Gas Transport in Glassy Polymers: Prediction of Diffusional Time Lag
Abstract
:1. Introduction
2. Theoretical Background
2.1. NELF Model
2.2. Transport Model
- (i)
- the thermodynamic factor α1;
- (ii)
- the mobility coefficient L1, which is a purely kinetic quantity.
2.3. Diffusional Time Lag
- (i)
- the problem of transient one-dimensional penetrant diffusion in a glassy polymeric membrane is solved numerically (finite elements method), considering the appropriate boundary conditions (for each upstream penetrant pressure) given by the solubility values provided by the NELF model, and using the local mobility values calculated from Equation (6);
- (ii)
- from the results obtained at each upstream pressure, the penetrant mass permeated per unit area Q(t) is calculated at any time according to Equation (9);
- (iii)
- the time lag is determined from the intercept on the time axis of the steady state line asymptotically reached by the quantity Q(t).
- (a)
- the solubility curve (penetrant concentration vs. pressure) is first inspected and described by the NELF model, which makes use of polymer density, as well as values of swelling (ksw) and binary interaction (k12) coefficients; when not available, the last two parameters are retrieved from the analysis of the experimental data;
- (b)
- steady state transport data (penetrant permeability vs. upstream pressure) is analyzed and modeled, using the solubility coefficient dependence of penetrant pressure provided by the NELF model, thus retrieving the parameters L10 and β;
- (c)
- transient transport properties (diffusional time lag vs. upstream pressure) are finally calculated in a predictive fashion, making use of the description of penetrant solubility and mobility obtained, with no additional parameters.
3. Results
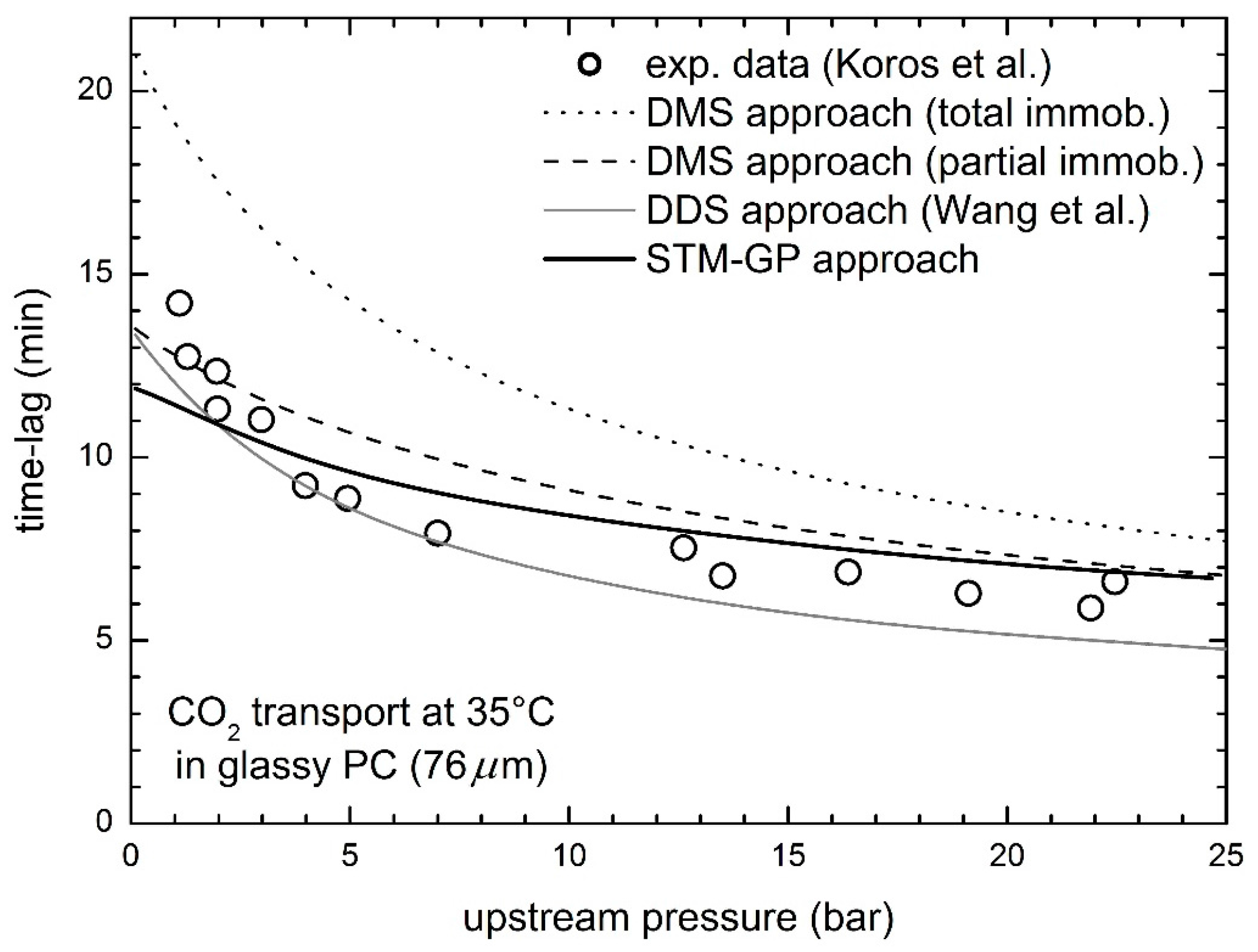
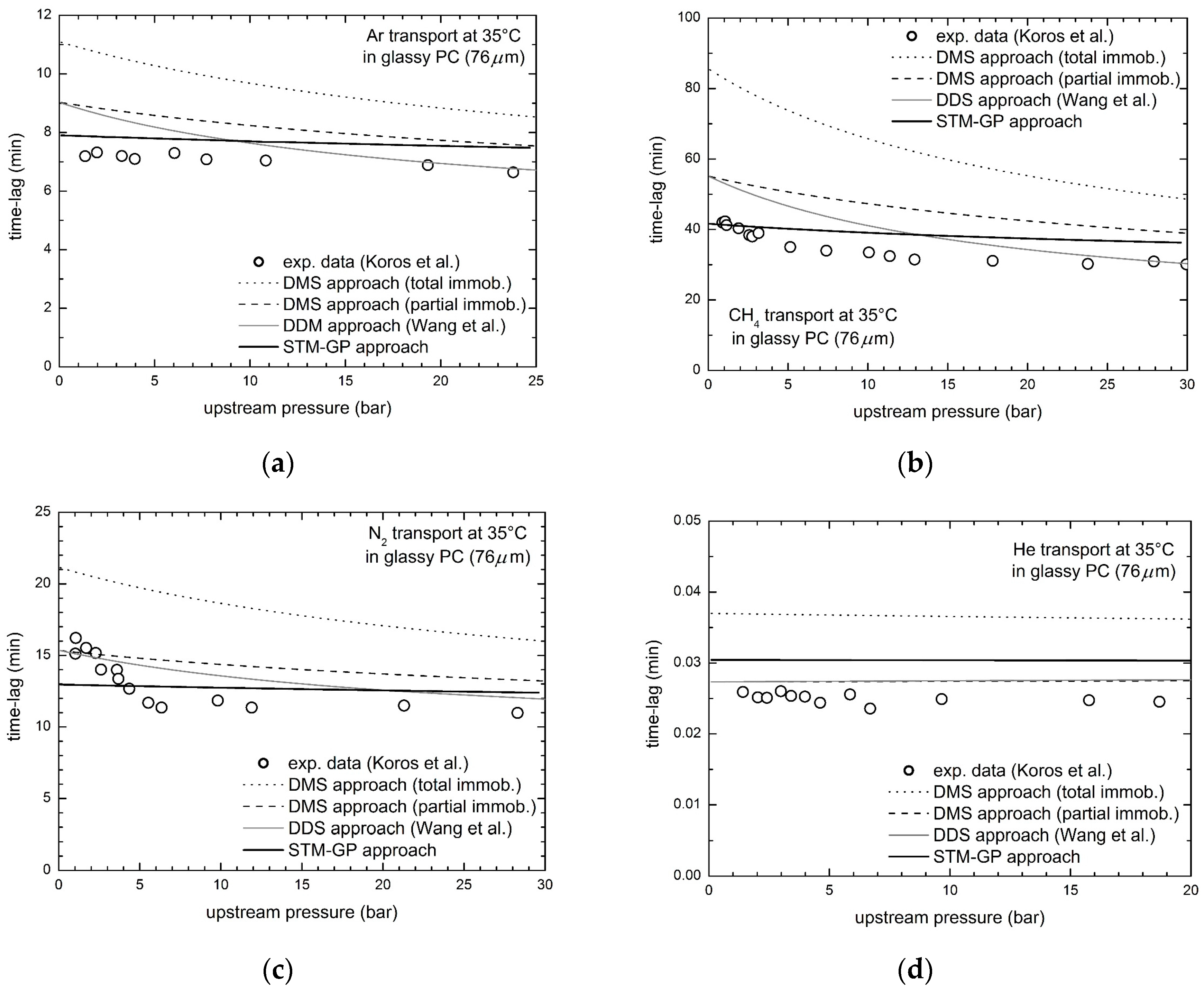
3.1. Gas Transport in Glassy Bisphenol a Polycarbonate (PC)
3.2. Gas Transport in Glassy PVC
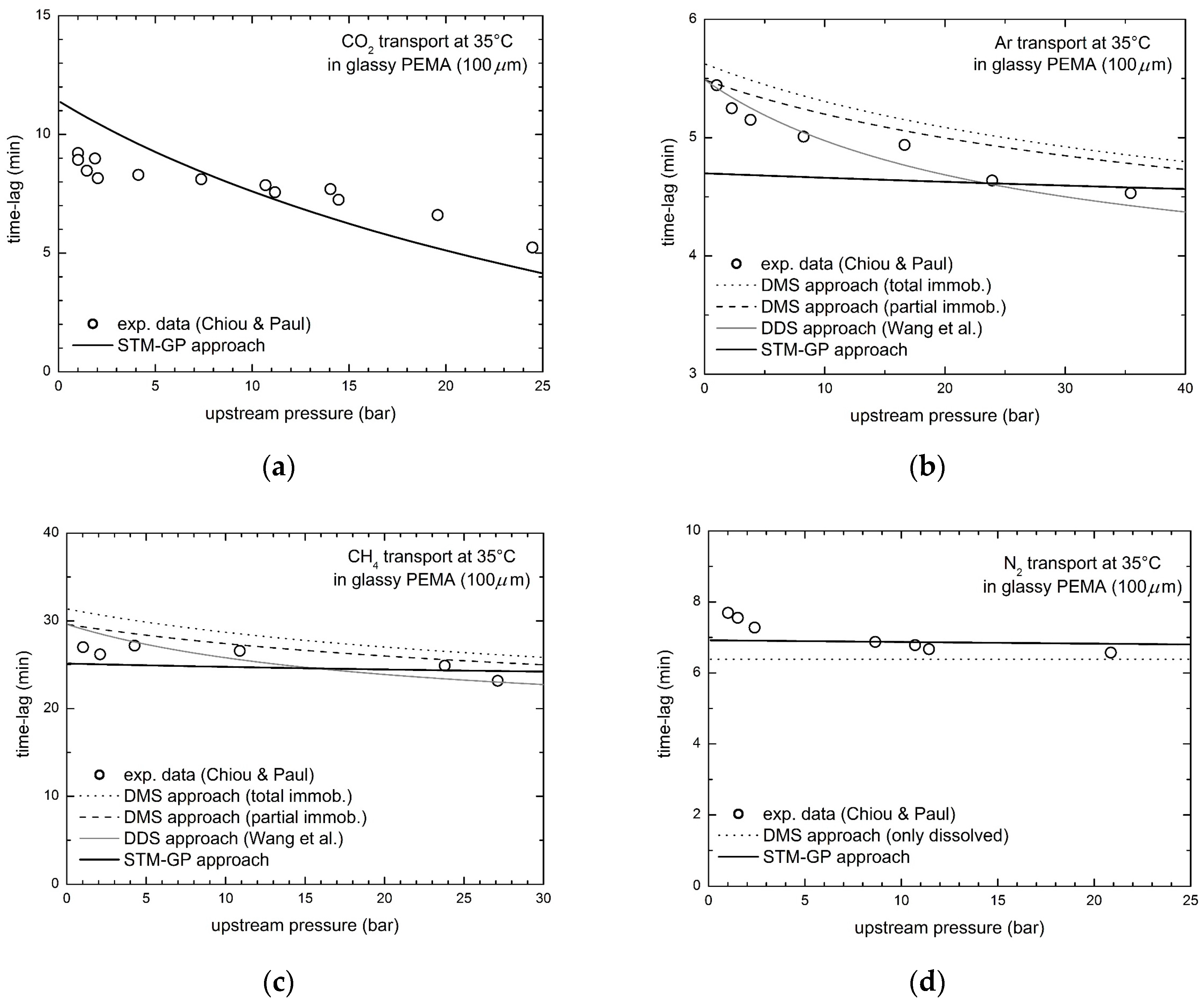
3.3. Gas Transport in Glassy Poly(Ethylmethacrylate) (PEMA)
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; He, G.; Wu, H.; Pan, F.; Jiang, Z. Facilitated transport of small molecules and ions for energy-efficient membranes. Chem. Soc. Rev. 2015, 44, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Sethu, S.P.; Ramos, M.; Robertson, J.; Al-Jumaily, A. A technical review on gas diffusion mechanism and medium of PEM fuel cell. Ionics 2015, 21, 1–18. [Google Scholar] [CrossRef]
- Teo, J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L.; Sung, T.W. Review on recent developments of fluorescent oxygen and carbon dioxide optical fiber sensors. Photonic Sens. 2011, 1, 234–250. [Google Scholar] [CrossRef]
- Rai, D.R.; Paul, S. Packaging requirements of highly respiring produce under modified atmosphere: A review. J. Food Sci. Technol. 2007, 44, 10–15. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Crank, J. Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Daynes, H.A. The process of diffusion through a rubber membrane. R. Soc. Proc. A 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Michaels, A.S.; Vieth, W.R.; Barrie, J.A. Solution of gases in polyethylene terephthalate. J. Appl. Phys. 1963, 34, 1–12. [Google Scholar] [CrossRef]
- Michaels, S.; Vieth, W.R.; Barrie, J.A. Diffusion of gases in polyethylene terephthalate. J. Appl. Phys. 1963, 34, 13–20. [Google Scholar] [CrossRef]
- Vieth, W.R.; Sladek, K.J. A model for diffusion in a glassy polymer. J. Colloid Sci. 1965, 20, 1014–1033. [Google Scholar] [CrossRef]
- Paul, D.R. Effect of immobilizing adsorption on the diffusion timelag. J. Polym. Sci. A 1969, 27, 1811–1818. [Google Scholar] [CrossRef]
- Paul, D.R.; Koros, W.J. Effect of partially immobilizing sorption of permeability and the diffusion timelag. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 675–685. [Google Scholar] [CrossRef]
- Tshudy, J.A.; Frankenberg, C.V. A model incorporating reversible immobilization for sorption and diffusion in glassy polymers. J. Polym. Sci. Polym. Phys. 1973, 11, 2027–2037. [Google Scholar] [CrossRef]
- Wang, L.; Corriou, J.P.; Castel, C.; Favre, E. Transport of gases in glassy polymers under transient conditions: Limit-behavior investigations of dual-mode sorption theory. Ind. Eng. Chem. Res. 2013, 52, 1089–1101. [Google Scholar] [CrossRef]
- Doghieri, F.; Biavati, D.; Sarti, G.C. Solubility and diffusivity of ethanol in PTMSP: Effects of activity and of polymer ageing. Ind. Eng. Chem. Res. 1996, 35, 2420–2430. [Google Scholar] [CrossRef]
- Galizia, M.; de Angelis, M.G.; Finkelshtein, E.; Yampolskii, Y.P.; Sarti, G.C. Sorption and transport of hydrocarbons and alcohols in addition-type poly(trimethyl silyl norbornene). I: Experimental data. J. Membr. Sci. 2011, 385–386, 141–153. [Google Scholar] [CrossRef]
- Chiou, J.S.; Paul, D.R. Sorption and transport of CO2 in PVF2/PMMA blends. J. Appl. Polym. Sci. 1986, 32, 2897–2918. [Google Scholar] [CrossRef]
- Bos, A.; Pünt, I.G.M.; Wessling, M.; Strathmann, H. CO2-induced plasticization phenomena in glassy polymers. J. Membr. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Bearman, R.J.; Kirkwood, J.G. Statistical mechanics of transport processes. XI. Equations of transport in multicomponent systems. J. Chem. Phys. 1958, 28, 136–145. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Permeability and diffusivity of CO2 in glassy polymers with and without plasticization. J. Membr. Sci. 2013, 435, 176–185. [Google Scholar] [CrossRef]
- Doghieri, F.; Sarti, G.C. Nonequilibrium lattice fluids: A predictive model for the solubility in glassy polymers. Macromolecules 1996, 29, 7885–7896. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C. Solubility of gases and liquids in glassy polymers. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Minelli, M.; Sarti, G.C. Permeability and solubility of carbon dioxide in different glassy polymer systems with and without plasticization. J. Membr. Sci. 2013, 444, 429–439. [Google Scholar] [CrossRef]
- Minelli, M. Modeling CO2 solubility and transport in poly(ethylene terephthalate) above and below the glass transition. J. Membr. Sci. 2014, 451, 305–311. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Gas permeability in glassy polymers: A thermodynamic approach. Fluid Phase Equilib. 2016, 424, 44–51. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Thermodynamic modeling of gas transport in glassy polymeric membranes. Membranes 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Minelli, M.; Sarti, G.C. Thermodynamic model for the permeability of light gases in glassy polymers. AIChE J. 2015, 61, 2776–2788. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Thermodynamic basis for vapor permeability in Ethyl Cellulose. J. Membr. Sci. 2015, 473, 137–145. [Google Scholar] [CrossRef]
- Minelli, M.; Sarti, G.C. Elementary prediction of gas permeability in glassy polymers. J. Membr. Sci. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Toni, E.; Minelli, M.; Sarti, G.C. A predictive model for the permeability of gas mixtures in glassy polymers. Fluid Phase Equilib. 2018, 455, 54–62. [Google Scholar] [CrossRef]
- Baschetti, M.G.; Doghieri, F.; Sarti, G.C. Solubility in glassy polymers: Correlations through the nonequilibrium lattice fluid model. Ind. Eng. Chem. Res. 2001, 40, 3027–3037. [Google Scholar] [CrossRef]
- Doghieri, F.; Sarti, G.C. Solubility, diffusivity, and mobility of n-pentane and ethanol in poly (1-trimethylsilyl-1-propyne). J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2245–2258. [Google Scholar] [CrossRef]
- Sarti, G.C.; de Angelis, M.G. Calculation of the solubility of liquids solutes in glassy polymers. AIChE J. 2012, 58, 292–301. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C. Solubility and diffusivity of gases in mixed matrix membranes containing hydrophobic fumed silica: Correlations and predictions based on the NELF model. Ind. Eng. Chem. Res. 2008, 47, 5214–5226. [Google Scholar] [CrossRef]
- Minelli, M.; Campagnoli, S.; de Angelis, M.G.; Sarti, F.G.C. Predictive model for the solubility of fluid mixtures in glassy polymers. Macromolecules 2011, 44, 4852–4862. [Google Scholar] [CrossRef]
- Minelli, M.; Friess, K.; Vopička, O.; de Angelis, M.G. Modeling gas and vapor sorption in a polymer of intrinsic microporosity (PIM-1). Fluid Phase Equilib. 2013, 347, 35–44. [Google Scholar] [CrossRef]
- Sanchez, C.; Lacombe, R.H. An elementary molecular theory of classical fluids. Pure fluids. J. Phys. Chem. 1976, 80, 2352–2362. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilib. 1989, 52, 31–38. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-chain SAFT: An equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Doghieri, F.; Quinzi, M.; Rethwisch, D.; Sarti, G.C. Advanced Materials for Membrane Separations; ACS Symposium Series; Pinnau, I., Freeman, B.D., Eds.; ACS: Washington, DC, USA, 2004; Volume 879, pp. 74–90. [Google Scholar]
- Sarti, G.C.; Doghieri, F. Predictions of the solubility of gases in glassy polymers based on the NELF model. Chem. Eng. Sci. 1998, 53, 3435–3447. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C.; Doghieri, F. NELF model prediction of the infinite dilution gas solubility in glassy polymers. J. Membr. Sci. 2007, 289, 106–122. [Google Scholar] [CrossRef]
- Rodgers, P.A. Pressure–volume–temperature relationships for polymeric liquids: A review of equations of state and their characteristic parameters for 56 polymers. J. Appl. Polym. Sci. 1993, 48, 1061–1080. [Google Scholar] [CrossRef]
- Sanchez, C.; Lacombe, R.H. Statistical thermodynamics of polymer solutions. Macromolecules 1978, 11, 1145–1156. [Google Scholar] [CrossRef]
- Minelli, M.; Doghieri, F. A predictive model for vapor solubility and volume dilation in glassy polymers. Ind. Eng. Chem. Res. 2012, 51, 16505–16516. [Google Scholar] [CrossRef]
- Minelli, M.; Doghieri, F. Predictive model for gas and vapor solubility and swelling in glassy polymers I: Application to different polymer/penetrant systems. Fluid Phase Equilib. 2014, 381, 1–11. [Google Scholar] [CrossRef]
- Koros, W.J.; Paul, D.R.; Rocha, A.A. Carbon dioxide sorption and transport in polycarbonate. J. Polym. Sci. Polym. Phys. Ed. 1976, 14, 687–702. [Google Scholar] [CrossRef]
- Koros, W.J.; Chan, A.H.; Paul, D.R. Sorption and transport of various gases in polycarbonate. J. Membr. Sci. 1977, 2, 165–190. [Google Scholar] [CrossRef]
- El-Hibri, M.J.; Paul, D.R. Effects of uniaxial drawing and heat-treatment on gas sorption and transport in PVC. J. Appl. Polym. Sci. 1985, 30, 3649–3678. [Google Scholar] [CrossRef]
- Chiou, J.S.; Paul, D.R. Gas sorption and permeation in poly(ethyl methacrylate). J. Membr. Sci. 1989, 45, 167–189. [Google Scholar] [CrossRef]



| Species | T* (K) | p* (MPa) | ρ* (g/cm3) | Ref. |
|---|---|---|---|---|
| CO2 | 300 | 630 | 1.515 | [24] |
| N2 | 145 | 160 | 0.943 | [44] |
| Ar | 190 | 180 | 1.400 | [45] |
| CH4 | 215 | 250 | 0.500 | [44] |
| He | 9.3 | 4 | 0.148 | [45] |
| PC | 755 | 534 | 1.275 | [24] |
| PVC | 680 | 620 | 1.487 | [30] |
| PEMA | 602 | 568 | 1.221 | [46] |
| Polymer | Gas | k12 | ksw (bar−1) |
|---|---|---|---|
| PC | CO2 | 0.022 | 0.00120 |
| N2 | −0.018 | 0.00004 | |
| Ar | 0.030 | 0.00009 | |
| CH4 | 0.035 | 0.00014 | |
| He | −1.090 | ≈0 | |
| PVC | CO2 | 0.080 | 0.00070 |
| N2 | 0.210 | ≈0 | |
| Ar | 0.206 | ≈0 | |
| CH4 | 0.142 | 0.00003 | |
| PEMA | CO2 | 0.025 | 0.00210 |
| N2 | 0.032 | 0.00003 | |
| Ar | 0.061 | 0.00009 | |
| CH4 | 0.017 | 0.00024 |
| Polymer | Gas | L10 (cm2/s) | β | Ref. |
|---|---|---|---|---|
| PC | CO2 | 1.3 × 10−8 | 16.2 | [23] |
| N2 | 1.2 × 10−8 | 0 | [30] | |
| Ar | 2.0 × 10−8 | 0 | [30] | |
| CH4 | 3.9 × 10−9 | 9 | [30] | |
| He | 5.2 × 10−6 | 0 | [30] | |
| PVC | CO2 | 1.5 × 10−9 | 22 | [30] |
| N2 | 1.8 × 10−9 | 0 | [30] | |
| Ar | 3.1 × 10−9 | 0 | [30] | |
| CH4 | 4.7 × 10−10 | 0 | [30] | |
| PEMA | CO2 | 2.5 × 10−8 | 39.5 | [23] |
| N2 | 4.2 × 10−8 | 0 | [30] | |
| Ar | 6.0 × 10−8 | 5 | [30] | |
| CH4 | 1.1 × 10−8 | 10 | [30] |
| Polymer | Gas | Relative Average Deviation εave | |||
|---|---|---|---|---|---|
| DMS-TI | DMS-PI | DDM | STM-GP | ||
| PC | CO2 | 45.2 | 13.5 | 11.4 | 11.0 |
| Ar | 40.4 | 18.3 | 11.8 | 9.3 | |
| CH4 | 92.6 | 30.0 | 21.6 | 12.2 | |
| N2 | 48.6 | 13.9 | 10.1 | 10.8 | |
| He | 47.6 | 9.4 | 9.6 | 21.5 | |
| PVC | CO2 | 8.2 | 4.6 | 17.2 | 4.3 |
| Ar | 26.9 | 24.4 | 18.4 | 7.4 | |
| CH4 | 39.2 | 18.6 | 7.9 | 5.7 | |
| N2 | 28.3 | 24.1 | 16.4 | 4.8 | |
| PEMA | CO2 | not applicable | 17.4 | ||
| Ar | 5.8 | 3.7 | 1.7 | 6.8 | |
| CH4 | 11.6 | 6.5 | 4.7 | 5.6 | |
| N2 | 10.3 | 10.3 | 10.3 | 4.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minelli, M.; Sarti, G.C. Gas Transport in Glassy Polymers: Prediction of Diffusional Time Lag. Membranes 2018, 8, 8. https://doi.org/10.3390/membranes8010008
Minelli M, Sarti GC. Gas Transport in Glassy Polymers: Prediction of Diffusional Time Lag. Membranes. 2018; 8(1):8. https://doi.org/10.3390/membranes8010008
Chicago/Turabian StyleMinelli, Matteo, and Giulio C. Sarti. 2018. "Gas Transport in Glassy Polymers: Prediction of Diffusional Time Lag" Membranes 8, no. 1: 8. https://doi.org/10.3390/membranes8010008






