Review of Membranes for Helium Separation and Purification
Abstract
:1. Introduction
2. Helium Sources
3. Polymeric Membranes
4. Inorganic Membranes
5. Membrane Processes
6. Conclusions
Author Contributions
Conflict of Interest
References
- Clarke, R.; Nuttall, W.; Glowacki, B. Endangered helium: Bursting the myth. Chem. Eng. 2013, 870, 32–36. [Google Scholar]
- Scholes, C.A. Helium: Is the party really over? Chem. Aust. 2011, 78, 18–20. [Google Scholar]
- Haussinger, P.; Glathaar, R.; Rhode, W.; Kick, H.; Benkmann, C.; Weber, J.; Wunschel, H.-J.; Stenke, V.; Leicht, E.; Stenger, H. Noble Gases. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 392–448. [Google Scholar]
- Peterson, J.B. Helium Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2001.
- Hamak, J.E. Helium Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2016.
- Rufford, T.E.; Chan, K.I.; Huang, S.H.; May, E.F. A review of conventional and emerging process technologies for the recovery of helium from natural gas. Adsorpt. Sci. Technol. 2014, 32, 49–72. [Google Scholar] [CrossRef] [Green Version]
- Kerry, F.G. Rare (Noble) Gases. In Industrial Gas Handbook Gas Separation and Purification; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Spillman, R. Economics of Gas Separation Membrane Processes. In Membrane Separations Technology; Noble, R.D., Stern, S.A., Eds.; Elsevier Science: Eastbourne, UK, 1995; pp. 589–667. [Google Scholar]
- Committee on the impact of selling the Federal helium reserve. The Impact of Selling the Federal Helium Reserve; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Zartman, R.E.; Wasserburg, G.J.; Reynolds, J.H. Helium, argon and carbon in some natural gases. J. Geophys. Res. 1961, 66, 277–306. [Google Scholar] [CrossRef]
- Stern, S.A.; Sinclair, T.F.; Gareis, P.J.; Vahldieck, N.P.; Mohr, P.H. Helium recovery by permeation. Ind. Eng. Chem. 1965, 57, 49–60. [Google Scholar] [CrossRef]
- Industries, E. Sepuran Noble. Available online: http://www.sepuran.com (accessed on 25 September 2016).
- Systems, I.G. Helium Recovery. Generon Membrane Technology. Available online: http://www.igs-global.com (accessed on 25 September 2016).
- Divex. Helipure Membrane Gas Separation System. Available online: http://www.divexglobal.com (accessed on 25 September 2016).
- Petropoulos, J.H. Mechanisms and Theories for Sorption and Diffusion of Gases in Polymers. In Polymeric Gas Separation; Paul, D.R., Yampol’skii, Y.P., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 17–81. [Google Scholar]
- Flory, P. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Scholes, C.A.; Tao, W.X.; Stevens, G.W.; Kentish, S.E. Sorption of methane, nitrogen, carbon dioxide and water in matrimid 5218. J. Appl. Polym. Sci. 2010, 117, 2284–2289. [Google Scholar] [CrossRef]
- Kamiya, Y.; Naito, Y.; Mizoguchi, K.; Terada, K.; Moreau, J. Thermodynamic intereactions in rubbery polymer/gas systems. J. Polym. Sci. Polym. Phys. B 1997, 35, 1049–1053. [Google Scholar] [CrossRef]
- Brandani, S.; Mangano, E.; Sarkisov, L. Net, excess and absolute adsorption and adsorption of helium. Adsorption 2016, 22, 261–276. [Google Scholar] [CrossRef]
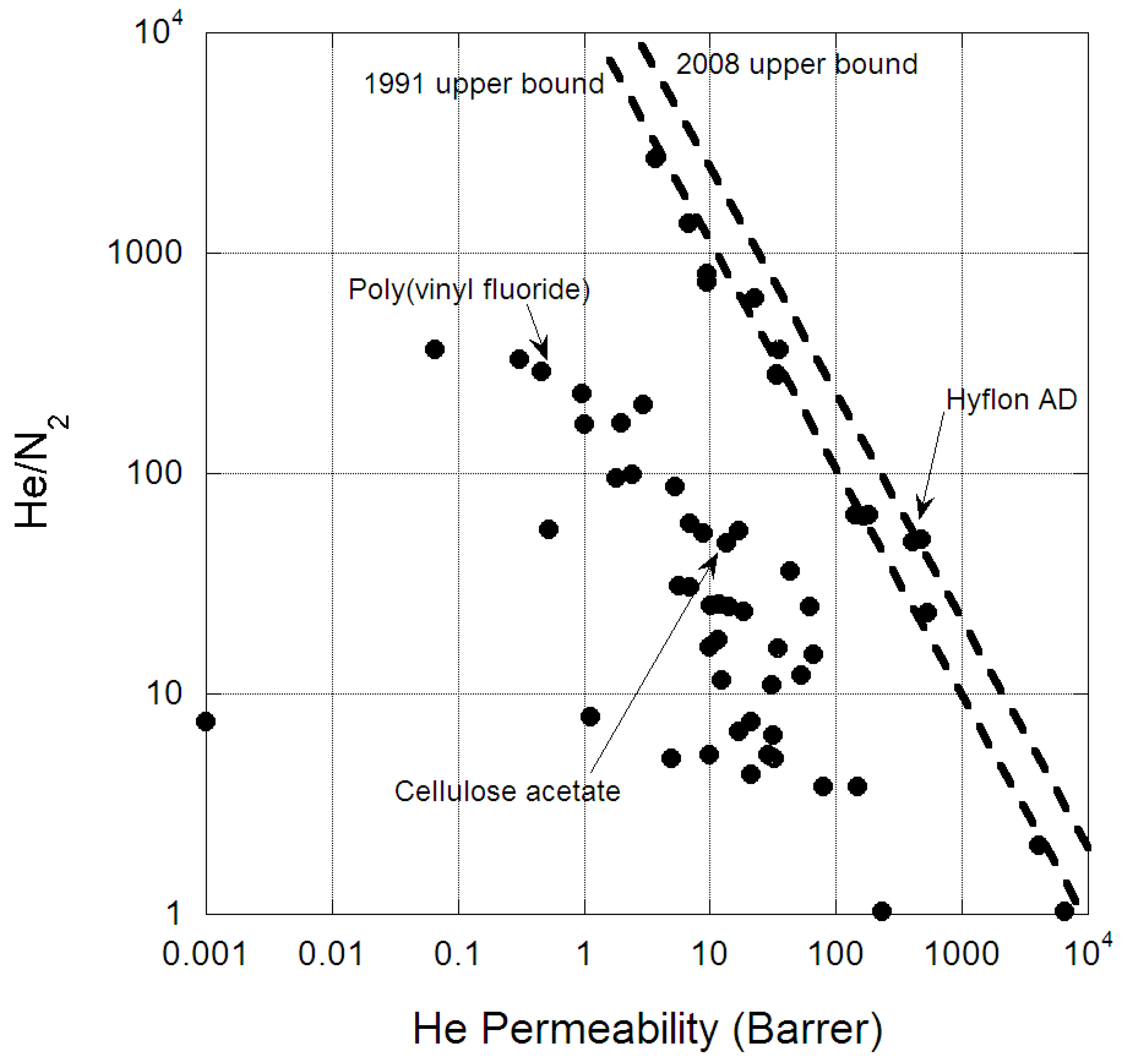
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W. Effects of minor components in carbon dioxide capture using polymeric gas separation membranes. Sep. Purif. Rev. 2009, 38, 1–44. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W. The effect of hydrogen sulfide, carbon monoxide and water on the performance of a pdms membrane in carbon dioxide/nitrogen separation. J. Membr. Sci. 2010, 350, 189–199. [Google Scholar] [CrossRef]
- Budd, P.M.; Makhseed, S.M.; Ghanem, B.S.; Msayib, K.J.; Tattershall, C.E.; McKeown, N.B. Microporous polymeric materials. Mater. Today 2004, 7, 40–46. [Google Scholar] [CrossRef]
- Hu, Y.; Shiotsuki, M.; Sanda, F.; Freeman, B.D.; Masuda, T. Synthesis and properties of indan-based polyacetylenes that feature the highest gas permeability among all the existing polymers. Macromolecules 2008, 41, 8525–8532. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Gantzel, P.K.; Merten, U. Gas separations with high-flux cellulose acetate membranes. Ind. Eng. Chem. Process Des. Dev. 1970, 9, 331–332. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Scholes, C.A.; Ghosh, U. Helium separation through polymeric membranes: Selectivity targets. J. Membr. Sci. 2016, 520, 221–230. [Google Scholar] [CrossRef]
- Takada, K.; Matsuya, H.; Masuda, T.; Higashimura, T. Gas permeability of polyacetylenes carrying substituents. J. Appl. Polym. Sci. 1985, 30, 1605–1616. [Google Scholar] [CrossRef]
- Toy, L.G.; Nagai, K.; Freeman, B.D.; Pinnau, I.; He, Z.; Masuda, T.; Teraguchi, M.; Yampolskii, Y.P. Pure gas and vapor permeation and sorption properties of poly[1-phenyl-2-[p-(trimethylsilyl)phenyl]acetylene] (ptmsdpa). Macromolecules 2000, 33, 2516–2524. [Google Scholar] [CrossRef]
- Min, K.E.; Paul, D.R. Effect of tacticity on permeation properites of poly(methyl metacrylate). J. Polym. Sci. B Polym. Phys. 1988, 26, 1021–1033. [Google Scholar] [CrossRef]
- Chiou, J.S.; Paul, D.R. Gas permeation in a dry nafion membrane. Ind. Eng. Chem. Res. 1988, 27, 2161–2164. [Google Scholar] [CrossRef]
- Pye, D.G.; Hoehn, H.H.; Panar, M. Measurement of gas permeability of polymers. I. Permeabilities in constant volume/variable pressure apparatus. J. Appl. Polym. Sci. 1976, 20, 1921–1931. [Google Scholar] [CrossRef]
- Kim, T.-H.; Koros, W.J.; Husk, G.R. Advanced gas separation membrane materials: Rigid aromatic polyimides. Sep. Sci. Technol. 1988, 23, 1611–1626. [Google Scholar] [CrossRef]
- Kim, J.H.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6fda-based polyimide membranes: Part 1. Without crosslinking. J. Membr. Sci. 2006, 282, 21–31. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Koros, W.J. Polypyrrolones for membrane gas separations. I. Structural comparison of gas transport and sorption properties. J. Polym. Sci. B Polym. Phys. 1999, 37, 1235–1249. [Google Scholar] [CrossRef]
- Guzman-Gutierrez, M.T.; Ruiz-Trevino, F.A.; Zolutukhin, M.; Hernandez-Lopez, S.; Scherf, U. Gas transport properties of high free volume polyarylates based on isophthalic/terephthalic acid chloride mixtures. J. Membr. Sci. 2007, 305, 347–352. [Google Scholar] [CrossRef]
- Macchione, M.; Jansen, J.C.; Luca, G.D.; Tocci, E.; Longeri, M.; Drioli, E. Experimental analysis and simulation of the gas transport in dense hyflon® AD60X membranes: Influence of residual solvent. Polymer 2007, 48, 2619–2635. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene. J. Membr. Sci. 1996, 109, 125–133. [Google Scholar] [CrossRef]
- Fitch, M.W.; Koros, W.J.; Nolen, R.L.; Carnes, J.R. Permeation of several gases through elastomers, with emphasis on the deuterium/hydrogen pair. J. Appl. Polym. Sci. 1993, 47, 1033–1046. [Google Scholar] [CrossRef]
- Teplyakov, V.V.; Paul, D.R.; Bespalova, N.B.; Finkel’shtein, E.S. Gas permeation in a fluorine-containing polynorbornene. Macromolecules 1992, 25, 4218–4219. [Google Scholar] [CrossRef]
- Mottern, M.L.; Shi, J.Y.; Shqau, K.; Yu, D.; Verweij, H. Microstructural Optimization of Thin Supported Inorganic Membranes for Gas and Water Purification. In Advanced Membrane Technology and Applications; Li, N.N., Fane, A.G., Ho, W.S.W., Matsuura, T., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 899–928. [Google Scholar]
- Zolandz, R.R.; Fleming, G.K. Definitions. In Membrane Handbook; Ho, W.S.W., Sirkar, K.K., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2001; pp. 19–24. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley & Sons: New York, NY, USA, 1973. [Google Scholar]
- Kanezashi, M.; Fujita, T.; Asaeda, M. Nickel-doped silica membranes for separation of helium from organic gas mixtures. Sep. Sci. Technol. 2005, 40, 225–238. [Google Scholar] [CrossRef]
- Van Veen, H.M.; Tol, J.P.B.M.; Engelen, C.W.R.; Veringa, H.J. High Temperature Gas Separation with Alumina Membranes. In Proceedings of the International Conference on Inorganic Membranes (ICIM2–91), Montpellier, France, 1–4 July 1991.
- Yoo, Y.; Varela-Guerrero, V.; Jeong, H.-K. Isoreticular metal-organic frameworks and their membranes with enhanced crack resistance and moisture stability by surfactant-assisted drying. Langmuir 2011, 27, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Tsapatsis, M. Microporous metal organic framework membrane on porous support using the seeded growth method. Chem. Mater. 2009, 21, 4920–4924. [Google Scholar] [CrossRef]
- Takamizawa, S.; Takasaki, Y.; Miyake, R. Single-crystal membrane for anisotropic and efficient gas permeation. J. Am. Chem. Soc. 2010, 132, 2862–2863. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhang, C.; Xiao, Y.; Huang, H.; Zhang, W.; Liu, D.; Zhong, C.; Yang, Q.; Yang, Z.; Lu, X. Helium recovery by a Cu-BTC metal-organic-framework membrane. Ind. Eng. Chem. Res. 2012, 51, 11274–11278. [Google Scholar] [CrossRef]
- Vaezi, M.J.; Bayat, Y.; Babaluo, A.A.; Shafiei, S. Separation of helium from gases using the synthesized hydroxy sodalite membrane. Sci. Iran. C 2016, 23, 1136–1143. [Google Scholar]
- Asaeda, M.; Yamasaki, S. Separation of inorganic/organic gas mixtures by porous silica membranes. Sep. Purif. Technol. 2001, 25, 151–159. [Google Scholar] [CrossRef]
- Peters, T.A.; Fontalvo, J.; Vorstman, M.A.G.; Benes, N.E.; van Dam, R.A.; Vroon, Z.A.E.P.; van Soest-Vercammen, E.L.J.; Keurentjes, J.T.F. Hollow fibre microporous silica membranes for gas separation and pervaporation. Synthesis, performance and stability. J. Membr. Sci. 2005, 248, 73–80. [Google Scholar] [CrossRef]
- Araki, S.; Mohri, N.; Yoshimitsu, Y.; Miyake, Y. Synthesis, characterization and gas permeation properties of a silica membrane prepared by high-pressure chemical vapor deposition. J. Membr. Sci. 2007, 290, 138–145. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the cost of CO2 capture from flue gases using membrane technology. Ind. Eng. Chem. Res. 2008, 47, 1562–1568. [Google Scholar] [CrossRef]
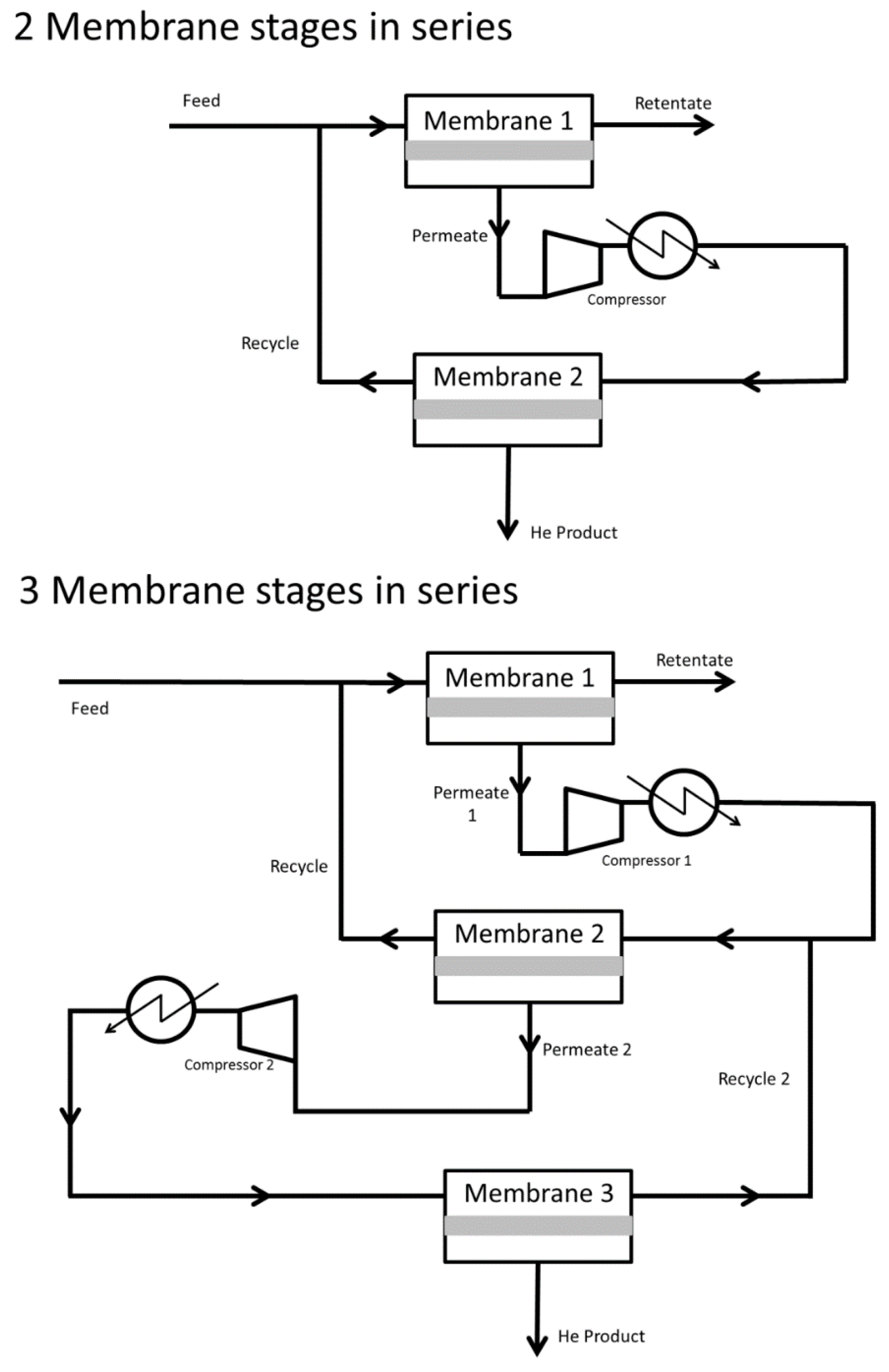
- Seok, D.R.; Kang, S.G.; Hwang, S.-T. Separation of helium and hydrocarbon mixtures by a two-membrane column. J. Membr. Sci. 1986, 27, 1–11. [Google Scholar] [CrossRef]
- Hale, P.W.; Lokhandwala, K.A. Helium Recovery from Gas Streams. U.S. Patent 20050217479 A1, 6 October 2005. [Google Scholar]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
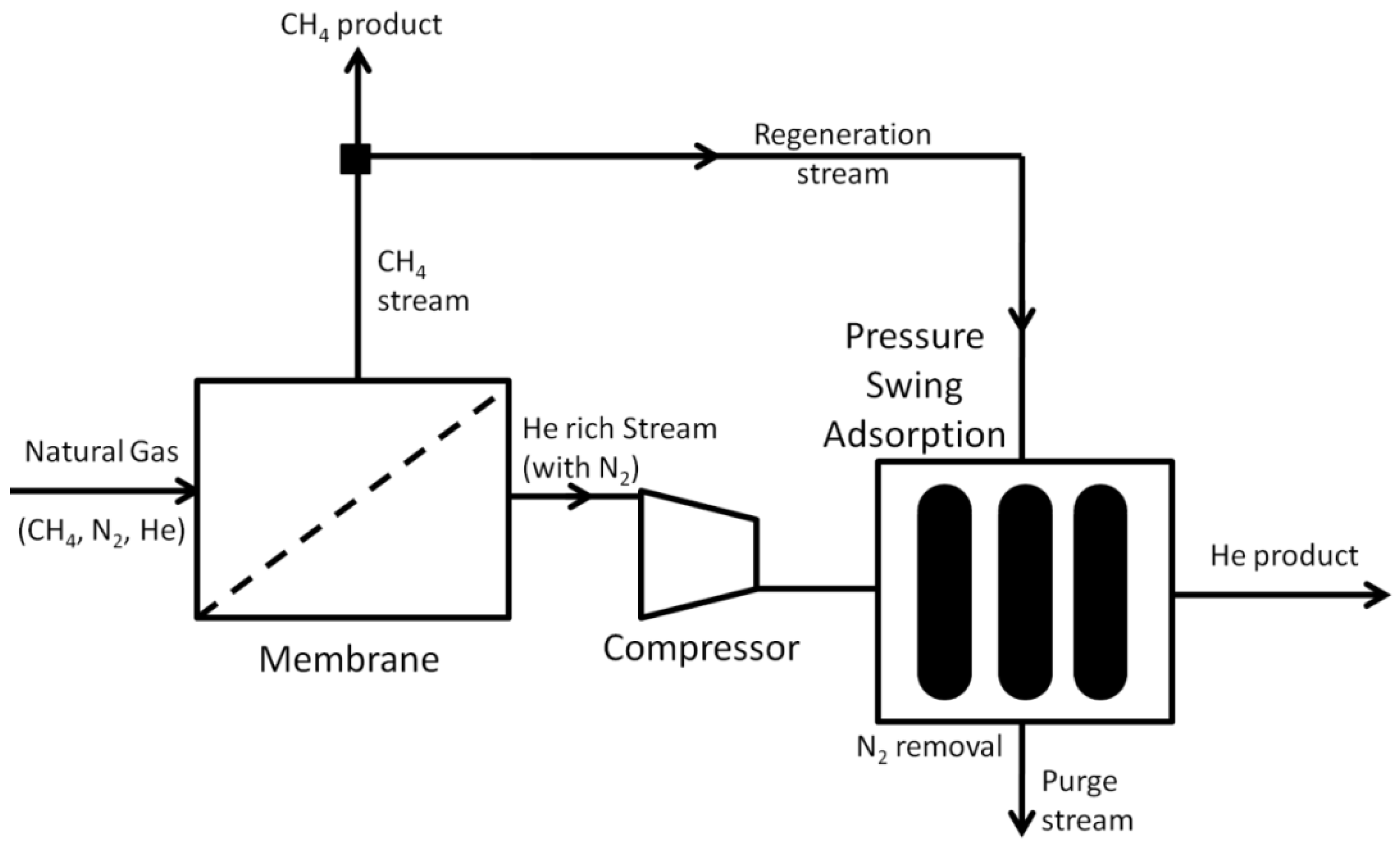
- Behling, R.-D.; Peinemann, K.V. A Process for the Separation/Recovery of Gases. U.S. Patent 6,179,900 B1, 30 January 2001. [Google Scholar]
- Choe, J.S.; Auvil, S.R.; Agrawal, R. Process for Separating Components of a Gas Stream. U.S. Patent 4,701,187 A, 20 October 1987. [Google Scholar]
- Doshi, K.J.; Werner, R.G.; Mitariten, M.J. Integrated Membrane/PSA Process and System. U.S. Patent 4,863,492, 5 September 1989. [Google Scholar]
- Jaynes, S.E. System and Process for Gas Recovery. U.S. Patent 6,517,791, 11 February 2003. [Google Scholar]
- Choe, J.S.; Agrawal, R.; Auvil, S.R. Process for Recovering Helium from a Multi-Component Gas Stream. U.S. Patent 4,717,407, 5 January 1988. [Google Scholar]
- Doshi, K.J. Enhanced Gas Separation Process. U.S. Patent 4,690,695, 1 September 1987. [Google Scholar]
- Stoner, G.; Reingold, H.E.I.; D’Amico, J.S.; Knaebel, K.S. Enhanced Helium Recovery. U.S. Patent 5,632,803, 27 May 1997. [Google Scholar]
- Doshi, K.J. Enhanced Hydrogen Recovery from Low Purity Gas Streams. U.S. Patent 4,398,926, 16 August 1983. [Google Scholar]
- Mehra, Y.R. Process for Recovering Helium from a Gas Stream. U.S. Patent 5,224,350, 6 July 1993. [Google Scholar]
- Schulte, T.R. Coolant Recovery Process. U.S. Patent 5,377,491, 3 January 1995. [Google Scholar]
- Lhote, B.; Queille, P.; Zumbrunn, J.-P.; Duchateau, E. Process and Apparatus for Heat Treating Articles While Hardening in Gaseous Medium. U.S. Patent 5,158,625, 27 October 1992. [Google Scholar]
| Natural Gas Field | He | CH4 | N2 | CO2 | C2+ |
|---|---|---|---|---|---|
| New Mexico, USA | 4.05 | 49 | 45 | 0.90 | 1.05 |
| Alaska, USA | 2.54 | 90.2 | 6.8 | 0.3 | – |
| Texas, USA | 1.17 | 66.2 | 31.1 | 0.10 | 1.43 |
| Alberta, Canada | 0.53 | 93 | 6 | 0.50 | – |
| Ostrow, Poland | 0.40 | 56 | 46 | 0.30 | 0.30 |
| North Field, Qatar | 0.03 | 79.5 | 5.19 | 3.68 | 8.85 |
| Palm Valley, Australia | 0.21 | 97.5 | 2.3 | 0.10 | – |
| Polymer | He Permeability | He/N2 | He/CH4 | Ref. | Citations |
|---|---|---|---|---|---|
| Poly(trimethylsilylpropyne) | 4100 | 2.05 | 0.98 | [30] | 5 |
| Poly(trimethylsilylpropyne) | 6500 | 1.03 | 0.433 | [31] | 74 |
| Substituted Poly(diphenylacetylene) | 11200 | 0.97 | 0.38 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 15800 | 1.01 | 0.46 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 12800 | 1.07 | 0.46 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 17800 | 1.07 | 0.51 | [25] | 33 |
| Substituted Poly(diphenylacetylene) | 13700 | 1.05 | 0.47 | [25] | 33 |
| Isotactic poly(methyl methacrylate) (PMMA) | 3.75 | 2679 | – | [32] | 51 |
| Atactic PMMA | 9.43 | 806 | – | [32] | 51 |
| Syndiotactic PMMA | 9.57 | 736 | – | [32] | 51 |
| Poly(trichloromonochloroethylene) poly diacetylene (PDA) | 34.1 | 284 | – | [15] | 1384 |
| Nafion 117 | 40.9 | – | 401 | [33] | 93 |
| Poly(trichloromonochloroethylene) | 34.1 | – | 406 | [15] | 1384 |
| Tetramethyl bis polycarbonate | 206 | – | 43.8 | [15] | 1384 |
| Poly(vinyl alcohol) | 0.0071 | – | – | [34] | 67 |
| Poly(vinyl alcohol) | 0.052 | – | – | [15] | 1384 |
| 6FDA-DAF polyimide | 98.5 | – | 156 | [35] | 73 |
| 6FDA/tetramethyl PDA polyimide | 530 | 23.2 | – | [15] | 19 |
| Polyimide (6FDA-6FpDA:DABA (2:1)) | 142 | 65 | – | [36] | 31 |
| Polyimide | 396 | – | – | [15] | 1384 |
| Polypyrrolone (6FDA/PMDA (10/90)-TAB) | 22.5 | 622 | 3041 | [37] | 55 |
| Polypyrrolone (6FDA/PMDA (25/75)-TAB) | 35.7 | 364 | 1594 | [37] | 55 |
| Polypyrrolone (6FDA-TAB) | 166 | 64.4 | 184 | [37] | 55 |
| Polyarylate (TMHFBPA I/T) | 182 | 64.8 | – | [38] | 6 |
| Hyflon AD | 405 | 48.8 | 167 | [16] | 1186 |
| Hyflon AD60X | 476 | 50.3 | 157 | [39] | 43 |
| Teflon AF-2400 | 3600 | – | 6 | [40] | 153 |
| Teflon FEP | 62 | 25 | 44 | [7] | 70 |
| Viton E60 fluoroelastomer | 30.5 | – | – | [41] | 19 |
| Viton fluoroelastomer | 43.9 | – | – | [41] | 19 |
| Cytop | 170 | – | – | [16] | 1186 |
| Fluorinated polynorbornene | 185 | – | – | [42] | 20 |
| Hostaflon perfluoroalkoxy alkane (PFA) | 43.9 | 35.9 | 41.8 | [43] | – |
| Poly(tetrafluoroethylene-co-ethylene) | 5.63 | 30.9 | – | [43] | – |
| Poly(trifluorochloroethylene-co-ethylene) | 5.33 | 87.5 | – | [43] | – |
| Polyvinyl fluoride | 1.8 | 95 | 280 | [7] | 70 |
| Poly(vinyl fluoride) | 0.46 | 289 | – | [43] | – |
| Low density polyethylene (LDPE) | 4.92 | 5.06 | 1.68 | [41] | 552 |
| High density polyethylene (HDPE) | 1.14 | 7.8 | 2.97 | [41] | 552 |
| Poly(ethylene-co-propylene) | 31.9 | 6.49 | – | [42] | 14 |
| Poly(ethylene-co-propylene) | 29 | 5.31 | – | [42] | 14 |
| Poly(ethylene-co-propylene) | 21.3 | 4.32 | – | [42] | 14 |
| Poly(propylene) | 0.373 | 0.85 | – | [43] | – |
| Trespaphan | 14.1 | 25 | – | [44] | – |
| Trespaphan | 11.96 | 25.3 | – | [44] | – |
| Trespaphan | 10.25 | 25.2 | – | [44] | – |
| Trespaphan | 11.6 | 17.6 | – | [44] | – |
| Poly(styrene) | 18.64 | 23.73 | – | [45] | 17 |
| Polystyrene | 35 | 16 | 15 | [7] | 70 |
| Poly(ethyl methacrylate) | 6.9 | 30.5 | – | [43] | – |
| Poly(vinyl acetate) | 12.57 | – | 398 | [46] | 418 |
| Poly(trifluorochloroethylene) | 6.79 | 1360 | – | [47] | – |
| Poly(vinyl alcohol) | 0.001 | 7.5 | – | [47] | – |
| Poly(vinyl benzoate) | 8.88 | 53.79 | – | [48] | 95 |
| Poly(vinyl chloride) | 2 | 168.5 | 71.4 | [43] | – |
| Saran | 0.31 | 330 | 260 | [21,47] | 47 |
| Poly(butadiene) | 32.6 | 5.06 | – | [42] | 14 |
| Poly(butadiene-co-acryonitrile) | 16.9 | 6.7 | – | [49] | 213 |
| Poly(butadiene-co-acryonitrile) | 12.3 | 11.5 | – | [49] | 213 |
| Poly(butadiene-co-acryonitrile) | 9.85 | 16.3 | – | [49] | 213 |
| Poly(oxydimethylsilylene) | 233 | 1.03 | – | [43] | – |
| Nylon 6 | 0.53 | 55.8 | – | [47] | – |
| Cellulose acetate | 13.6 | 48.6 | – | [47] | – |
| Cellulose nitrate | 6.9 | 59.5 | – | [50] | 16 |
| Ethyl cellulose | 53.4 | 12.1 | – | [50] | 16 |
| Polyvinyl fluoride | 0.97 | 231 | – | [51] | 16 |
| Polyvinylidene chloride | 0.066 | 366 | – | [51] | 16 |
| Nylon 6 | 2.43 | 98.8 | – | [51] | 16 |
| Mylar | 1.002 | 167 | 170 | [21,51] | 16 |
| Polyethylene terephthalate | 2.967 | 206 | – | [51] | 16 |
| Cellulose acetate | 1990 | – | 11.8 | [26] | 13 |
| Silicone rubber | 356 | – | 0.34 | [26] | 13 |
| Phenylene silicone rubber | 150 | 3.8 | 0.75 | [7] | 70 |
| Nitrile silicone rubber | 79 | 3.8 | 0.79 | [7] | 70 |
| Polycarbonate | 67 | 15 | 19 | [7] | 70 |
| Trithene B | 34 | 280 | 400 | [7] | 70 |
| Ethyl cellulose | 31 | 11 | 4.9 | [7] | 70 |
| Ethylene-vinyl acetate | 21 | 7.5 | 1.9 | [7] | 70 |
| Viton A | 17 | 55 | 110 | [7] | 70 |
| Polyvinyl chloride | 14 | – | 7 | [7] | 70 |
| Material | He Permeance | He/N2 | He/CH4 | Ref. | Citations |
|---|---|---|---|---|---|
| Ni doped silica | 3466 | – | 600 (300 °C) | [46] | 31 |
| Porous Alumina | 86,190 | – | – | [47] | – |
| Isoreticular Metal-Organic framework (IRMOF-3) | 2986 | 2.5 | 1.6 | [48] | 44 |
| IRMOF-3 and -6 | 2389 | 2.6 | 1.3 | [48] | 44 |
| Metal-Organic framework (MMOF) | 32.9 | 3.5 | [49] | 176 | |
| [Cu2(bza)4(pyz)]n | 8.1 | 3.9 | 7.3 | [50] | 32 |
| [Cu2(bza)4(pyz)]n | 1.76 | – | – | [50] | 32 |
| Cu-BTC | 4181 | 2.6 | 2.07 | [51] | 19 |
| Hydroxy sodalite | – | 8.8 | 5 | [52] | – |
| Vycor Glas | 4.8 Barrer | 7619 | – | [11] | 70 |
| Microporous Silica | 2933 | 31 | 147 | [53] | 84 |
| Microporous Silica | 6570 | 560 | – | [54] | 65 |
| Microporous Silica | 89.6 | – | 5000 | [55] | 44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholes, C.A.; Ghosh, U.K. Review of Membranes for Helium Separation and Purification. Membranes 2017, 7, 9. https://doi.org/10.3390/membranes7010009
Scholes CA, Ghosh UK. Review of Membranes for Helium Separation and Purification. Membranes. 2017; 7(1):9. https://doi.org/10.3390/membranes7010009
Chicago/Turabian StyleScholes, Colin A., and Ujjal K. Ghosh. 2017. "Review of Membranes for Helium Separation and Purification" Membranes 7, no. 1: 9. https://doi.org/10.3390/membranes7010009






