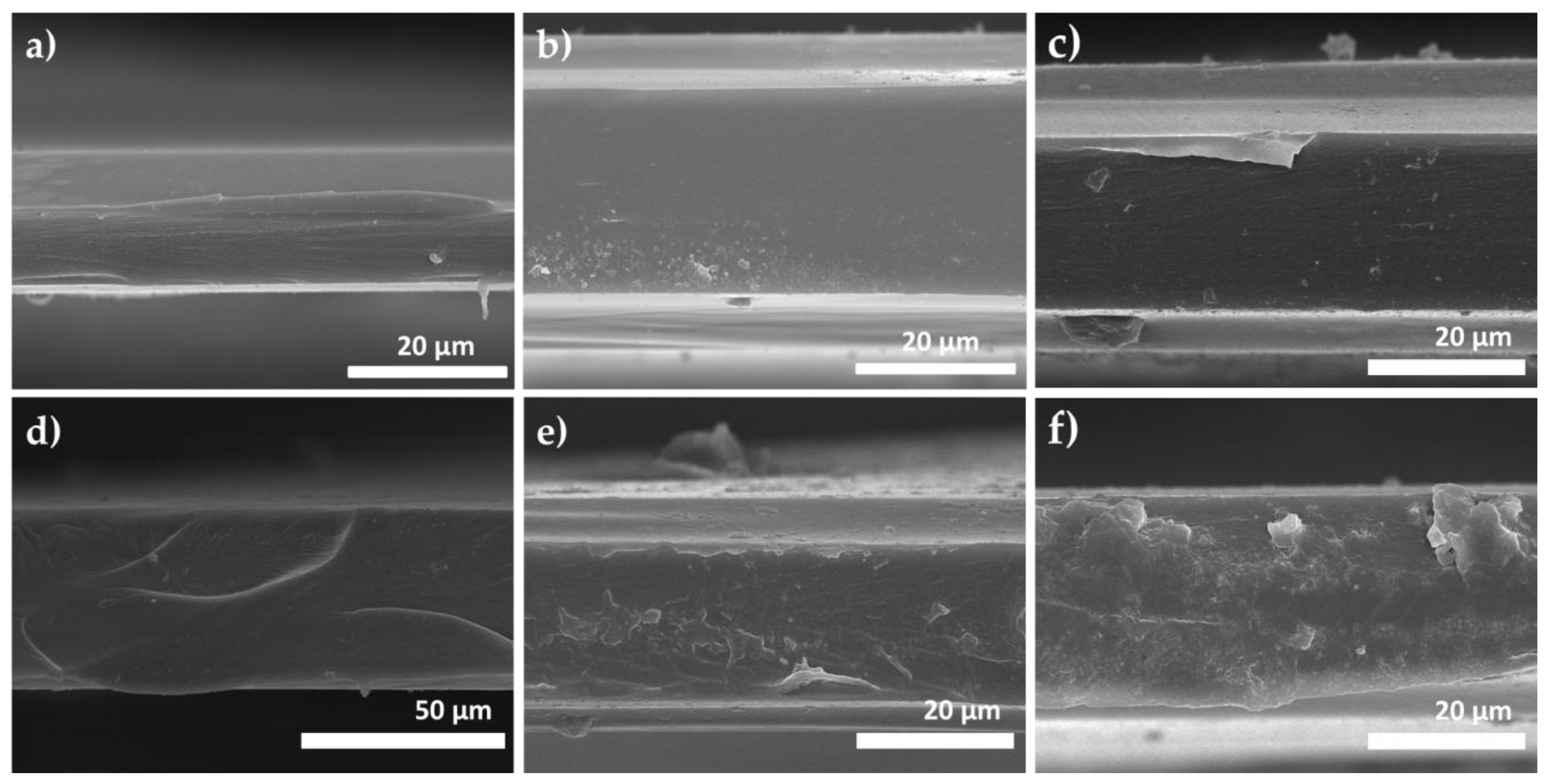
3.1. Membrane Characterizations
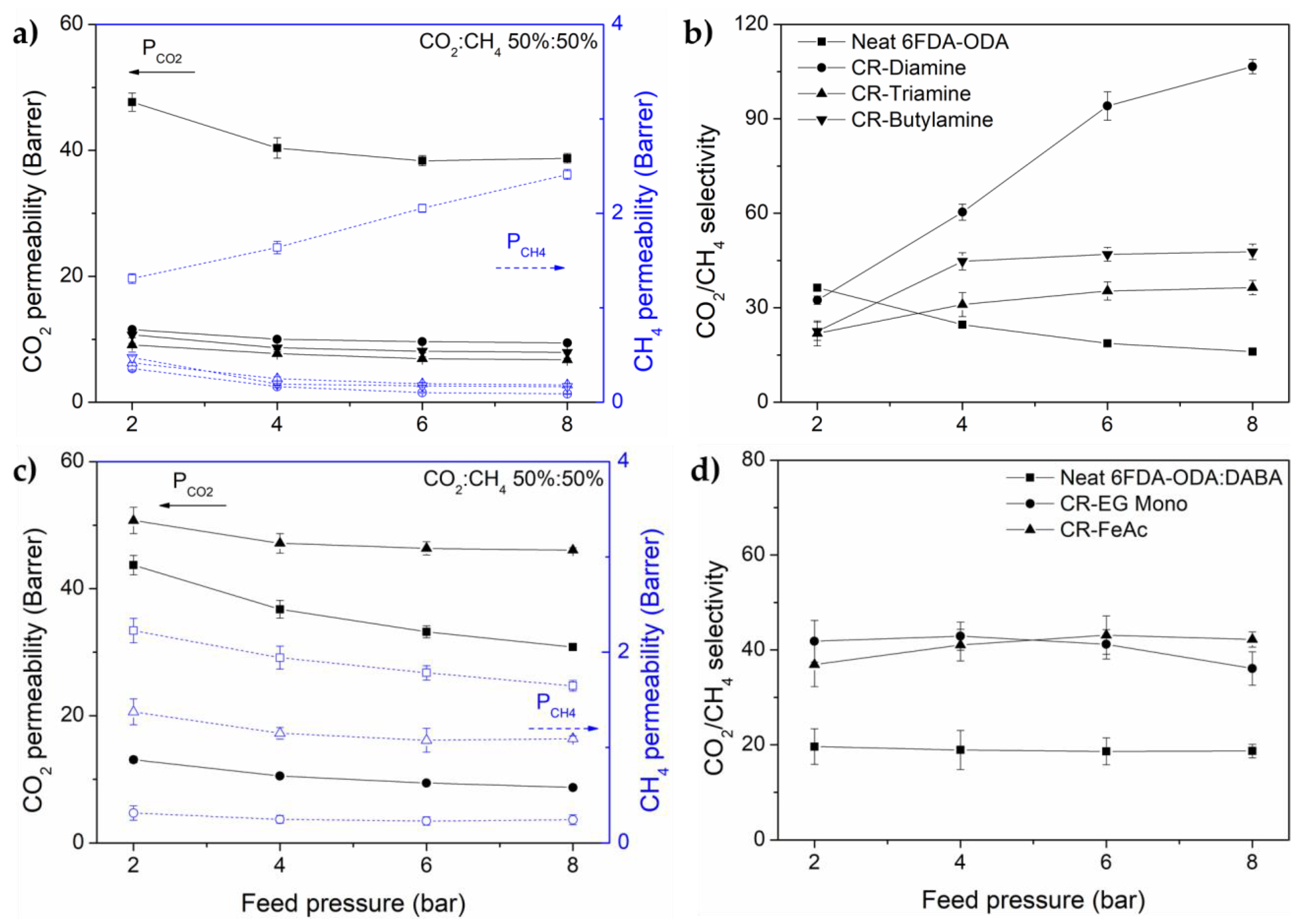
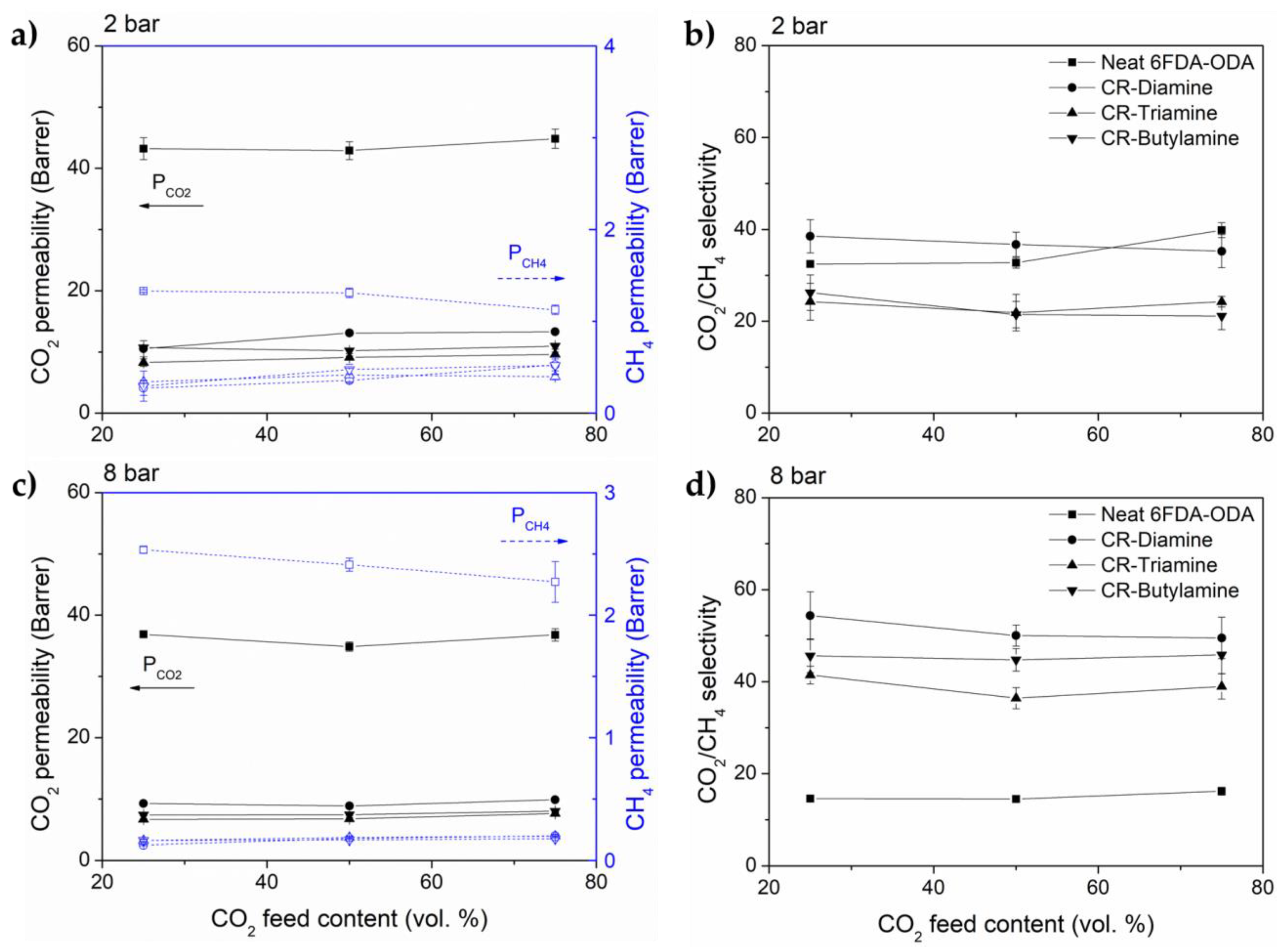
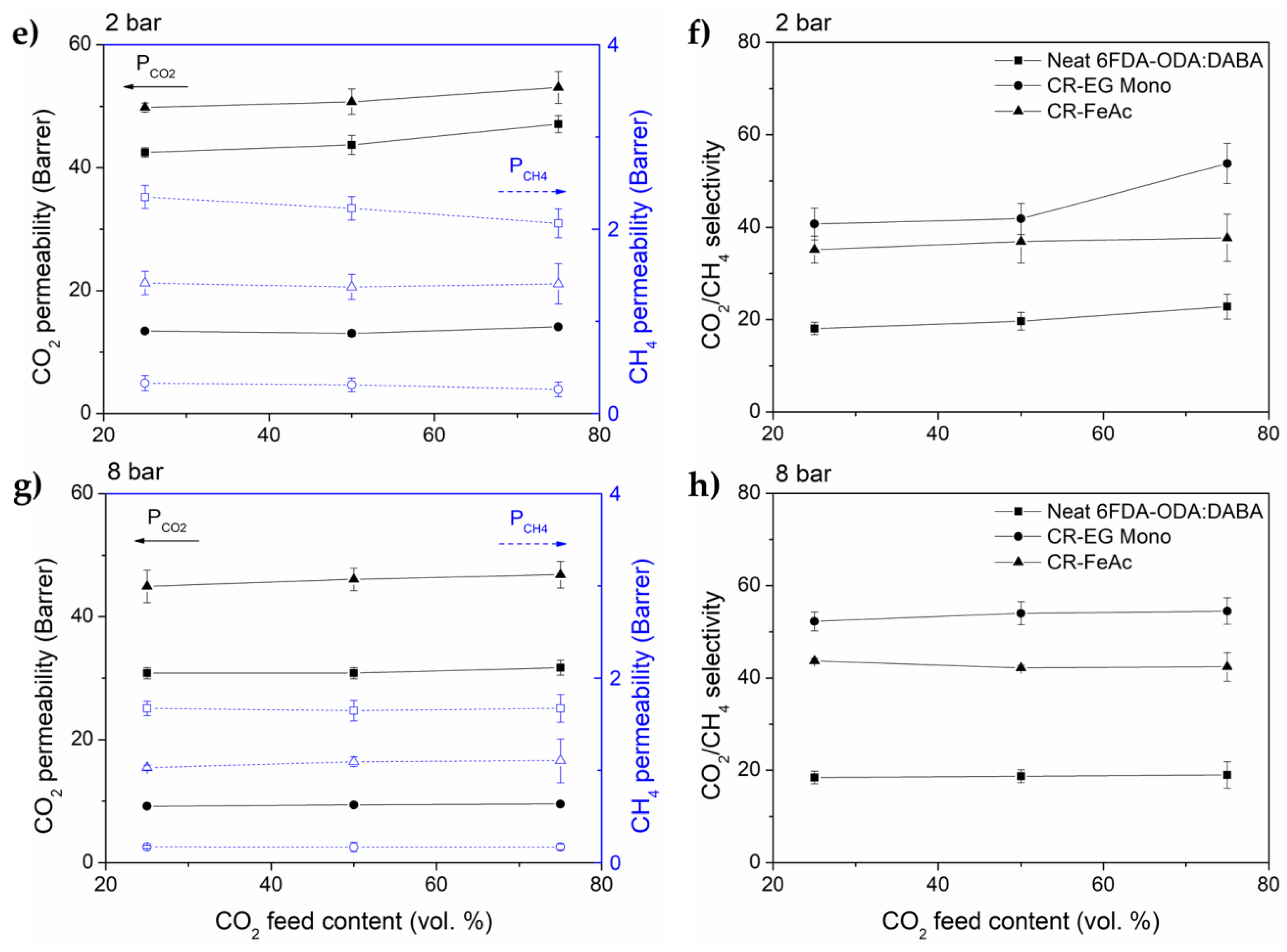
3.2. Gas Transport Properties
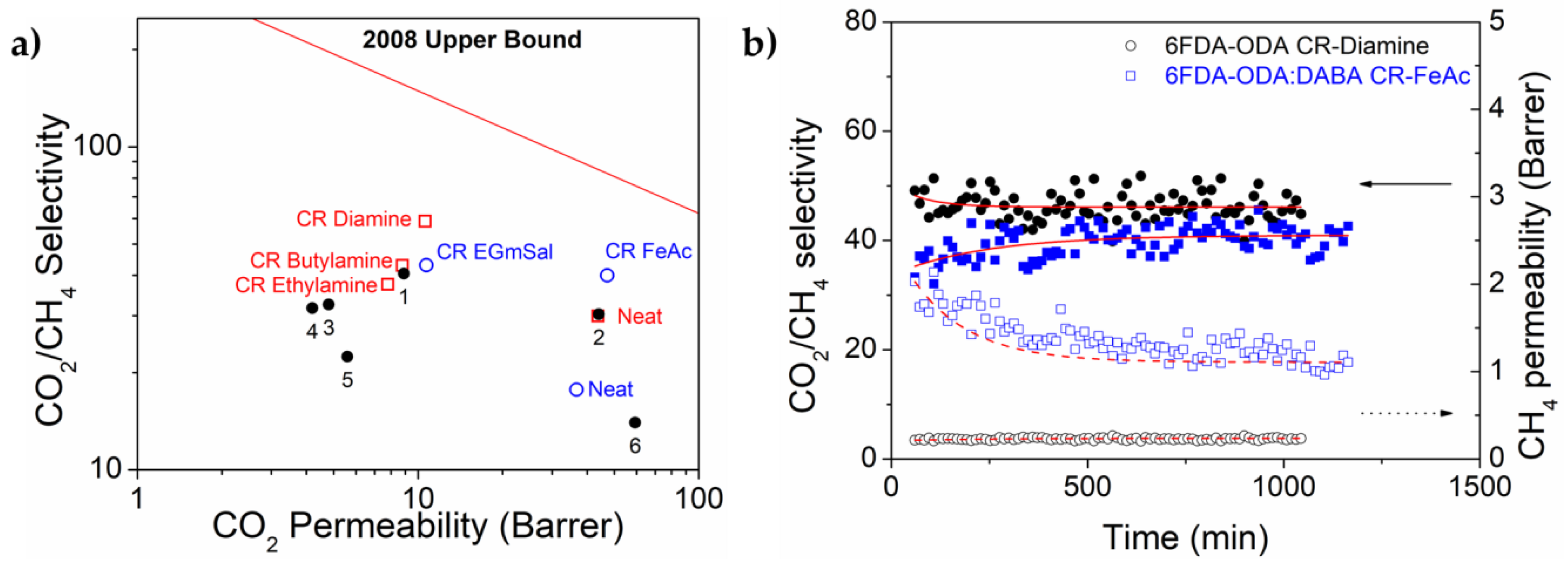
3.3. Performance Benchmarking and Its Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Chua, M.L.; Xiao, Y.C.; Chung, T.S. Modifying the molecular structure and gas separation performance of thermally labile polyimide-based membranes for enhanced natural gas purification. Chem. Eng. Sci. 2013, 104, 1056–1064. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Martin-Gil, V.; Ahmad, M.Z.; Fíla, V. Matrimid® 5218 in preparation of membranes for gas separation: Current state-of-the-art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Membr. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, R.Y.M.; Feng, X. Synthetic 6FDA-ODA copolyimide membranes for gas separation and pervaporation: Functional groups and separation properties. Polymer 2007, 48, 5355–5368. [Google Scholar] [CrossRef]
- Le, N.L.; Wang, Y.; Chung, T.S. Synthesis, cross-linking modifications of 6FDA-NDA/DABA polyimide membranes for ethanol dehydration via pervaporation. J. Membr. Sci. 2012, 415–416, 109–121. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef]
- Kim, J.H.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes. Part 1. Without crosslinking. J. Membr. Sci. 2006, 282, 21–31. [Google Scholar] [CrossRef]
- Visser, T.; Masetto, N.; Wessling, M. Materials dependence of mixed gas plasticization behavior in asymmetric membranes. J. Membr. Sci. 2007, 306, 16–28. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Y.; Ding, X.; Zhou, M.; Yuan, Q. Effects of cross-linkers with different molecular weights in cross-linked Matrimid 5218 and test temperature on gas transport properties. J. Membr. Sci. 2008, 323, 176–184. [Google Scholar] [CrossRef]
- Eguchi, H.; Kim, D.J.; Koros, W.J. Chemically cross-linkable polyimide membranes for improved transport plasticization resistance for natural gas separation. Polym. J. 2015, 58, 121–129. [Google Scholar] [CrossRef]
- Low, B.T.; Xiao, Y.; Chung, T.S.; Liu, Y. Simultaneous occurrence of chemical grafting, cross-linking, and etching on the surface of polyimide membranes and their impact on H2/CO2 separation. Macromolecules 2008, 41, 1297–1309. [Google Scholar] [CrossRef]
- Staudt-bickel, C.; Koros, W.J. Improvement of CO2/CH4 separation characteristics of polyimides by chemical crosslinking. J. Membr. Sci. 1999, 155, 145–154. [Google Scholar] [CrossRef]
- Hess, S.; Staudt, C. Variation of esterfication conditions to optimize solid-state crosslinking reaction of DABA-containing copolyimide membranes for gas separations. Desalination 2007, 217, 8–16. [Google Scholar] [CrossRef]
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. Solid-state covalent cross-linking of polyimide membranes for carbon dioxide plasticization reduction. Macromolecules 2003, 36, 1882–1888. [Google Scholar] [CrossRef]
- Chua, M.-L.; Xiao, Y.; Chung, T. Using iron (III) acetylacetonate as both a cross-linker and micropore former to develop polyimide membranes with enhanced gas separation performance. Sep. Purif. Technol. 2014, 133, 120–128. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, C.C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub-Tg cross-linking of a polyimide membrane for enhanced CO2 plasticization resistance for natural gas separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]
- Cao, C.; Wang, R.; Chung, T.S.; Liu, Y. Formation of high-performance 6FDA-2,6-DAT asymmetric composite hollow fiber membranes for CO2/CH4 separation. J. Membr. Sci. 2002, 209, 309–319. [Google Scholar] [CrossRef]
- Horn, N.R. A critical review of free volume and occupied volume calculation methods. J. Membr. Sci. 2016, 518, 289–294. [Google Scholar] [CrossRef]
- Hrabánek, P.; Zikánová, A.; Bernauer, B.; Fíla, V.; Kočiřík, M. Butane isomer separation with composite zeolite MFI mebranes. Desalination 2009, 245, 437–443. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Martin-gil, V.; Perfilov, V.; Sysel, P.; Fila, V. Investigation of a new co-polyimide, 6FDA-bisP and its ZIF-8 mixed matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 207, 523–534. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; Fila, V.; Coronas, J. Enhancement of CO2/CH4 separation performances of 6FDA-based co-polyimides mixed matrix membranes embedded with UiO-66 nanoparticles. Sep. Purif. Technol. 2018, 192, 465–474. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413–414, 48–61. [Google Scholar] [CrossRef]
- Park, J.; Paul, D.R. Correlation and Prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Hou, L.; Strounina, E.; Rudolph, V.; Zhu, Z. Mixed matrix membranes with strengthened MOFs/polymer interfacial interaction and improved membrane performance. ACS Appl. Mater. Interfaces 2014, 6, 5609–5618. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Bailo, F.; Caro, G.; Etxeberria, M.; Karvan, O.; Tellez, C.; Coronas, J. MOF-polymer enhanced compatibility: Post-annealed zeolite imidazolate framework membranes inside polyimide hollow fibers. RSC Adv. 2016, 6, 5881–5889. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ismail, A.F.; Matsuura, T. Prediction of gas permeability in mixed matrix membranes using theoretical models. J. Membr. Sci. 2010, 347, 53–61. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of uio-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.H.; Suh, M.P. Enhancing CO2 separation ability of a metal-organic framework by post-synthetic ligand exchange with flexible aliphatic carboxylates. Chem. Eur. J. 2014, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.L.; Xiao, Y.C.; Chung, T.S. Effects of thermally labile saccharide units on the gas separation performance of highly permeable polyimide membranes. J. Membr. Sci. 2012, 415–416, 375–382. [Google Scholar] [CrossRef]
- Bachman, J.E.; Smith, Z.P.; Li, T.; Xu, T.; Long, J.R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals. Nat. Mater. 2016, 15, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Visser, T.; Koops, G.H.; Wessling, M. On the subtle balance between competitive sorption and plasticization effects in asymmetric hollow fiber gas separation membranes. J. Membr. Sci. 2005, 252, 265–277. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer-MOF membranes for gas separation at elevated pressures. J. Membr. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Stannett, V. The transport of gases in synthetic polymeric membranes—An historic perspective. J. Membr. Sci. 1978, 3, 97–115. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Cakal, U.; Yilmaz, L.; Kalipcilar, H. Effect of feed gas composition on the separation of CO2/CH4 mixtures by PES-SAPO 34-HMA mixed matrix membranes. J. Membr. Sci. 2012, 417–418, 45–51. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
| Membranes | Td (°C) a | Tg (°C) | Density (g cm−3) | FFV b |
|---|---|---|---|---|
| Neat 6FDA-ODA | ||||
| [24] | 545 | 303 | 1.435 | 0.161 |
| [25] | 536 | 294 | 1.455 | 0.169 |
| This study | 549 | 309 | 1.413 | 0.174 |
| CR Diamine | 563 | 313 | 1.434 | 0.162 |
| CR Ethylamine | 567 | 318 | 1.451 | 0.152 |
| CR Butylamine | 569 | 322 | 1.471 | 0.140 |
| Neat 6FDA-ODA:DABA (8:2) | ||||
| This study | 538 | 263/327 | 1.366 | 0.148 |
| CR EG Mono | 545 | 316 | 1.379 | 0.140 |
| CR FeAc | 548 | 313 | 1.378 | 0.141 |
| Membranes | Neat 6FDA-ODA | CR-Diamine | CR-Triamine | CR-Butylamine |
| Permeability (Barrer) | ||||
| CO2 | 43.8 ± 1.6 | 10.6 ± 0.2 | 7.8 ± 0.4 | 8.8 ± 0.3 |
| CH4 | 1.5 ± 0.1 | 0.2 ± 0.0 * | 0.2 ± 0.0 * | 0.2 ± 0.0 * |
| Selectivity, αCO2/CH4 | 29.9 ± 1.2 | 58.8 ± 2.6 | 37.5 ± 3.8 | 42.9 ± 2.7 |
| Membranes | Neat 6FDA-ODA:DABA | CR-EG Mono | CR-FeAc | |
| Permeability (Barrer) | ||||
| CO2 | 36.7 ± 1.4 | 10.7 ± 0.3 | 47.2 ± 1.5 | |
| CH4 | 2.1 ± 0.1 | 0.2 ± 0.0 * | 1.2 ± 0.1 | |
| Selectivity, αCO2/CH4 | 17.7 ± 4.1 | 43.0 ± 3.4 | 40.0 ± 3.2 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.Z.; Pelletier, H.; Martin-Gil, V.; Castro-Muñoz, R.; Fila, V. Chemical Crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for Improved CO2/CH4 Separation. Membranes 2018, 8, 67. https://doi.org/10.3390/membranes8030067
Ahmad MZ, Pelletier H, Martin-Gil V, Castro-Muñoz R, Fila V. Chemical Crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for Improved CO2/CH4 Separation. Membranes. 2018; 8(3):67. https://doi.org/10.3390/membranes8030067
Chicago/Turabian StyleAhmad, Mohd Zamidi, Henri Pelletier, Violeta Martin-Gil, Roberto Castro-Muñoz, and Vlastimil Fila. 2018. "Chemical Crosslinking of 6FDA-ODA and 6FDA-ODA:DABA for Improved CO2/CH4 Separation" Membranes 8, no. 3: 67. https://doi.org/10.3390/membranes8030067





