Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review
Abstract
:1. Introduction
2. Electro-Conductive Membranes
2.1. Electro-Conductive Polymers
- Intrinsic electro-conductive polymers, characterized by conjugated π-π or p-π systems;
- Redox polymers that possess redox potentials within their structure groups (reduction/oxidation capacity).
2.2. Preparation of Electro-Responsive Polymer Membranes
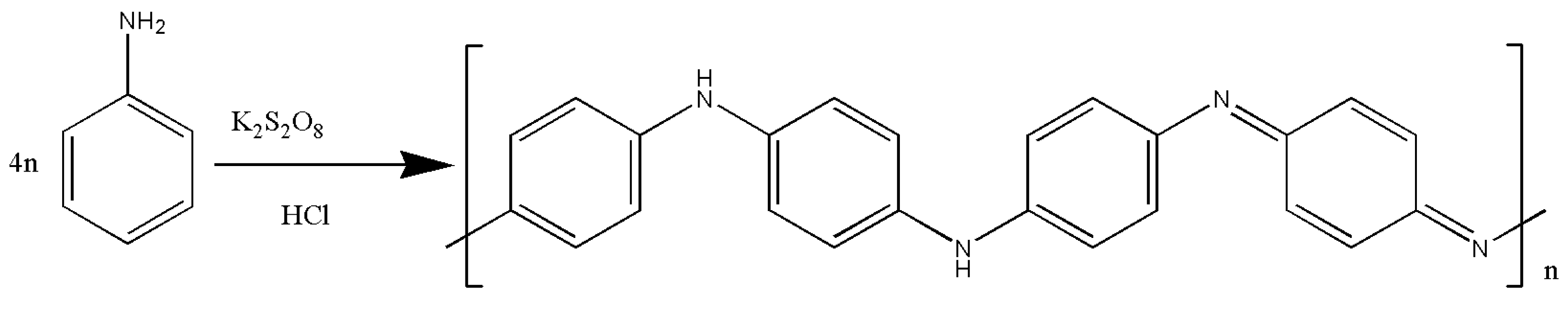
2.2.1. Responsive Membranes Based on Polyaniline
2.2.2. Responsive Membranes Based on Polypyrrole
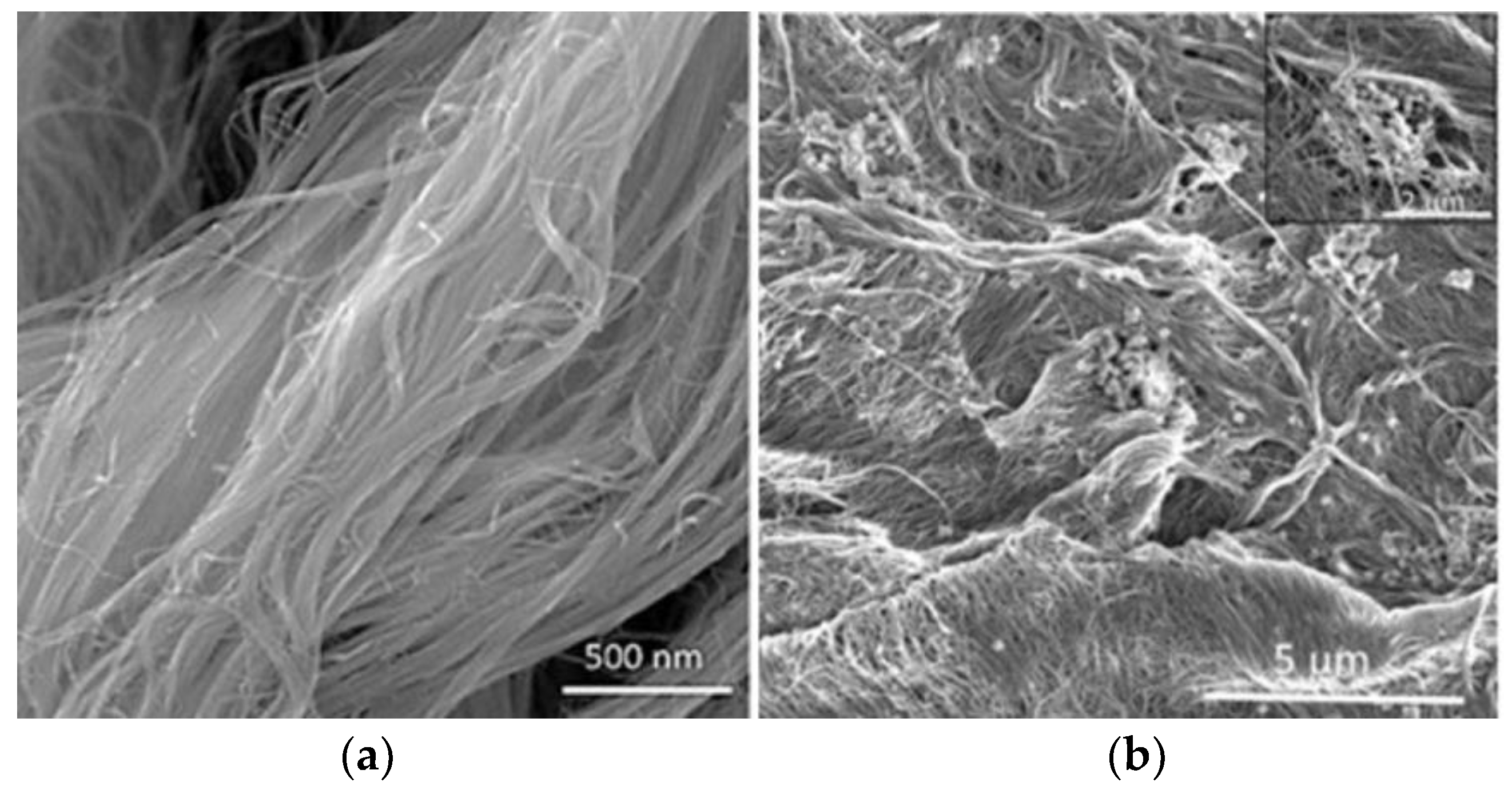
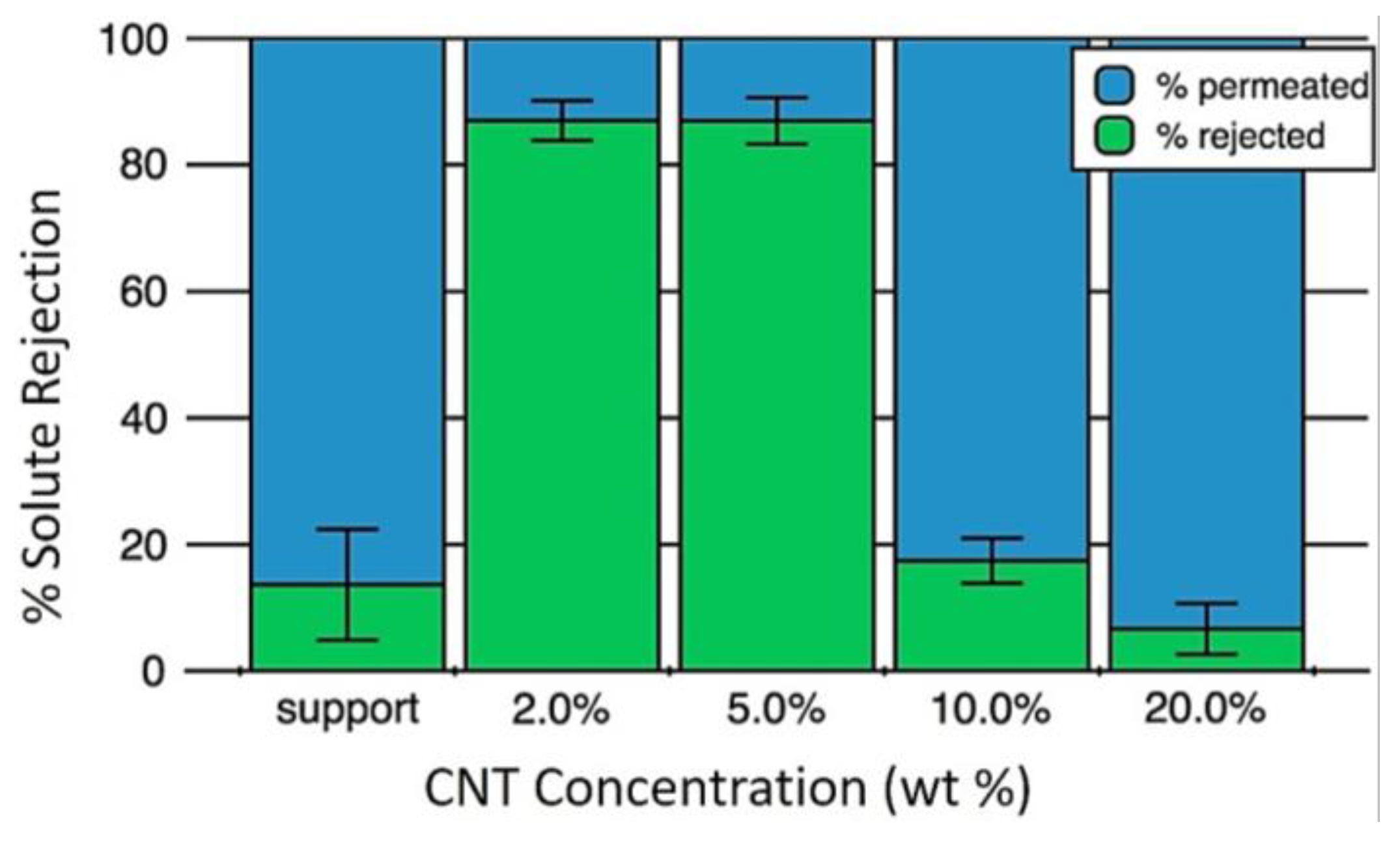
2.2.3. Responsive Membranes Based on Carbon Nanotubes
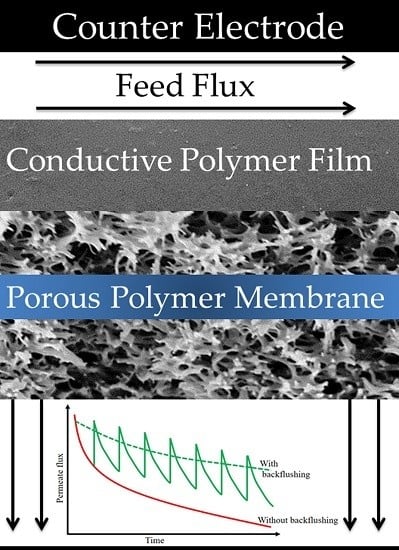
2.3. Electro-Conductive Membranes for Permeation Enhancement
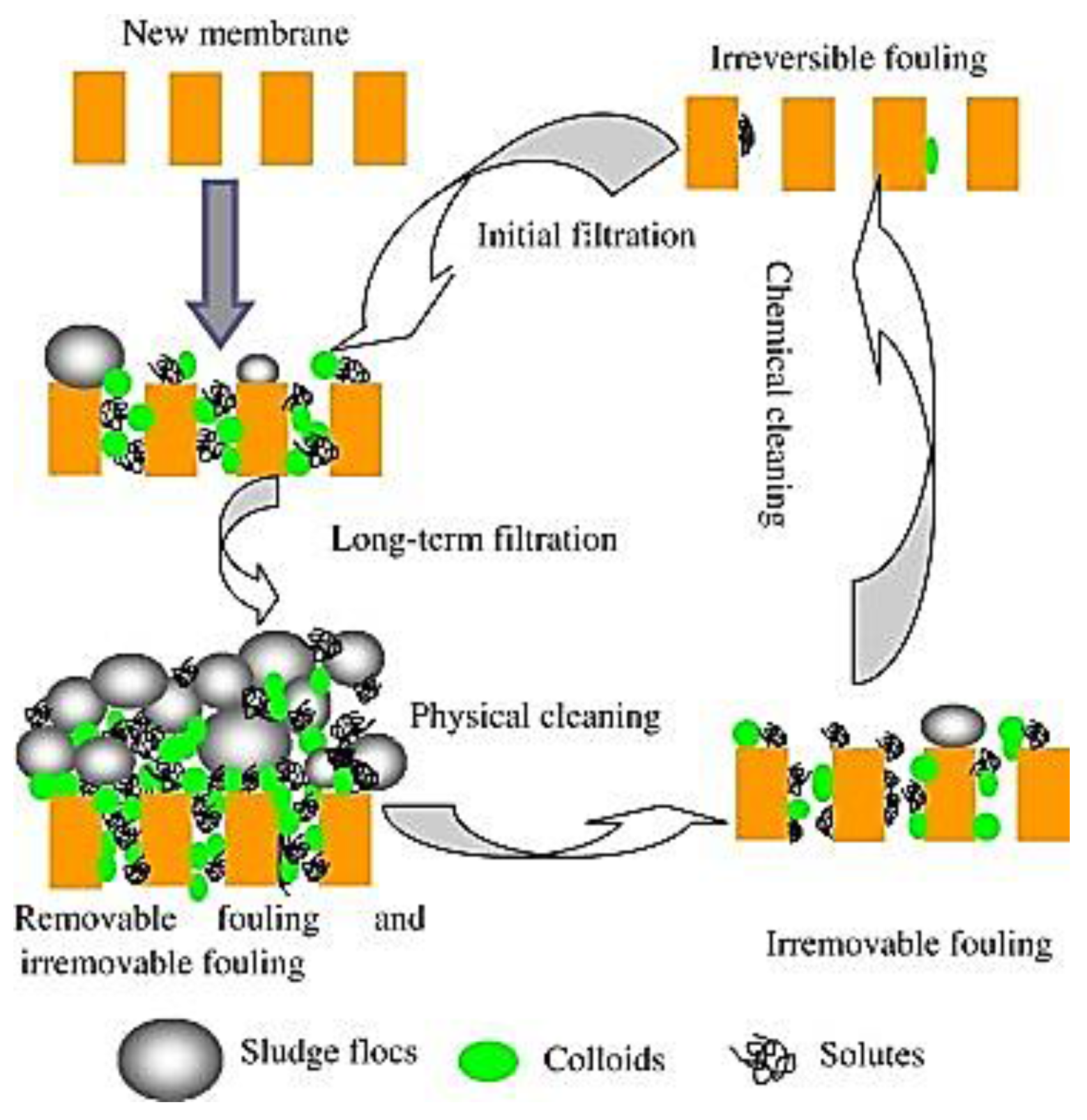
3. Membrane Fouling
- a lowering in productivity because of longer filtration times,
- an alteration of membrane selectivity as a consequence of a change in pore size,
- an increase of operational costs [97].
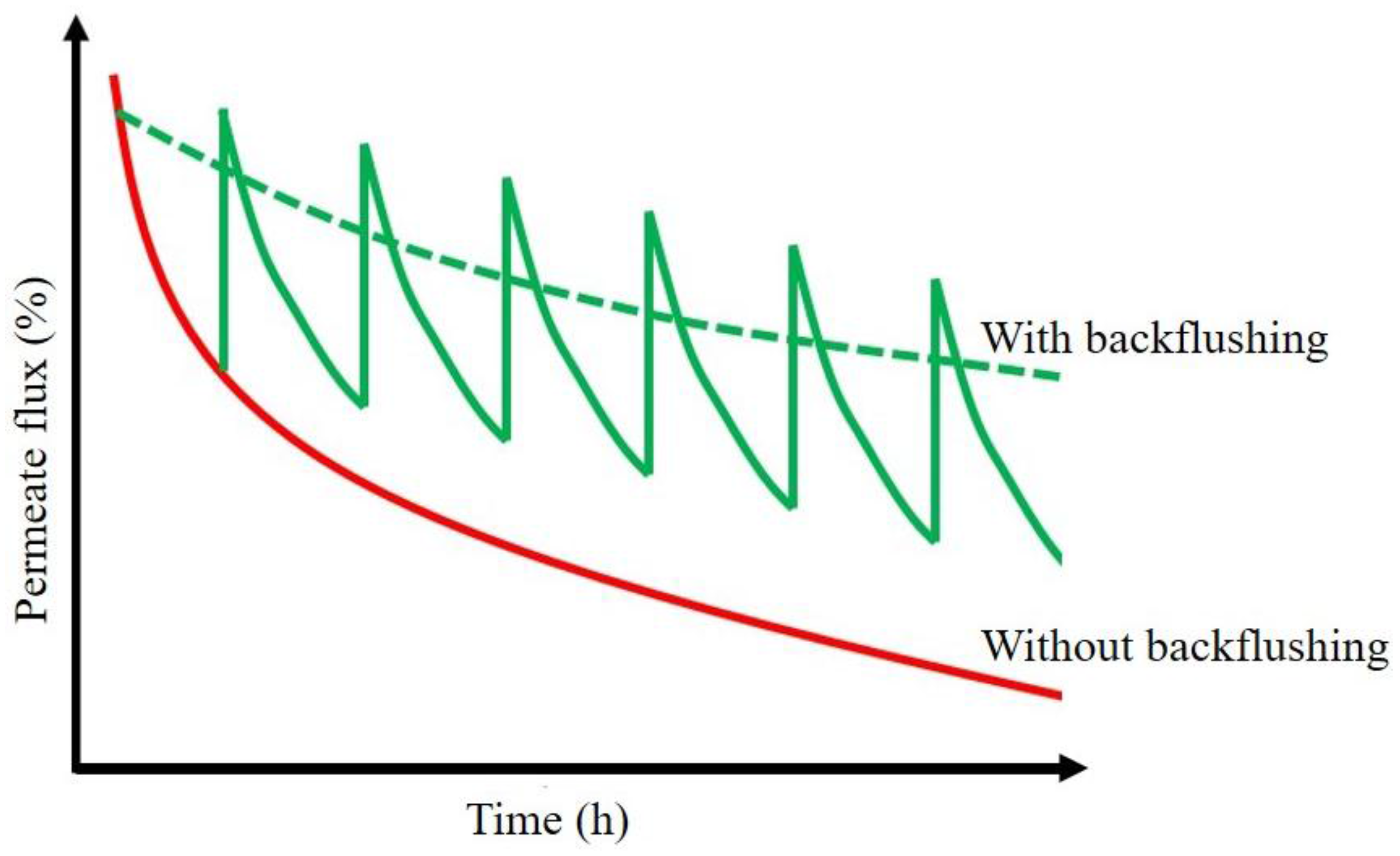
3.1. Novel Approaches to Mitigate Fouling
- a decrease in the membrane hydrophobicity,
- a reduction of the surface roughness,
- an increase in the membrane selectivity,
- a modification of the surface charge.
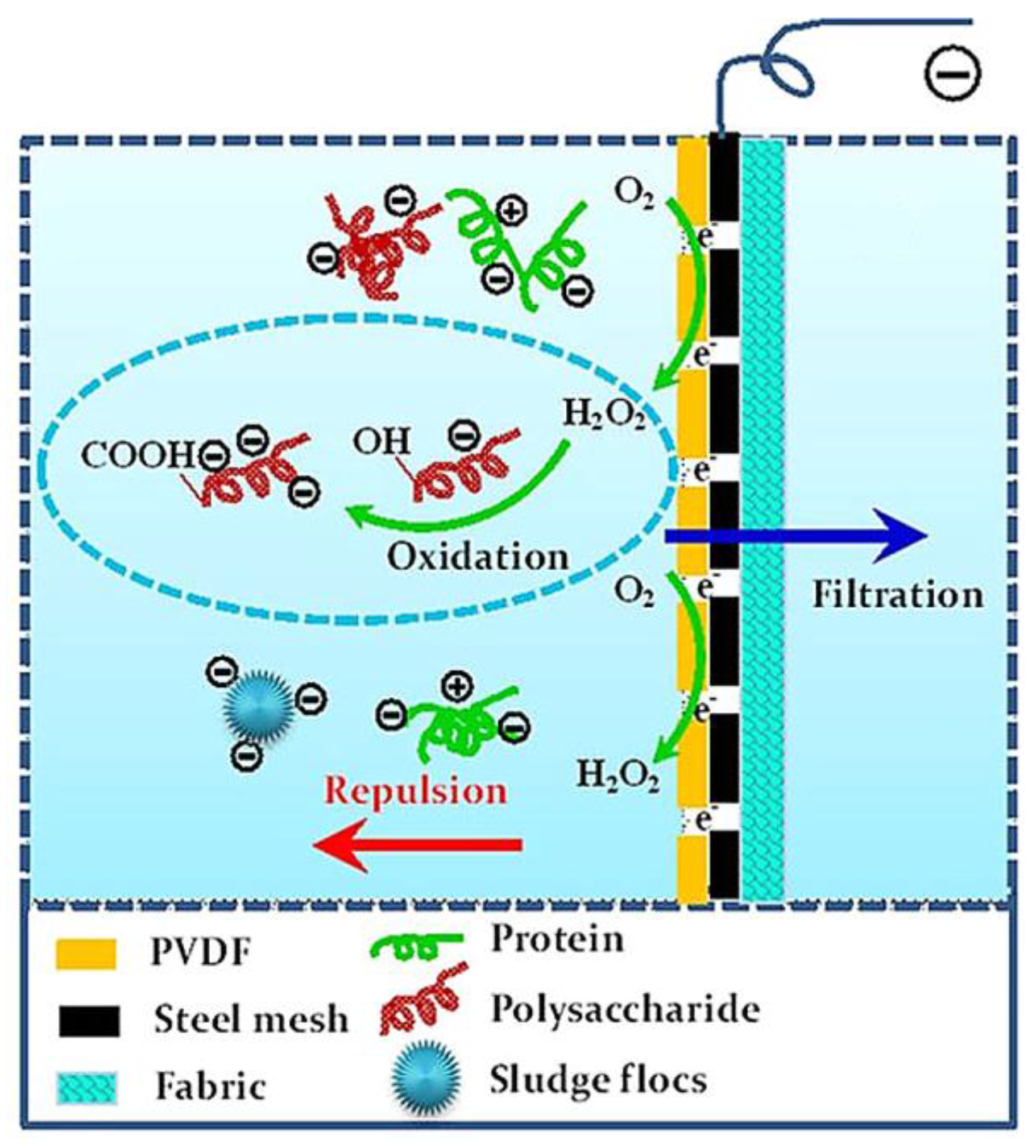
3.1.1. Electrostatic Repulsion and Hydrogen Peroxide Generation
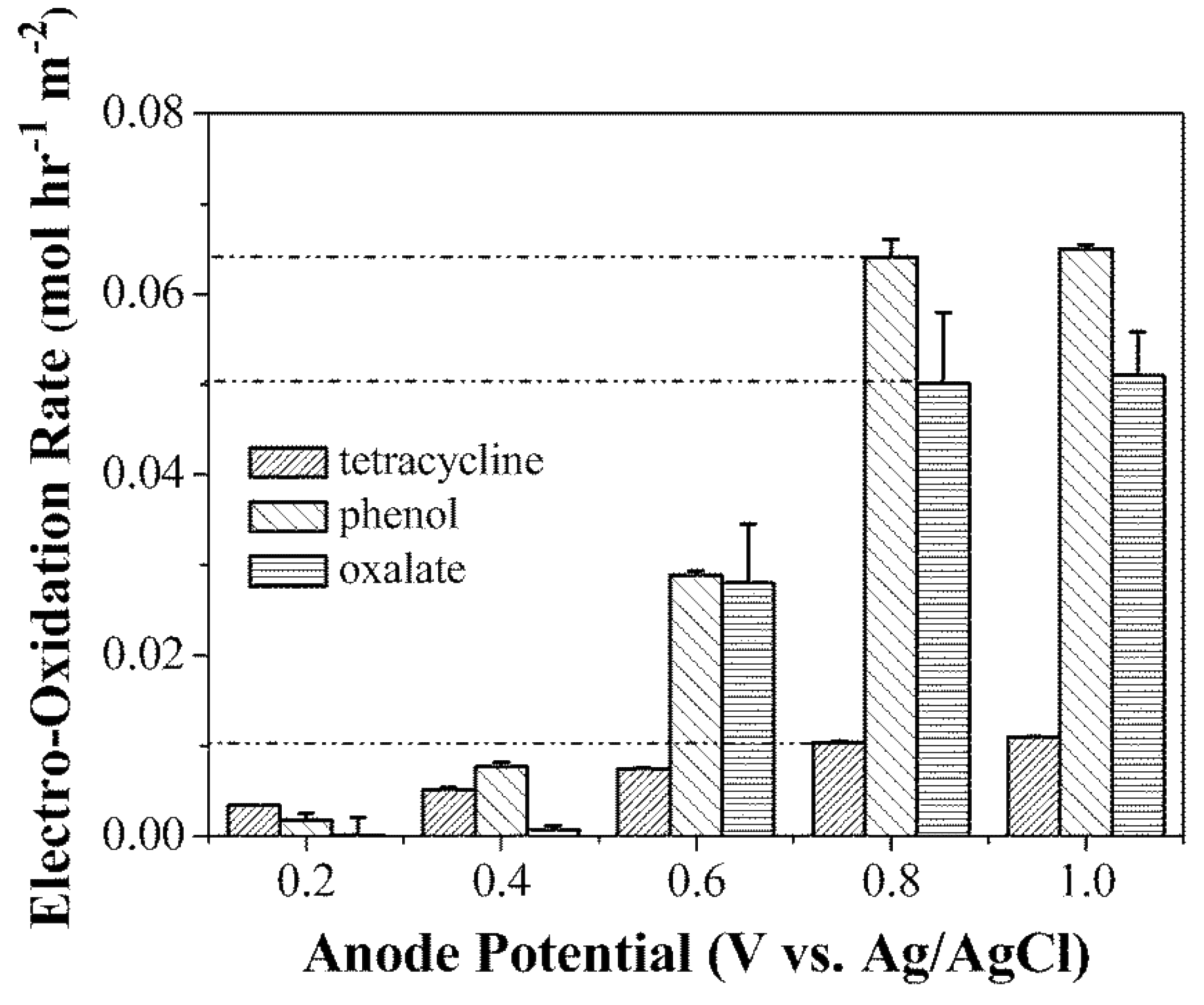
3.1.2. Electrochemical Oxidation
3.2. Biofouling Mitigation with Electro-Responsive Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giorno, L. Permselectivity. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-44323-1. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4247-2. [Google Scholar]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Liu, J.; Wu, D.; Liu, M.; Gao, C. Surface modification of thin-film composite polyamide reverse osmosis membranes with thermoresponsive polymer (TRP) for improved fouling resistance and cleaning efficiency. Sep. Purif. Technol. 2011, 76, 283–291. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Schäfer, T.; Wickramasinghe, S.R.; Daunert, S. Responsive Membranes and Materials; Wiley: New York, NY, USA, 2012; ISBN 978-0-470-97430-8. [Google Scholar] [CrossRef]
- Nicoletta, F.P.; Cupelli, D.; Formoso, P.; De Filpo, G.; Colella, V.; Gugliuzza, A. Light responsive polymer membranes: A review. Membranes 2012, 2, 134–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiang, T.; Yue, W.; Li, H.; Liang, S.; Sun, S.; Zhao, C. Preparation and characterization of pH-sensitive polyethersulfone hollow fiber membranes modified by poly(methyl methylacrylate-co-4-vinylpyridine) copolymer. J. Membr. Sci. 2012, 423–424, 275–283. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Wang, Y. Swelling-induced mesoporous block copolymer membranes with intrinsically active surfaces for size-selective separation. J. Mater. Chem. 2012, 22, 20542. [Google Scholar] [CrossRef]
- Frost, S.; Ulbricht, M. Thermoresponsive ultrafiltration membranes for the switchable permeation and fractionation of nanoparticles. J. Membr. Sci. 2013, 448, 1–11. [Google Scholar] [CrossRef]
- Gugliuzza, A. Intelligent membranes: Dream or reality? Membranes 2013, 3, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-responsive polymer membranes of slide-ring gels with movable cross-links. Adv. Mater. 2013, 25, 4636–4640. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, J.; Xiang, T.; Wang, R.; Wang, D.; Sun, S.; Zhao, C. Preparation and characterization of pH- and thermo-sensitive polyethersulfone hollow fiber membranes modified with P(NIPAAm-MAA-MMA)terpolymer. Desalination 2013, 309, 1–10. [Google Scholar] [CrossRef]
- Qiu, X.; Yu, H.; Karunakaran, M.; Pradeep, N.; Nunes, S.P.; Peinemann, K-V. Selective separation of similarly sized proteins with tunable nanoporous block copolymer membranes. ACS Nano 2013, 7, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Himstedt, H.M.; Ulbricht, M.; Qian, X.; Wickramasinghe, S.R. Designing magnetic field responsive nanofiltration membranes. J. Membr. Sci. 2013, 430, 70–78. [Google Scholar] [CrossRef]
- Chen, P.R.; Chuang, Y.J. The development of conductive nanoporous chitosan polymer membrane for selective transport of charged molecules. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Bi, S.; Fu, W.; Shi, C.; Chen, L. Conferring pH-sensitivity on poly (vinylidene fluoride) membrane by poly (acrylic acid-co-butyl acrylate) microgels. React. Funct. Polym. 2014, 74, 58–66. [Google Scholar] [CrossRef]
- Ma, S.; Meng, J.; Li, J.; Zhang, Y.; Ni, L. Synthesis of catalytic polypropylene membranes enabling visible-light-driven photocatalytic degradation of dyes in water. J. Membr. Sci. 2014, 453, 221–229. [Google Scholar] [CrossRef]
- Xiao, L.; Isner, A.; Waldrop, K.; Saad, A.; Takigawa, D.; Bhattacharyya, D. Development of bench and full-scale temperature and pH responsive functionalized PVDF membranes with tunable properties. J. Membr. Sci. 2014, 457, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kaner, P.; Bengani-Lutz, P.; Sadeghi, I.; Asatekin, A. Responsive filtration membranes by polymer self-assembly. Technology 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Meng, H.; Hu, J. A brief review of stimulus—Active polymers responsive to thermal, light, magnetic, electric, and water/solvent stimuli. J. Intell. Mater. Syst. Struct. 2010, 21, 859–885. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Arias-Pardilla, J. Biomimetic electrochemistry from conducting polymers. A review: Artificial muscles, smart membranes, smart drug delivery and computer/neuron interfaces. Electrochim. Acta 2012, 84, 112–128. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio) sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Glavas, L.; Albertsson, A-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Asatekin, A.; Mayes, A.M. Polymer filtration membranes. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Bikales, N.M., Eds.; Wiley: New York, NY, USA, 2009; ISBN 9780471440260. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Wiley: New York, NY, USA, 2004; ISBN 9780470743720. [Google Scholar] [CrossRef]
- Majidi Salehi, S.; Di Profio, G.; Fontananova, E.; Nicoletta, F.P.; Curcio, E.; De Filpo, G. Membrane distillation by novel hydrogel composite membranes. J. Membr. Sci. 2016, 504, 220–229. [Google Scholar] [CrossRef]
- Di Profio, G.; Polino, M.; Nicoletta, F.P.; Belviso, B.D.; Caliandro, R.; Fontananova, E.; De Filpo, G.; Curcio, E.; Drioli, E. Tailored hydrogel membranes for efficient protein crystallization. Adv. Funct. Mater. 2014, 24, 1582–1590. [Google Scholar] [CrossRef]
- Hernandez, S.; Saad, A.; Ormsbee, L.; Bhattacharyya, D. Nanocomposite and responsive membranes for water treatment. In Emerging Membrane Technology For Sustainable Water Treatment; Hankins, N.P., Singh, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 389–431. ISBN 978-0-444-63312-5. [Google Scholar] [CrossRef]
- Asatekin, A.; Vannucci, C. Self-assembled polymer nanostructures for liquid filtration membranes: A review. Nanosci. Nanotechnol. Lett. 2015, 7, 21–32. [Google Scholar] [CrossRef]
- Bengani, P.; Kou, Y.; Asatekin, A. Zwitterionic copolymer self-assembly for fouling resistant, high flux membranes with size-based small molecule selectivity. J. Membr. Sci. 2015, 493, 755–765. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B.L. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Batrinescu, G.; Constantin, L.A.; Cuciureanu, A.; Constantin, M.A. Conductive polymer-based membranes. In Conducting Polymers; Yilmaz, F., Ed.; Intech (Open Science): Rijeka, Croatia, 2016; ISBN 978-953-51-2691-1. [Google Scholar] [CrossRef]
- Wesson, R.D.; Dow, E.S.; Williams, S.R. Responsive membranes/material-based separations: Research and development needs. In Responsive Membranes and Materials; Bhattacharyya, D., Schäfer, T., Wickramasinghe, S.R., Daunert, S., Eds.; Wiley: New York, NY, USA, 2013; pp. 385–393. ISBN 978-0-470-97430-8. [Google Scholar] [CrossRef]
- Chen, H.; Hsieh, Y.L. Dual temperature- and pH-sensitive hydrogels from interpenetrating networks and copolymerization of N-isopropylacrylamide and sodium acrylate. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 3293–3301. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S. A short review of membrane fouling in forward osmosis processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O.; Brown, A.A.; Huck, W.T.S. Tunable wettability by clicking counterions into polyelectrolyte brushes. Adv. Mater. 2007, 19, 151–154. [Google Scholar] [CrossRef]
- Husson, S.M. Synthesis aspects in the design of responsive membranes. In Responsive Membranes and Materials; Bhattacharyya, D., Schäfer, T., Wickramasinghe, S.R., Daunert, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 73–96. ISBN 978-0-470-97430-8. [Google Scholar] [CrossRef]
- Yang, Q.; Wickramasinghe, S.R. Responsive membranes for water treatment. In Responsive Membranes and Materials; Bhattacharyya, D., Schäfer, T., Wickramasinghe, S.R., Daunert, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 978-0-470-97430-8. [Google Scholar] [CrossRef]
- Mendes, P.M. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 2008, 37, 2512–2529. [Google Scholar] [CrossRef] [PubMed]
- Kastantin, M.; Tirrell, M. Helix formation in the polymer brush. Macromolecules 2011, 44, 4977–4987. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.F.; Banerjee, P.; Mayes, A.M. Preparation of protein-resistant surfaces on poly(vinylidene fluoride) membranes via surface segregation. Macromolecules 1999, 32, 1643–1650. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, P.; Won, Y.Y.; Akthakul, A.; Acar, M.H.; Mayes, A.M. ATRP of amphiphilic graft copolymers based on PVDF and their use as membrane additives. Macromolecules 2002, 35, 7652–7661. [Google Scholar] [CrossRef]
- Jung, B. Preparation of hydrophilic polyacrylonitrile blend membranes for ultrafiltration. J. Membr. Sci. 2004, 229, 129–136. [Google Scholar] [CrossRef]
- Asatekin, A.; Kang, S.; Elimelech, M.; Mayes, A.M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly(ethylene oxide) as an additive. J. Membr. Sci. 2007, 298, 136–146. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Su, Y.L.; Sun, Q.; Ma, X.L.; Jiang, Z.Y. Generation of anti-biofouling ultrafiltration membrane surface by blending novel branched amphiphilic polymers with polyethersulfone. J. Membr. Sci. 2006, 286, 228–236. [Google Scholar] [CrossRef]
- Barghi, H.; Taherzadeha, M.J. Synthesis of an electroconductive membrane using poly(hydroxymethyl-3,4-ethylenedioxythiophene-cotetramethylene-N-hydroxyethyl adipamide). J. Mater. Chem. C 2013, 1, 6347–6354. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Cuciureanu, A.; Batrinescu, G.; Badea, N.N.; Radu, D.A. The influence of changing the polyaniline and polysulphone ratio on composite Psf–PANI membranes performances. Mater. Plast. 2010, 47, 416–420. Available online: http://www.revmaterialeplastice.ro/article_eng.asp?ID=2758 (accessed on 16 June 2017).
- Guimaraes, I.S.; Hidalgo, A.A.; Cunha, H.N.; Santos, L.M.; Santos, J.A.V.; Santos, J.R., Jr. Thermal and morphological characterization of conducting polyaniline/polystyrene blends. Synth. Met. 2012, 162, 705–709. [Google Scholar] [CrossRef]
- Yang, J.; Hou, J.; Zhu, W.; Xu, M.; Wan, M. Substituted polyaniline-polypropylene film composite: Preparation and properties. Synth. Met. 1996, 80, 283–289. [Google Scholar] [CrossRef]
- Mattoso, L.H.; Medeiros, E.S.; Baker, D.A.; Avloni, J.; Wood, D.F.; Orts, W.J. Electrically conductive nanocomposite made from cellulose nanofibrils and polyaniline. J. Nanosc. Nanotech. 2009, 9, 2017–2022. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchova, M.; Cirici-Marjanovic, G.; Sapurina, I. Polymerization of aniline on aniline membranes. J. Phys. Chem. B 2007, 111, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, A.A.; Hyland, M.M.; Patterson, D.A. Effects of various polymerization techniques on PANI deposition at the surface of cellulose ester microporous membrane: XPS and electrical studies. Synth. Met. 2012, 162, 958–967. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Qaiser, A.A.; Javaid, A.; Saeed, F. In situ solution-phase polymerization and chemical vapor deposition of polyaniline on microporous cellulose ester membranes: AFM and electrical conductivity studies. Synth. Met. 2015, 200, 164–171. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, X.G.; Yang, Y.L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Gao, B.; Yang, F. Integration of bio-electrochemical cell in membrane bioreactor for membrane cathode fouling reduction through electricity generation. J. Membr. Sci. 2013, 430, 196–202. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Bo, G.; Yang, F.; Crittenden, C.Y. Conductive and hydrophilic polypyrrole modified membrane cathodes and fouling reduction in MBR. J. Membr. Sci. 2013, 429, 252–258. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, F.; Liu, J.; Yang, F. Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating. J. Membr. Sci. 2013, 437, 99–107. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Choi, C.H.; Gao, N.; Yang, E.H. Tunable wetting mechanism of polypyrrole surfaces and low-voltage droplet manipulation via redox. Langmuir 2011, 27, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, L.; Yang, F.; Ren, N. E-Fenton degradation of MB during filtration with Gr/PPy modified membrane cathode. Chem. Eng. J. 2013, 230, 491–498. [Google Scholar] [CrossRef]
- Wang, C.; Song, H.; Zhang, Q.; Wang, B.; Li, A. Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated efficient and the fouled electrode regeneration. Desalination 2015, 365, 407–415. [Google Scholar] [CrossRef]
- Wang, S.; Liang, S.; Liang, P.; Zhang, X.; Sun, J.; Wu, S.; Huang, X. In-situ combined dual-layer CNT/PVDF membrane for electrically enhanced fouling resistance. J. Membr. Sci. 2015, 491, 37–44. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.-Q.; Yuan, G.-E.; Liu, H.-Y.; Liu, J.-D.; Yang, F.-L. Anti-fouling performance and mechanism of anthraquinone/polypyrrole composite modified membrane cathode in a novel MFC–aerobic MBR coupled system. RSC Adv. 2015, 5, 22533–22543. [Google Scholar] [CrossRef]
- Jeon, G.; Yang, S.Y.; Byun, J.; Kim, J.K. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011, 11, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Picci, N.; Nicoletta, F.P.; Iemma, F.; Hampel, S. On demand delivery of ionic drugs from electro-responsive CNT hybrid films. RSC Adv. 2015, 5, 44902–44911. [Google Scholar] [CrossRef]
- Formoso, P.; Muzzalupo, R.; Tavano, L.; De Filpo, G.; Nicoletta, F.P. Nanotechnology for the environment and medicine. Mini-Rev. Med. Chem. 2016, 16, 668–675. [Google Scholar] [CrossRef] [PubMed]
- De Filpo, G.; Siprova, S.; Chidichimo, G.; Mashin, A.; Nicoletta, F.P.; Cupelli, D. Alignment of single-walled carbon nanotubes in polymer dispersed liquid crystals. Liq. Cryst. 2012, 39, 359–364. [Google Scholar] [CrossRef]
- De Filpo, G.; Mormile, S.; Nicoletta, F.P.; Chidichimo, G. Fast, self-supplied, all-solid photoelectrochromic film. J. Power Sources 2010, 195, 4365–4369. [Google Scholar] [CrossRef]
- Kudryashov, M.A.; Mashin, A.I.; Tyurin, A.S.; Chidichimo, G.; De Filpo, G. Metal-polymer composite films based on polyacrylonitrile and silver nanoparticles. Preparation and properties. J. Surf. Investig X-ray Synchrotron Neutron Tech. 2010, 4, 437–441. [Google Scholar] [CrossRef]
- Kudryashov, M.A.; Mashin, A.I.; Logunov, A.A.; Chidichimo, G.; De Filpo, G. Filpo Frequency dependence of the electrical conductivity in Ag/PAN nanocomposites. Tech. Phys. 2012, 57, 965–970. [Google Scholar] [CrossRef]
- Tyurin, A.; De Filpo, G.; Cupelli, D.; Nicoletta, F.P.; Mashin, A.I.; Chidichimo, G. Particle size tuning in silver-polyacrylonitrile nanocomposites. eXPRESS Polym. Lett. 2010, 4, 71–78. [Google Scholar] [CrossRef]
- Shawky, H.; Chae, S.-R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 72, 46–50. [Google Scholar] [CrossRef]
- De Lannoy, C.F.; Jassby, D.; Gloe, K.; Gordon, A.D.; Wiesner, M.R. Aquatic biofouling prevention by electrically charged nanocomposite polymer thin film membranes. Environ. Sci. Technol. 2013, 47, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.R.; Trivedi, J.J.; Devmurari, C.V.; Mohan, D.J.; Singh, P.; Rao, A.P.; Joshi, S.V.; Ghosh, P.K. Fouling resistant membranes in desalination and water recovery. Desalination 2005, 183, 301–306. [Google Scholar] [CrossRef]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 22, 4567–4586. [Google Scholar] [CrossRef]
- Gullinkala, J.; Escobar, I. A green membrane functionalization method to decrease natural organic matter fouling. J. Membr. Sci. 2010, 360, 155–164. [Google Scholar] [CrossRef]
- Cai, G.; Gorey, C.; Zaky, A.; Escobar, I.; Grunden, C. Thermally responsive membrane-based microbiological sensing component for early detection of membrane biofouling. Desalination 2011, 270, 116–123. [Google Scholar] [CrossRef]
- Chen, Y.; Bose, A.; Bothun, G. Controlled release from bilayer-decorated magneto-liposomes via electromagnetic heating. ACS Nano 2010, 4, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, R.; Fraser, S.; Hilt, J. Synthesis and temperature response analysis of magnetic-hydrogel nanocomposites. J. Biomed. Mater. Res. Part A 2007, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Preiss, M.; Bothun, G. Stimuli-responsive liposome-nanoparticle assemblies. Expert Opin. Drug Deliv. 2011, 8, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.; Huck, W.T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Xie, R.; Ju, X.J.; Chu, L.Y. Stimuli responsive smart gating membranes. Chem. Soc. Rev. 2016, 45, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lee, A.; Chen, J.; Cadene, M.; Chait, B.T.; MacKinnon, R. The open pore conformation of potassium channels. Nature 2002, 417, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Meng, J.; Zhang, P.; Yang, G.; Su, B.; Sun, K.; Chen, L.; Han, D.; Wang, S.; et al. Hydrophobic interaction-mediated capture and release of cancer cells on thermoresponsive nanostructured surfaces. Adv. Mater. 2013, 25, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lalia, B.S.; Ahmed, F.E.; Shah, T.; Hilal, N.; Hashaikeh, R. Electrically conductive membranes based on carbon nanostructures for self-cleaning or biofouling. Desalination 2015, 360, 8–12. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Holloway, B.C.; Outlaw, R.A.; Manos, D.M.; Zhao, X. Carbon Nanostructures and Methods of Making and Using the Same. Patent WO2005084172 A3, 19 October 2006. [Google Scholar]
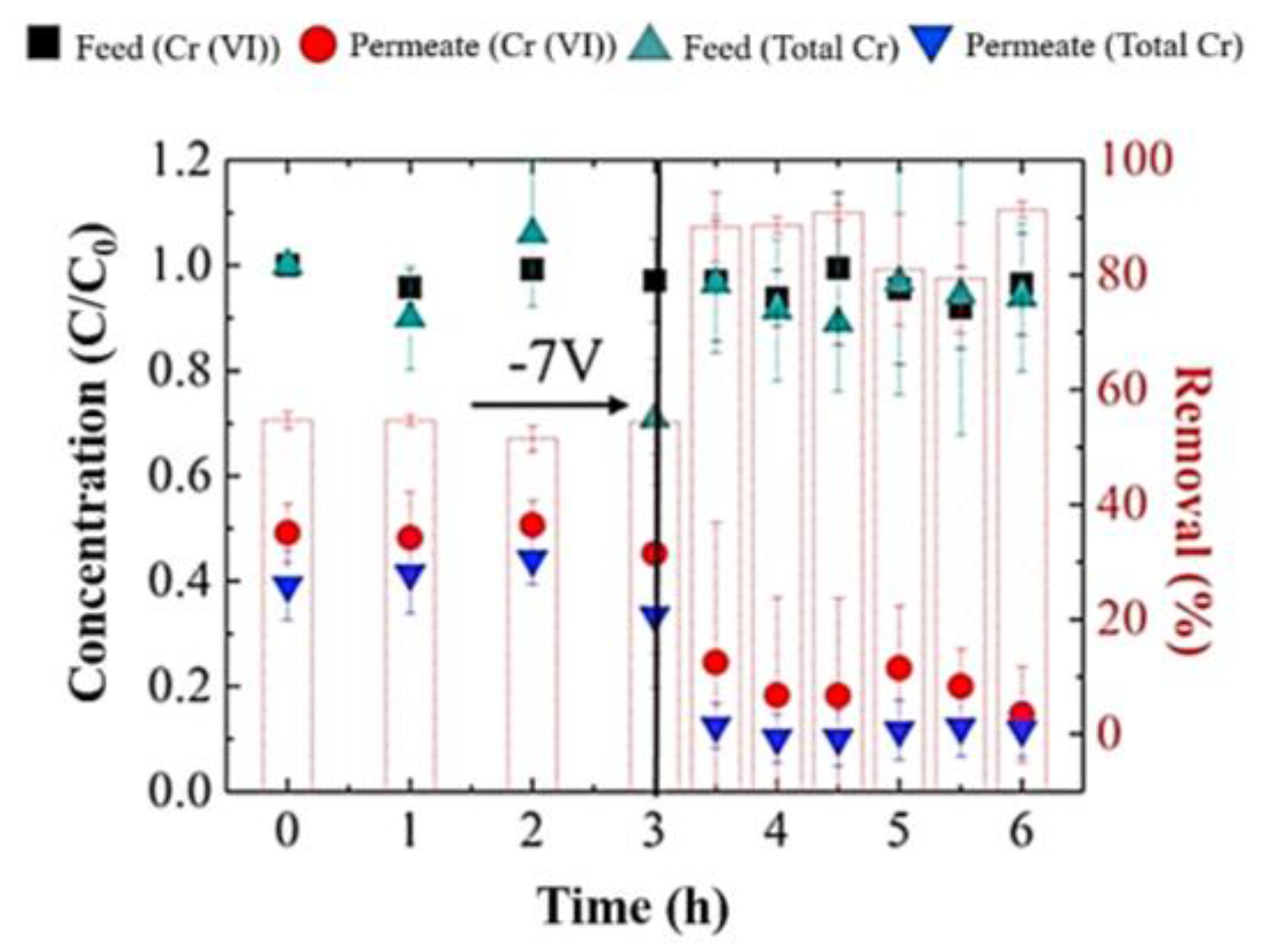
- Duan, W.; Chen, G.; Chen, C.; Sanghvi, R.; Iddya, A.; Walker, S.; Liu, H.; Ronen, A.; Jassby, D. Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J. Membr. Sci. 2017, 531, 160–171. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Highly efficient and robust oxygen evolution catalysts achieved by anchoring nanocrystalline cobalt oxides onto mildly oxidized multiwalled carbon nanotubes. J. Mater. Chem. A 2013, 1, 12053–12059. [Google Scholar] [CrossRef]
- Liu, H.; Vajpayee, A.; Vecitis, C.D. Bismuth-doped tin oxide-coated carbon nanotube network: Improved anode stability and efficiency for flow-through organic electrooxidation. ACS Appl. Mater. Interfaces 2013, 5, 10054–10066. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hankins, N.P. Introduction to membrane processes for water treatment. In Emerging Membrane Technology for Sustainable Water Treatment; Hankins, N.P., Singh, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 15–52. ISBN 978-0-444-63312-5. [Google Scholar] [CrossRef]
- Gorey, C.; Hausman, R.; Escobar, I.C. Functionalization of polymeric membranes and feed spacers for fouling control in drinking water treatment applications. In Responsive Membranes and Materials; Bhattacharyya, D., Schäfer, T., Wickramasinghe, S.R., Daunert, S., Eds.; John Wiley: New York, NY, USA, 2014; pp. 163–186. ISBN 9780470974308. [Google Scholar] [CrossRef]
- Ulbricht, M.; Richau, K.; Kamusewitz, H. Chemically and morphologically defined ultrafiltration membrane surfaces prepared by heterogeneous photo-initiated graft polymerization. Colloid Surf. A 1998, 138, 353–366. [Google Scholar] [CrossRef]
- Sun, M.P.; Su, Y.L.; Mu, C.X.; Jiang, Z.Y. Improved antifouling property of PES ultrafiltration membranes using additive of silica-PVP nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Nils, J.A.; Digiano, F.A. Influence of NOM composition on NF. J. Am. Water Works Assoc. 1996, 88, 53–66. Available online: http://www.jstor.org/stable/41295534 (accessed on 16 June 2017).
- Pabby, A.K.; Rizvi, S.S.H.; Sastre, A.M. Membranes in chemical and pharmaceutical industries and in conservation of natural resources: An introduction. In Handbook on Membrane Separations Chemical Pharmaceutical Food and Biotechnological Applications; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780849395499. Available online: https://www.crcpress.com/Handbook-of-Membrane-Separations-Chemical-Pharmaceutical-Food-and-Biotechnological/Pabby-Rizvi-Requena/p/book/9781466555563 (accessed on 16 June 2017).
- Akhtar, S.; Hawes, C.; Dudley, L.; Reed, I.; Stratford, P. Coatings reduce the fouling of microfiltration membranes. J. Membr. Sci. 1995, 107, 209–218. [Google Scholar] [CrossRef]
- Chen, J.; Kim, S.L.; Ting, Y.P. Optimization of membrane physical and chemical cleaning by a statistically designed approach. J. Membr. Sci. 2003, 219, 27–45. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Influence of formulated chemical cleaning reagents on the surface properties and separation efficiency of nanofiltration membranes. J. Membr. Sci. 2013, 432, 73–82. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric RO and NF membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Rautenbach, R.; Linn, T.; Al-Gobaisi, D.M.K. Present and future pretreatment concepts and strategies for reliable and low-maintenance RO seawater desalination. Desalination 1997, 110, 97–106. [Google Scholar] [CrossRef]
- Chellam, S.; Wiesner, M.R. Particle back-transport and permeate flux behaviour in cross-flow membrane filters. Environ. Sci. Technol. 1997, 31, 819–824. [Google Scholar] [CrossRef]
- Elimelech, M.; Philip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone)membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modification for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Peldszus, S.; Huck, P.M.; Feng, X. Modification of membrane surface via microswelling for fouling control in drinking water treatment. J. Membr. Sci. 2014, 475, 488–495. [Google Scholar] [CrossRef]
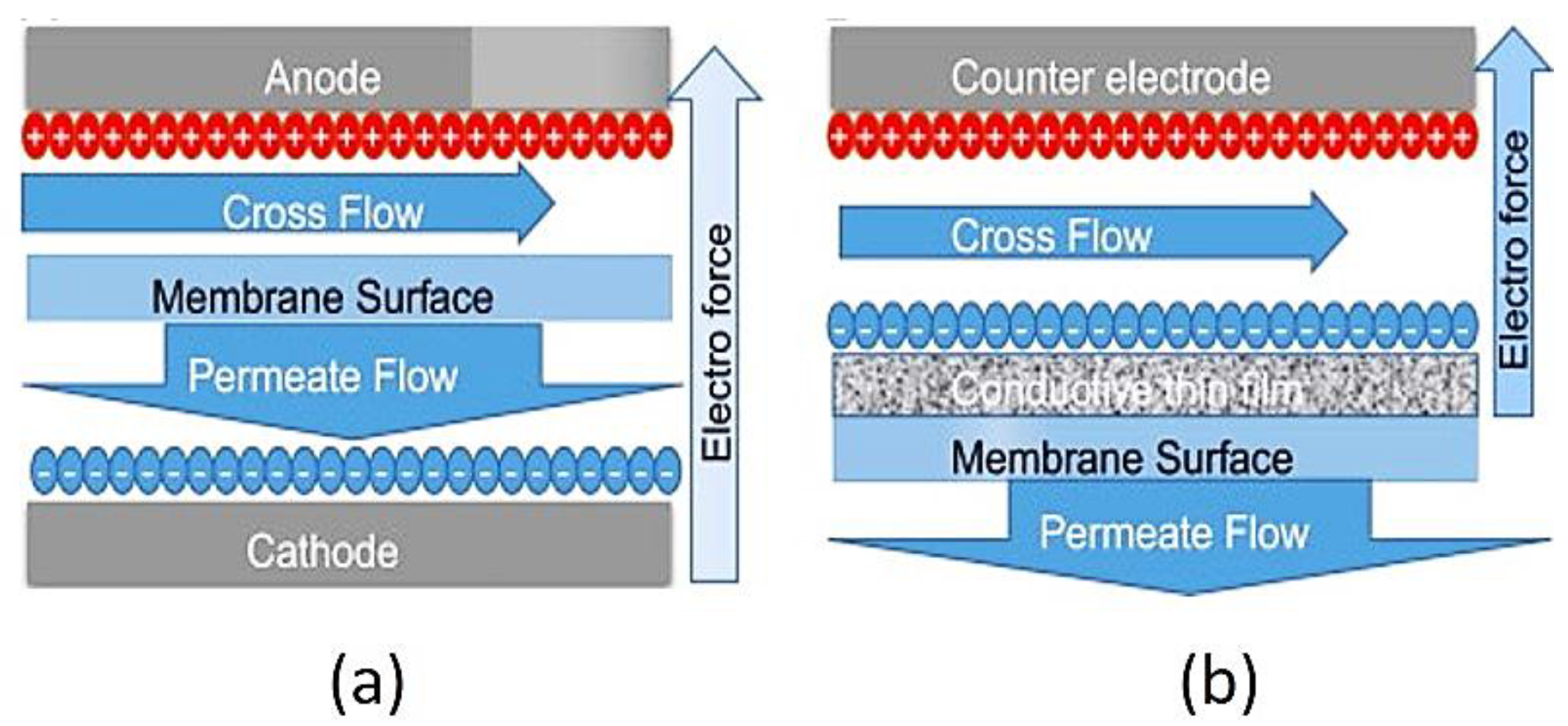
- Ronen, A.; Walker, S.L.; Jassby, D. Electroconductive and electroresponsive membranes for water treatment. Rev. Chem. Eng. 2016, 32, 533–550. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Dong, Y.; Mao, H.; Sun, J.; Chen, S.; Craig, V.S.J.; Hu, J. Cleaning using nanobubbles: Defouling by electrochemical generation of bubbles. J. Colloid Interface Sci. 2008, 328, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ci, L.; Chen, L.; Nayak, S.; Ajayan, P.M.; Koratkar, N. Polarity-dependent electrochemically controlled transport of water through carbon nanotube membranes. Nano Lett. 2007, 7, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guillen, G.; Hoek, E.M.V. Direct observation of microbial adhesion to membranes. Environ. Sci. Technol. 2005, 39, 6461–6469. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.M.; Chaplin, B.P. Porous substoichiometric TiO2 anodes as reactive electrochemical membranes for water treatment. Environ. Sci. Technol. 2013, 47, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Z.; Zhang, J.; Zhang, X.; Ma, J.; Wu, Z. A novel composite conductive microfiltration membrane and its antifouling performance with an external electric field in membrane bioreactors. Sci. Rep. 2015, 5, 9268. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzza, A. Smart membrane surfaces: Wettability amplification and self-healing. In Smart Membranes and Sensors Synthesis Characterization and Applications; Gugliuzza, A., Ed.; Wiley: New York, NY, USA, 2014; pp. 161–184. ISBN 978-1-118-42379-0. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Gao, B.; Yang, F.; Chellam, S. Fouling reductions in a membrane bioreactor using an intermittent electric field and cathodic membrane modified by vapor phase polymerized pyrrole. J. Membr. Sci. 2012, 394–395, 202–208. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Q.; Vecitis, C.D. CNT-PVDF composite flow-through electrode for single-pass sequential reduction-oxidation. J. Mater. Chem. A 2014, 2, 6185–6190. [Google Scholar] [CrossRef]
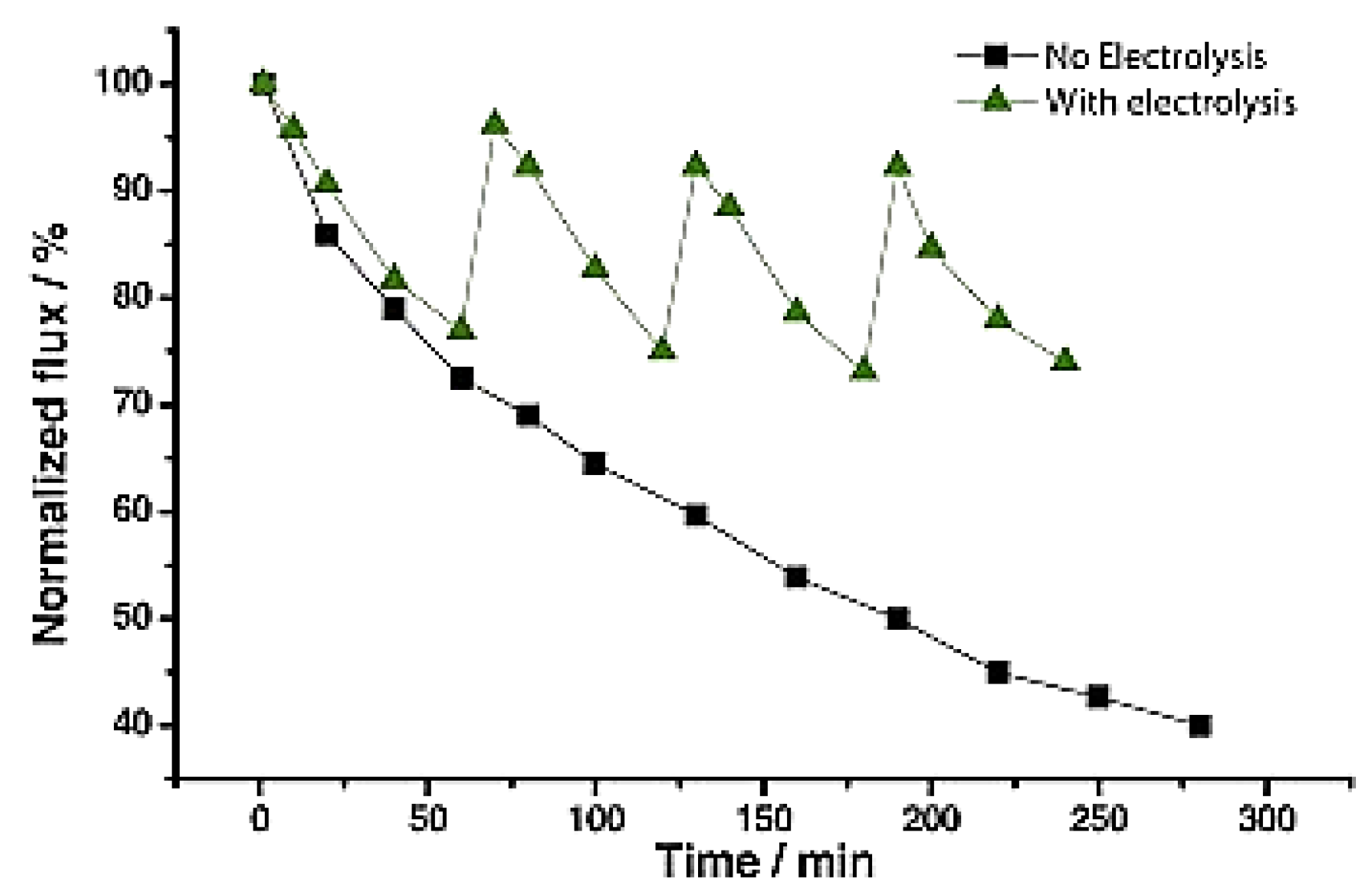
- Ronen, A.; Duan, W.; Wheeldon, I.; Walker, S.L.; Jassby, D. Microbial attachment inhibition through low voltage electrochemical reactions on electrically conducting membranes. Environ. Sci. Technol. 2015, 49, 12741–12750. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Dudchenko, A.; Mende, E.; Flyer, C.; Zhu, X.; Jassby, D. Electrochemical mineral scale prevention and removal on electrically conducting carbon nanotube–polyamide reverse osmosis membranes. Environ. Sci. Processes Impacts 2014, 16, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ronen, A.; Walker, S.L.; Jassby, D. Polyaniline-coated carbon nanotubes ultra-filtration membranes: Enhanced anodic stability for in situ cleaning and electro-oxidation processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Schnoor, M.H.; Rahaman, S.; Schiffman, J.D.; Elimelech, M. Electrochemical multiwalled carbon nanotube filter for viral and bacterial removal and inactivation. Environ. Sci. Technol. 2011, 45, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Hong, S.; Bae, T.; Tak, T.; Hong, S.; Randall, A. Fouling control in activated sludge submerged hollow fiber membrane bioreactors. Desalination 2002, 143, 219–228. [Google Scholar] [CrossRef]
- Sofia, A.; Ng, W.J.; Ong, S. Engineering design approaches for minimum fouling in submerged MBR. Desalination 2004, 160, 67–74. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Valle de Leon, J.; Dudchenko, A.; Yao, S.; Corbala-Delgado, J.; Yan, A.; Matsumoto, M.; Jassby, D. Treating anaerobic sequencing batch reactor effluent with electrically conducting ultrafiltration and nanofiltration membranes for fouling control. J. Membr. Sci. 2016, 504, 104–112. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; Chen, Z.; Su, X.; Hinds, B.J. Fouling characteristics and electrochemical recovery of carbon nanotube membranes. Adv. Funct. Mater. 2013, 23, 1500–1506. [Google Scholar] [CrossRef]
- Camalet, J.L.; Lacroix, J.C.; Aeiyach, S.; Chane-Ching, K.; Lacaze, P.C. Electrosynthesis of adherent polyaniline films on iron and mild steel in aqueous oxalic acid medium. Synth. Met. 1998, 93, 133–142. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Oliveira Brett, A.-M.C.F.; Pereira, J.L.C.; Rebelo, C. Properties of polyaniline formed at tin dioxide electrodes in weak acid solution: Effect of the counterion. J. Appl. Electrochem. 1993, 23, 332–338. [Google Scholar] [CrossRef]
- Murugesan, R.; Subramanian, E. Effect of organic dopants on electrodeposition and characteristics of polyaniline under the varying influence of H2SO4 and HClO4 electrolyte media. Mater. Chem. Phys. 2003, 80, 731–739. [Google Scholar] [CrossRef]
- Gloukhovski, R.; Oren, Y.; Linder, C.; Freger, V. Thin-film composite nanofiltration membranes prepared by electropolymerization. J. Appl. Electrochem. 2008, 38, 759–766. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Various Types of Dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in Water by UV-Irradiated Titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Jiang, Y.; Biswas, P.; Fortner, J.D. A review of recent developments in graphene-enabled membranes for water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 915–922. [Google Scholar] [CrossRef]
- De Lannoy, C.-F.; Soyer, E.; Wiesner, M.R. Optimizing carbon nanotube-reinforced polysulfone ultrafiltration membranes through carboxylic acid functionalization. J. Membr. Sci. 2013, 447, 395–402. [Google Scholar] [CrossRef]
- De Lannoy, CF.; Jassby, D.; Davis, D.D.; Wiesner, M.R. A highly electrically conductive polymer–multiwalled carbon nanotube nanocomposite membrane. J. Membr. Sci. 2012, 415–416, 718–724. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.H.D.; Xia, Q.; Ma, Y.; Yu, Y.; Yung, L.Y.L.; Xie, J.; Ong, C.N.; Vecitis, C.D.; Zhou, Z. A graphene-based electrochemical filter for water purification. J. Mater. Chem. A 2014, 2, 16554–16562. [Google Scholar] [CrossRef]
- Liu, Y.; Rosenfield, E.; Hu, M.; Mi, B. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 2013, 47, 2949–2958. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.L.; Yu, D.G.; Yang, M.C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Tang, L.; Gu, W.; Yi, P.; Bitter, J.L.; Hong, J.Y.; Fairbrother, D.H.; Chen, K.L. Bacterial anti-adhesive properties of polysulfone membranes modified with polyelectrolyte multilayers. J. Membr. Sci. 2013, 446, 201–211. [Google Scholar] [CrossRef]
- Diagne, F.; Malaisamy, R.; Boddie, V.; Holbrook, R.D.; Eribo, B.; Jones, K.L.; States, U. Polyelectrolyte and silver nanoparticle modification of microfiltration membranes to mitigate organic and bacterial fouling. Environ. Sci. Technol. 2012, 46, 4025–4033. [Google Scholar] [CrossRef] [PubMed]
- Gall, I.; Herzberg, M.; Oren, Y. The effect of electric fields on bacterial attachment to conductive surfaces. Soft Matter 2013, 9, 2443. [Google Scholar] [CrossRef]
- Miller, D.J.; Araújo, P.A.; Correia, P.B.; Ramsey, M.M.; Kruithof, J.C.; van Loosdrecht, M.C.M.; Freeman, B.D.; Paul, D.R.; Whiteley, M.; Vrouwenvelder, J.S. Short-term adhesion and long-term biofouling testing of polydopamine and poly(ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 2012, 46, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, D.; Sheng, X.; Cohen, R.E.; Rubner, M.F. Two-level antibacterial coating with both release-killing and contact-killing capabilities. Langmuir 2006, 22, 9820–9823. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lyon, D.; Li, Q.; Alvarez, P.J.J. Effect of soil sorption and aquatic natural organic matter on the antibacterial activity of a fullerene water suspension. Environ. Toxicol. Chem. 2008, 27, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Yoon, H.; Shim, S.; Choi, J.; Yoon, J. Electroconductive feed spacer as a tool for biofouling control in a membrane system for water treatment. Environ. Sci. Technol. Lett. 2014, 1, 179–184. [Google Scholar] [CrossRef]


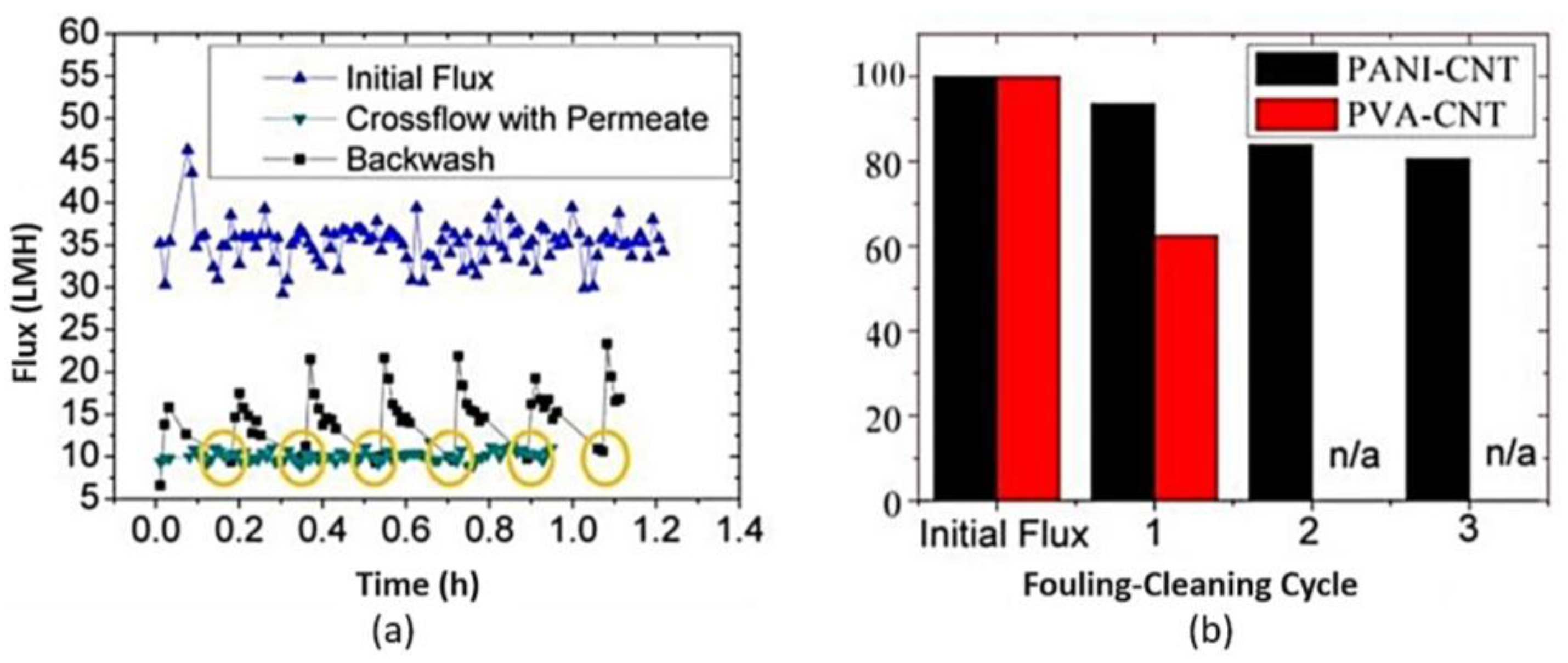
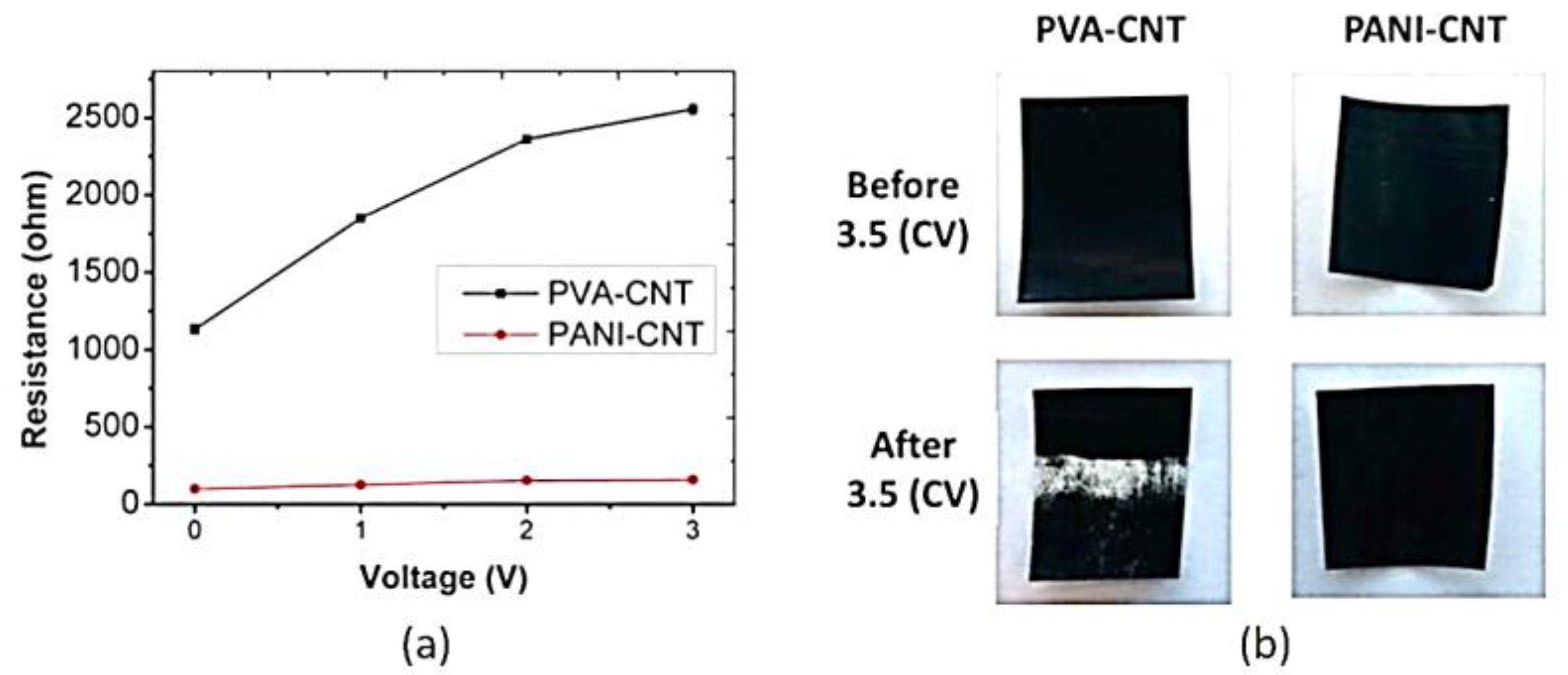
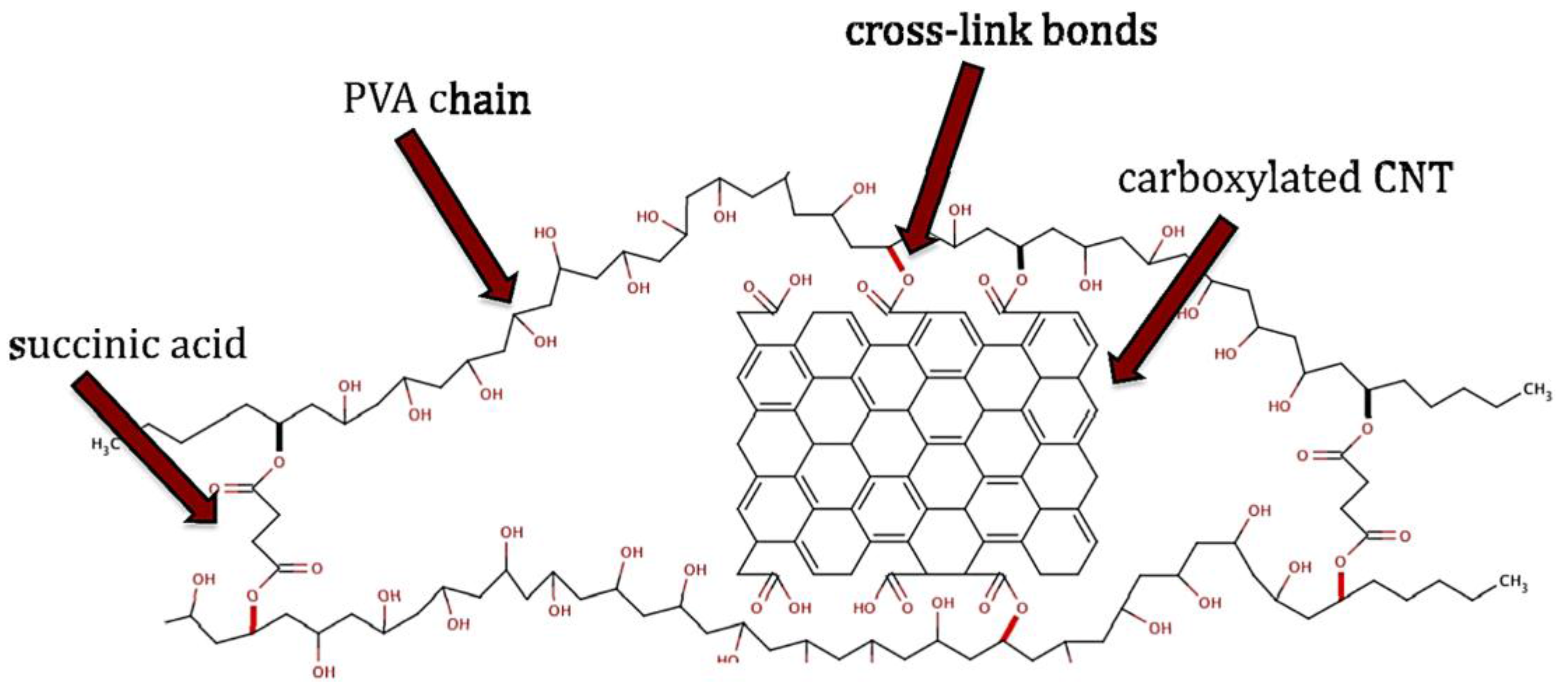


| Chemical Name | Abbreviation |
|---|---|
| Polyacetylene | PAc |
| Polyaniline | PANI |
| Polyazulene | PAZ |
| Polybutadiene | PBD |
| Polyisopren | PIP |
| Poly(isothianaphtene) | PITN |
| Polyfuran | PFu |
| Poly(α-naphthylamine) | PNA |
| Poly(p-phenylene) | PPP |
| Polythiophene | PTh |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review. Membranes 2017, 7, 39. https://doi.org/10.3390/membranes7030039
Formoso P, Pantuso E, De Filpo G, Nicoletta FP. Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review. Membranes. 2017; 7(3):39. https://doi.org/10.3390/membranes7030039
Chicago/Turabian StyleFormoso, Patrizia, Elvira Pantuso, Giovanni De Filpo, and Fiore Pasquale Nicoletta. 2017. "Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review" Membranes 7, no. 3: 39. https://doi.org/10.3390/membranes7030039