Polymeric and Lipid Membranes—From Spheres to Flat Membranes and vice versa
Abstract
:1. Introduction
2. Remote Control of the Permeability of Membranes—Release of Encapsulated Cargo from Capsules
3. Release Stimulated by Laser and IR Light
4. Laser Induced
5. US-Induced
6. Microwave
7. Mechanical
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LbL membrane | layer-by-layer membrane |
| FO membrane | forward osmosis membrane |
| W/O/W emulsion | water-in-oil-in-water emulsion |
| UV | ultraviolet |
| NIR | near infrared |
| CLSM | confocal laser scanning microscopy |
| TEM | transmission electron microscopy |
| AFM | atomic-force microscopy |
| PS core | polystyrene core |
| NP | nanoparticle |
| CD | carbon dot |
| FITC | fluorescein isothiocyanate |
| MF | Melamine formaldehyde |
| HA/PLL | Hyaluronic Acid/Poly(l-lysine) |
| DES, DS | dextran sulfate |
| PDADMA | poly(diallyldimethylammonium chloride) |
| P(Am-DDA) | poly(acrylamide-co-diallyl-dimethylammonium chloride) |
| PAH | poly(allylamine hydrochloride) |
| PSS | poly(4-styrenesulfonic acid) sodium salt |
References
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Qi, S.; Tang, C.Y. Synthesis of high flux forward osmosis membranes by chemically crosslinked layer-by-layer polyelectrolytes. J. Membr. Sci. 2011, 381, 74–80. [Google Scholar] [CrossRef]
- Qi, S.; Qiu, C.Q.; Zhao, Y.; Tang, C.Y. Double-skinned forward osmosis membranes based on layer-by-layer assembly—FO performance and fouling behavior. J. Membr. Sci. 2012, 405–406, 20–29. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Zuo, J.; Chung, T.-S. Highly crosslinked layer-by-layer polyelectrolyte FO membranes: Understanding effects of salt concentration and deposition time on FO performance. J. Membr. Sci. 2013, 427, 411–421. [Google Scholar] [CrossRef]
- Dai, J.; Jensen, A.W.; Mohanty, D.K.; Erndt, J.; Bruening, M.L. Controlling the permeability of multilayered polyelectrolyte films through derivatization, cross-linking, and hydrolysis. Langmuir 2001, 17, 931–937. [Google Scholar] [CrossRef]
- Bruening, M.L.; Dotzauer, D.M.; Jain, P.; Ouyang, L.; Baker, G.L. Creation of functional membranes using polyelectrolyte multilayers and polymer brushes. Langmuir 2008, 24, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; An, Q.F.; Ji, Y.; Qian, J.; Gao, C. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J. Membr. Sci. 2011, 379, 19–45. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- De Grooth, J.; Haakmeester, B.; Wever, C.; Potreck, J.; de Vos, W.M.; Nijmeijer, K. Long term physical and chemical stability of polyelectrolyte multilayer membranes. J. Membr. Sci. 2015, 489, 153–159. [Google Scholar] [CrossRef]
- Liu, X.; Qi, S.; Li, Y.; Yang, L.; Cao, B.; Tang, C.Y. Synthesis and characterization of novel antibacterial silver nanocomposite nanofiltration and forward osmosis membranes based on layer-by-layer assembly. Water Res. 2013, 47, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhin, M.V.; Man, S.M.; Sadovoy, A.V.; Low, H.Y.; Sukhorukov, G.B. Peculiarities of polyelectrolyte multilayer assembly on patterned surfaces. Langmuir 2011, 27, 8430–8436. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhin, M.V.; Man, S.M.; Tonoyan, A.; Low, H.Y.; Sukhorukov, G.B. Adhesion of polyelectrolyte multilayers: Sealing and transfer of microchamber arrays. Langmuir 2012, 28, 5678–5686. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhin, M.V.; Gorelik, S.R.; Man, S.M.; Subramanian, G.S.; Antipina, M.N.; Low, H.Y.; Sukhorukov, G.B. Individually addressable patterned multilayer microchambers for site-specific release-on-demand. Macromol. Rapid Commun. 2013, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Antipina, M.N.; Sukhorukov, G.B. Remote control over guidance and release properties of composite polyelectrolyte based capsules. Adv. Drug Deliv. Rev. 2011, 63, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Antipina, M.N.; Kiryukhin, M.V.; Skirtach, A.G.; Sukhorukov, G.B. Micropackaging via layer-by-layer assembly: Microcapsules and microchamber arrays. Int. Mater. Rev. 2014, 59, 224–244. [Google Scholar] [CrossRef]
- Delcea, M.; Mohwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Koumoto, K.; Iseya, S.; Sakai, M.; Matsuda, A.; Caruso, F. Tunable UV-responsive organic−inorganic hybrid capsules. Chem. Mater. 2009, 21, 195–197. [Google Scholar] [CrossRef]
- Bédard, M.F.; Sadasivan, S.; Sukhorukov, G.B.; Skirtach, A.G. Assembling polyelectrolytes and porphyrins into hollow capsules with laser-responsive oxidative properties. J. Mater. Chem. 2009, 19, 2226–2233. [Google Scholar] [CrossRef]
- Erokhina, S.; Benassi, L.; Bianchini, P.; Diaspro, A.; Erokhin, V.; Fontana, M.P. Light-driven release from polymeric microcapsules functionalized with bacteriorhodopsin. J. Am. Chem. Soc. 2009, 131, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Desai, T.A. Microfabricated drug delivery systems: From particles to pores. Adv. Drug Deliv. Rev. 2003, 55, 315–328. [Google Scholar] [CrossRef]
- Marchenko, I.; Yashchenok, A.; Borodina, T.; Bukreeva, T.; Konrad, M.; Möhwald, H.; Skirtach, A. Controlled enzyme-catalyzed degradation of polymeric capsules templated on CaCO3: Influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J. Control. Release 2012, 162, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Antipov, A.A.; Shchukin, D.G.; Sukhorukov, G.B. Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir 2004, 20, 6988–6992. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.; Yashchenok, A.M.; Atkin, V.; Lapanje, A.; Gorin, D.A.; Sukhorukov, G.B.; Parakhonskiy, B.V. Hollow silver alginate microspheres for drug delivery and surface enhanced Raman scattering detection. RSC Adv. 2016, 6, 20447–20452. [Google Scholar] [CrossRef]
- Jiang, C.; Markutsya, S.; Pikus, Y.; Tsukruk, V.V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nat. Mater. 2004, 3, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.S.; Fedoruk, M.; Horton, M.R.; Rädler, J.O.; Stefani, F.D.; Feldmann, J. Controlled nanometric phase transitions of phospholipid membranes by plasmonic heating of single gold nanoparticles. Nano Lett. 2009, 9, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Gorin, D.A.; Portnov, S.A.; Inozemtseva, O.A.; Luklinska, Z.; Yashchenok, A.M.; Pavlov, A.M.; Skirtach, A.G.; Möhwald, H.; Sukhorukov, G.B. Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899. [Google Scholar] [CrossRef] [PubMed]
- Vöpel, T.; Scholz, R.; Davico, L.; Groß, M.; Büning, S.; Kareth, S.; Weidner, E.; Ebbinghaus, S. Infrared laser triggered release of bioactive compounds from single hard shell microcapsules. Chem. Commun. 2015, 51, 6913–6916. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; Delcea, M.; Möhwald, H.; Skirtach, A.G. Remote near-IR light activation of a hyaluronic acid/poly(l-lysine) multilayered film and film-entrapped microcapsules. ACS Appl. Mater. Interfaces 2009, 1, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, C.; Frueh, J.; Sun, J.; He, Q. Remote-controllable explosive polymer multilayer tubes for rapid cancer cell killing. Macromol. Rapid Commun. 2015, 36, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Carregal-Romero, S.; Rejman, J.; Braeckmans, K.; De Smedt, S.C.; Parak, W.J. Light-addressable capsules as caged compound matrix for controlled triggering of cytosolic reactions. Angew. Chem. Int. Ed. 2013, 52, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, T.A.; Gorin, D.A.; Fernandes, P.; Kessel, S.; Khomutov, G.B.; Fery, A.; Shchukin, D.G.; Möhwald, H. Nanocomposite microcontainers with high ultrasound sensitivity. Adv. Funct. Mater. 2010, 20, 1189–1195. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Saez, V.; Cobley, A.; Graves, J.; Sukhorukov, G.B.; Mason, T.J. Controlled protein release from microcapsules with composite shells using high frequency ultrasound—Potential for in vivo medical use. Soft Matter 2011, 7, 4341. [Google Scholar] [CrossRef]
- Gao, H.; Sapelkin, A.V.; Titirici, M.M.; Sukhorukov, G.B. In situ synthesis of fluorescent carbon dots/polyelectrolyte nanocomposite microcapsules with reduced permeability and ultrasound sensitivity. ACS Nano 2016, 10, 9608–9615. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.; Saveleva, M.; Abalymov, A.; Atkin, V.; Wuytens, P.C.; Kamyshinsky, R.; Vasiliev, A.L.; Gorin, D.A.; Sukhorukov, G.B.; Skirtach, A.G.; et al. Silver alginate hydrogel micro- and nano- containers for theranostics: Synthesis, encapsulation, remote release and detection. ACS Appl. Mater. Interfaces 2017, 9, 21949–21958. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-J.; Shi, H.; Ma, K.; Xie, M.; Tang, L.-L.; Shen, S.; Li, B.; Wang, X.-S.; Jin, Y. Polyelectrolyte capsules packaging BSA gels for pH-controlled drug loading and release and their antitumor activity. Acta Biomater. 2013, 9, 6123–6133. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ono, T.; Kashiwagi, Y.; Takahashi, S.; Sato, K.; Anzai, J. pH-dependent release of insulin from layer-by-layer-deposited polyelectrolyte microcapsules. Polymers (Basel) 2015, 7, 1269–1278. [Google Scholar] [CrossRef]
- Nugraha, C.; Bora, M.; Venkatraman, S.S. Release retardation of model protein on polyelectrolyte-coated PLGA nano- and microparticles. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liao, W.-C.; Sohn, Y.S.; Nechushtai, R.; Lu, C.-H.; Willner, I. Light-responsive and pH-responsive DNA microcapsules for controlled release of loads. J. Am. Chem. Soc. 2016, 138, 8936–8945. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Riutin, M.; Parak, W.J.; Willner, I. Programmed pH-responsive microcapsules for the controlled release of CdSe/ZnS quantum Dots. ACS Nano 2016, 10, 8683–8689. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sulejmanovic, D.; Moore, T.; Colvin, D.C.; Qi, B.; Mefford, O.T.; Gore, J.C.; Alexis, F.; Hwu, S.-J.; Anker, J.N. Iron-loaded magnetic nanocapsules for pH-triggered drug release and MRI imaging. Chem. Mater. 2014, 26, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.; Li, D.; Long, Y.; Song, K.; Tung, C.-H. Magnetic compression of polyelectrolyte microcapsules for controlled release. Langmuir 2015, 31, 11195–11199. [Google Scholar] [CrossRef] [PubMed]
- Carregal-Romero, S.; Guardia, P.; Yu, X.; Hartmann, R.; Pellegrino, T.; Parak, W.J. Magnetically triggered release of molecular cargo from iron oxide nanoparticle loaded microcapsules. Nanoscale 2015, 7, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Gonzalez, E.; Abbasi, A.Z.; Parak, W.J.; Puntes, V. Synthesis and evaluation of gold nanoparticle-modified polyelectrolyte capsules under microwave irradiation for remotely controlled release for cargo. J. Mater. Chem. 2011, 21, 11468. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Piludu, M.; Miguel, M.G.; Lindman, B.; Ceglie, A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015, 447, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Gorin, D.A.; Bäumler, H.; Skirtach, A.G. Temperature rise around nanoparticles. J. Therm. Anal. Calorim. 2017, 127, 895–904. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Möhwald, H.; Sukhorukov, G.B. The role of metal nanoparticles in remote release of encapsulated materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Depero, L.E. Using plasmonic heating of gold nanoparticles to generate local SER(R)S-active TiO2 spots. Chem. Commun. 2009, 2359–2361. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Karageorgiev, P.; Bédard, M.F.; Sukhorukov, G.B.; Möhwald, H. Reversibly permeable nanomembranes of polymeric microcapsules. J. Am. Chem. Soc. 2008, 130, 11572–11573. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, T.V.; Parakhonskiy, B.V.; Skirtach, A.G.; Susha, A.S.; Sukhorukov, G.B. Preparation of polyelectrolyte microcapsules with silver and gold nanoparticles in a shell and the remote destruction of microcapsules under laser irradiation. Crystallogr. Rep. 2006, 51, 863–869. [Google Scholar] [CrossRef]
- Bukreeva, T.V.; Parakhonskiy, B.V.; Marchenko, I.V.; Khlebtsov, B.N.; Khlebtsov, N.G.; Dementieva, O.V.; Savvateev, M.N.; Feigin, L.A.; Kovalchuk, M.V. Polyelectrolyte microcapsules with a shell containing silver and gold nanoparticles, based on calcium carbonate and polystyrene cores. Nanotechnol. Russ. 2008, 3, 85–93. [Google Scholar] [CrossRef]
- Bédard, M.F.; Skirtach, A.G.; Sukhorukov, G.B. Optically driven encapsulation using novel polymeric hollow shells containing an azobenzene polymer. Macromol. Rapid Commun. 2007, 28, 1517–1521. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Bedard, M.; Bukreeva, T.V.; Sukhorukov, G.B.; Möhwald, H.; Skirtach, A.G. Nanoparticles on polyelectrolytes at low concentration: controlling concentration and size. J. Phys. Chem. C 2010, 114, 1996–2002. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Déjugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Sukhorukov, G.B. Nanoparticles distribution control by polymers: aggregates versus nonaggregates. J. Phys. Chem. C 2007, 111, 555–564. [Google Scholar] [CrossRef]
- Bédard, M.F.; Braun, D.; Sukhorukov, G.B.; Skirtach, A.G. Toward self-assembly of nanoparticles on polymeric microshells: Near-IR release and permeability. ACS Nano 2008, 2, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Bukreeva, T.V.; Marchenko, I.V.; Parakhonskiy, B.V.; Grigor’ev, Y.V. Formation of silver nanoparticles on shells of polyelectrolyte capsules using silver-mirror reaction. Colloid J. 2009, 71, 596. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Konrad, M.; Skirtach, A.G. Colloidal micro- and nano-particles as templates for polyelectrolyte multilayer capsules. Adv. Colloid Interface Sci. 2014, 207, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Palankar, R.; Pinchasik, B.-E.; Khlebtsov, B.N.; Kolesnikova, T.A.; Mowald, H.; Winterhalter, M.; Skirtach, A.G. Nanoplasmonically-induced defects in lipid membrane monitored by ion current: Transient nanopores versus membrane rupture. Nano Lett. 2014, 14, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Belkin, M.; Aksimentiev, A. Molecular dynamics simulation of DNA capture and transport in heated nanopores. ACS Appl. Mater. Interfaces 2016, 8, 12599–12608. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.S.; Pfeiffer, T.; Fedoruk, M.; Lutich, A.A.; Feldmann, J. Single-step injection of gold nanoparticles through phospholipid membranes. ACS Nano 2011, 5, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Csaki, A.; Garwe, F.; Steinbrück, A.; Maubach, G.; Festag, G.; Weise, A.; Riemann, I.; König, K.; Fritzsche, W. A parallel approach for subwavelength molecular surgery using gene-specific positioned metal nanoparticles as laser light antennas. Nano Lett. 2007, 7, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Giner-Casares, J.J.; Liz-Marzán, L.M. Plasmonic nanoparticles in 2D for biological applications: Toward active multipurpose platforms. Nano Today 2014, 9, 365–377. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Hastings, C.L.; Ozbakir, B.; O’Donnell, K.E.; O’Brien, F.J.; Storm, G.; Hennink, W.E.; Duffy, G.P.; Ruiz-Hernández, E. Hyperthermia-induced drug delivery from thermosensitive liposomes encapsulated in an injectable hydrogel for local chemotherapy. Adv. Healthc. Mater. 2014, 3, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xiao, Y.; Pan, M.; Li, F.; Duan, W.; Meng, L.; Liu, X.; Yan, F.; Zheng, H. Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J. Control. Release 2016, 243, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J.H.; Nordlander, P.; Weiss, P.S. Virtual issue on plasmonics. ACS Nano 2011, 5, 4245–4248. [Google Scholar] [CrossRef] [PubMed]
- Preiss, M.R.; Bothun, G.D. Stimuli-responsive liposome-nanoparticle assemblies. Expert Opin. Drug Deliv. 2011, 8, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.-A.; Tsai, P.-J.; Wang, Y.-C.; Wang, Y.-J.; Lo, L.-W.; Yang, C.-S. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology 2009, 20, 135101. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E. Nanoparticle-triggered release from lipid membrane vesicles. New Biotechnol. 2015, 32, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Gorin, D.A.; Möhwald, H. Ultrasonically induced opening of polyelectrolyte microcontainers. Langmuir 2006, 22, 7400–7404. [Google Scholar] [CrossRef] [PubMed]
- Yashchenok, A.M.; Delcea, M.; Videnova, K.; Jares-Erijman, E.A.; Jovin, T.M.; Konrad, M.; Möhwald, H.; Skirtach, A.G. Enzyme reaction in the pores of CaCO3 particles upon ultrasound disruption of attached substrate-filled liposomes. Angew. Chem. Int. Ed. Engl. 2010, 49, 8116–8120. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.M.; Schelkopf, S.R.; Jackson, A.C.; Landry, A.M.; Braun, P.V.; Moore, J.S. Microcapsules containing suspensions of carbon nanotubes. J. Mater. Chem. 2009, 19, 6093. [Google Scholar] [CrossRef]
- Fernandes, P.A.L.; Delcea, M.; Skirtach, A.G.; Möhwald, H.; Fery, A. Quantification of release from microcapsules upon mechanical deformation with AFM. Soft Matter 2010, 6, 1879. [Google Scholar] [CrossRef]
- Palankar, R.; Pinchasik, B.-E.; Schmidt, S.; De Geest, B.G.; Fery, A.; Möhwald, H.; Skirtach, A.G.; Delcea, M. Mechanical strength and intracellular uptake of CaCO3-templated LbL capsules composed of biodegradable polyelectrolytes: The influence of the number of layers. J. Mater. Chem. B 2013, 1, 1175. [Google Scholar] [CrossRef]
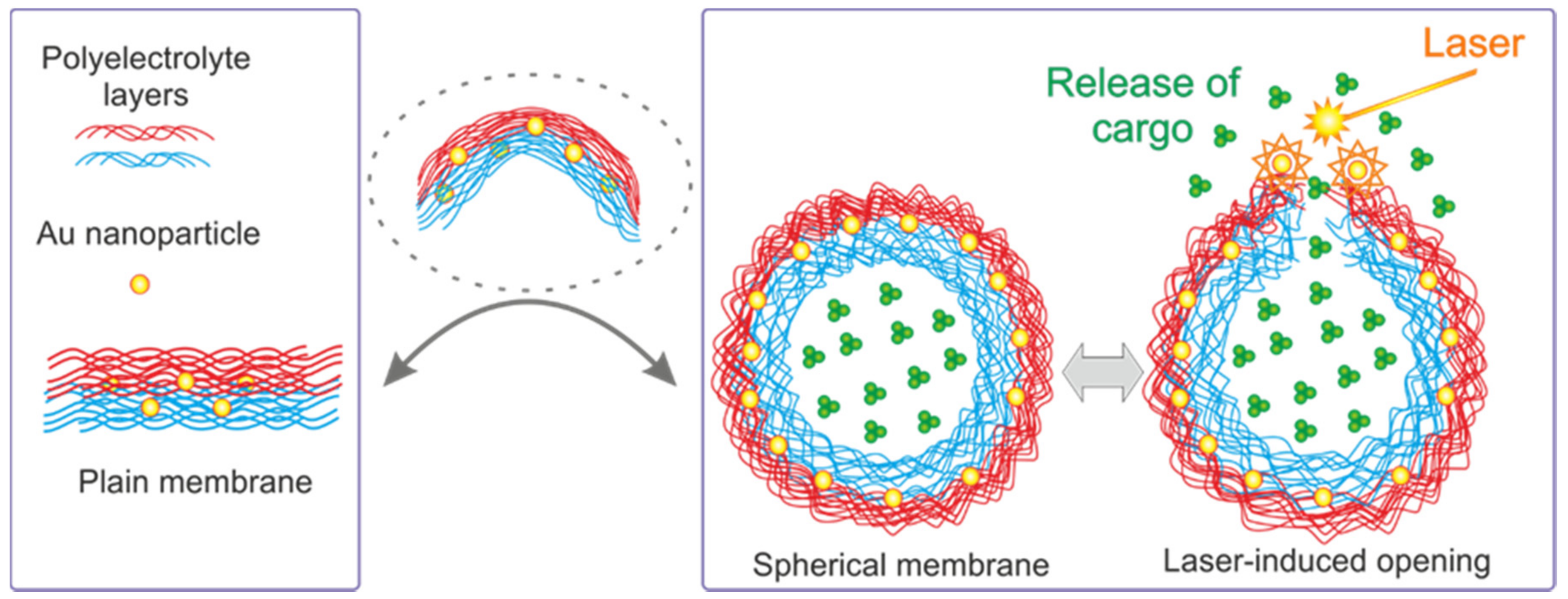


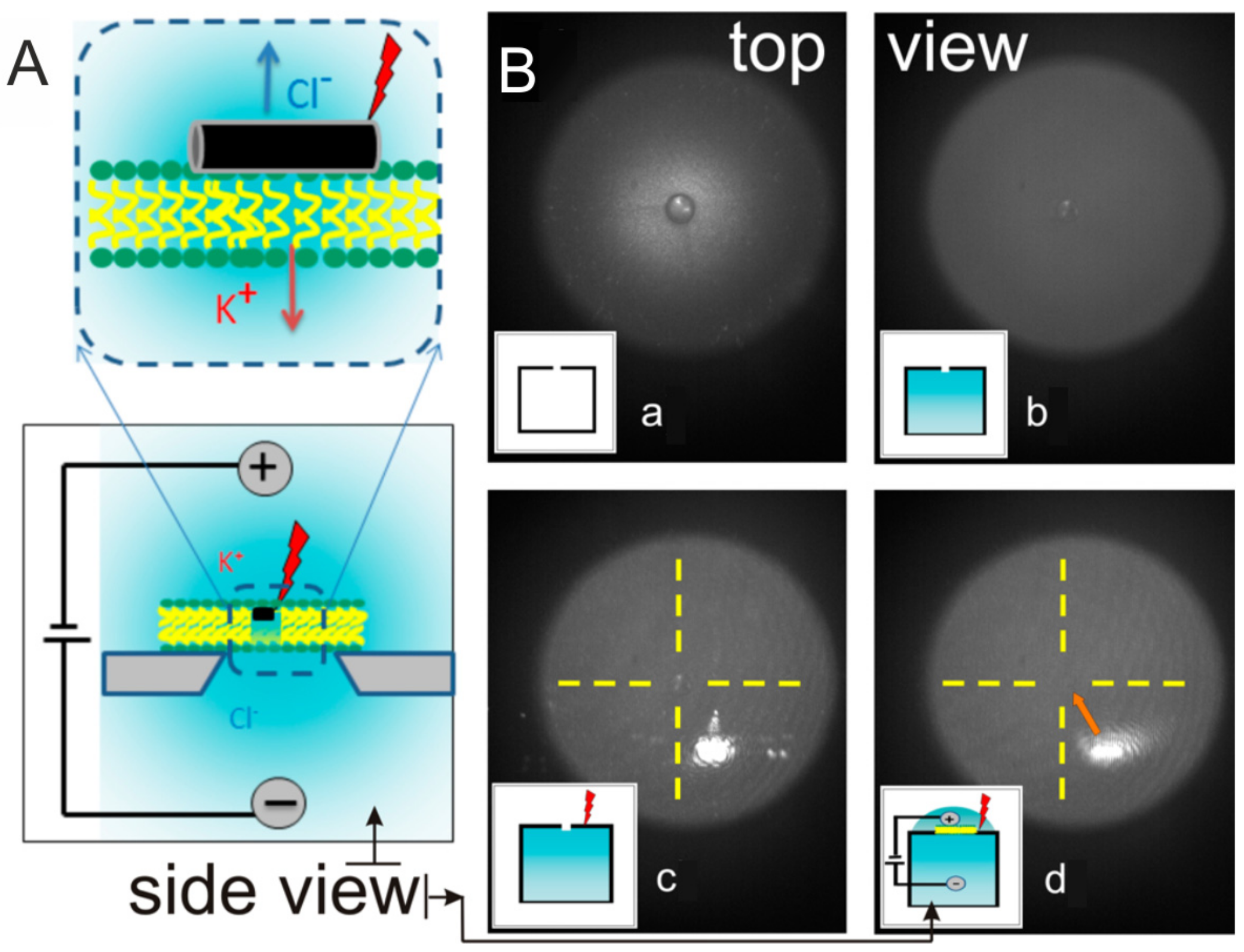
| Technique of Release | Membrane Material | Functionalization | Core/Template |
|---|---|---|---|
| Laser-induced, laser heating | PAH/PSS | Ag nanoparticles [23], Au nanoparticles [24], Bacteriorhodopsin [20] | MF latex particles [23,24], CaCO3 particles [20] |
| Alginate | Ag nanoparticles [25] | CaCO3 particles [25] | |
| Phospholipids [26] | Au nanoparticles [27] | – | |
| Poly(arginine)/DES | Fe3O4 nanoparticles and Au nanoparticles [28] | CaCO3 particles [28] | |
| NIR irradiation | PAH/PSS Witepsol W 31 (W/O/W emulsion) [29], (HA/PLL)24/PLL [30] | Gold nanoparticles [30,31,32] | Porous polycarbonate membrane [31], CaCO3 particles [32] |
| Ultrasonic irradiation | PAH/PSS | ZnO particles [33], Au nanoparticles [34], Fluorescent carbon dots (CDs) [35] | CaCO3 particles [33,34,35] |
| Silver alginate | Ag nanopartiles [36] | CaCO3 particles [36] | |
| pH-induced | PAH/PSS | – | CaCO3 particles [37,38], PLGA [39] |
| PLL/DES [39] | – | PLGA [39] | |
| PAH/PVS [38] | – | CaCO3 particles [38] | |
| PAH/DES [38] | – | CaCO3 particles [38] | |
| PAH/nucleic acid [40,41] | – | CaCO3 particles [40,41] | |
| Alginate/Poly-L-ysine | Au nanoparticles [42] | Fe-SiO2 [42] | |
| Magnetic Field | PDADMA/PSS [43], PSS/PAH/P(Am-DDA) [44] | Iron oxide nanocubes [44] | Fe(CO)5@SiO2 [43], CaCO3 particles [44] |
| Microwave irradiation | PAH/PSS | Au nanoparticles [45] | CaCO3 particles [45] |
| Induced by nonionic surfactant | Chitosan/alginate [46] | – | Liposomes [46] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saveleva, M.S.; Lengert, E.V.; Gorin, D.A.; Parakhonskiy, B.V.; Skirtach, A.G. Polymeric and Lipid Membranes—From Spheres to Flat Membranes and vice versa. Membranes 2017, 7, 44. https://doi.org/10.3390/membranes7030044
Saveleva MS, Lengert EV, Gorin DA, Parakhonskiy BV, Skirtach AG. Polymeric and Lipid Membranes—From Spheres to Flat Membranes and vice versa. Membranes. 2017; 7(3):44. https://doi.org/10.3390/membranes7030044
Chicago/Turabian StyleSaveleva, Mariia S., Ekaterina V. Lengert, Dmitry A. Gorin, Bogdan V. Parakhonskiy, and Andre G. Skirtach. 2017. "Polymeric and Lipid Membranes—From Spheres to Flat Membranes and vice versa" Membranes 7, no. 3: 44. https://doi.org/10.3390/membranes7030044







