Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes
Abstract
:1. Introduction
2. Results and Discussion
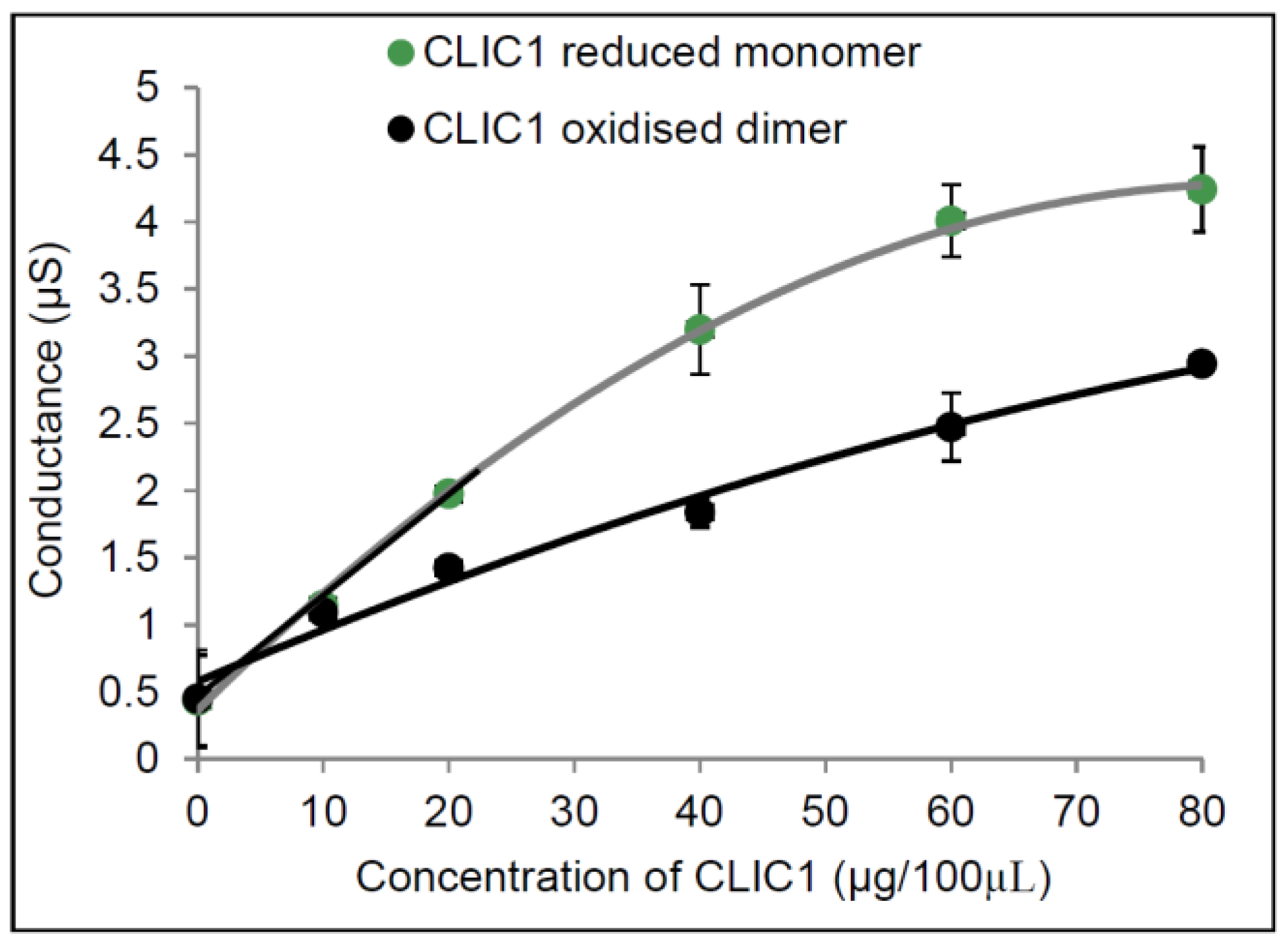
2.1. Conductance Properties of CLIC1 Reduced Monomer and CLIC1 Oxidised Dimer in tBLMs
2.2. Defining the Role of Redox Sensitive Residues within CLIC1
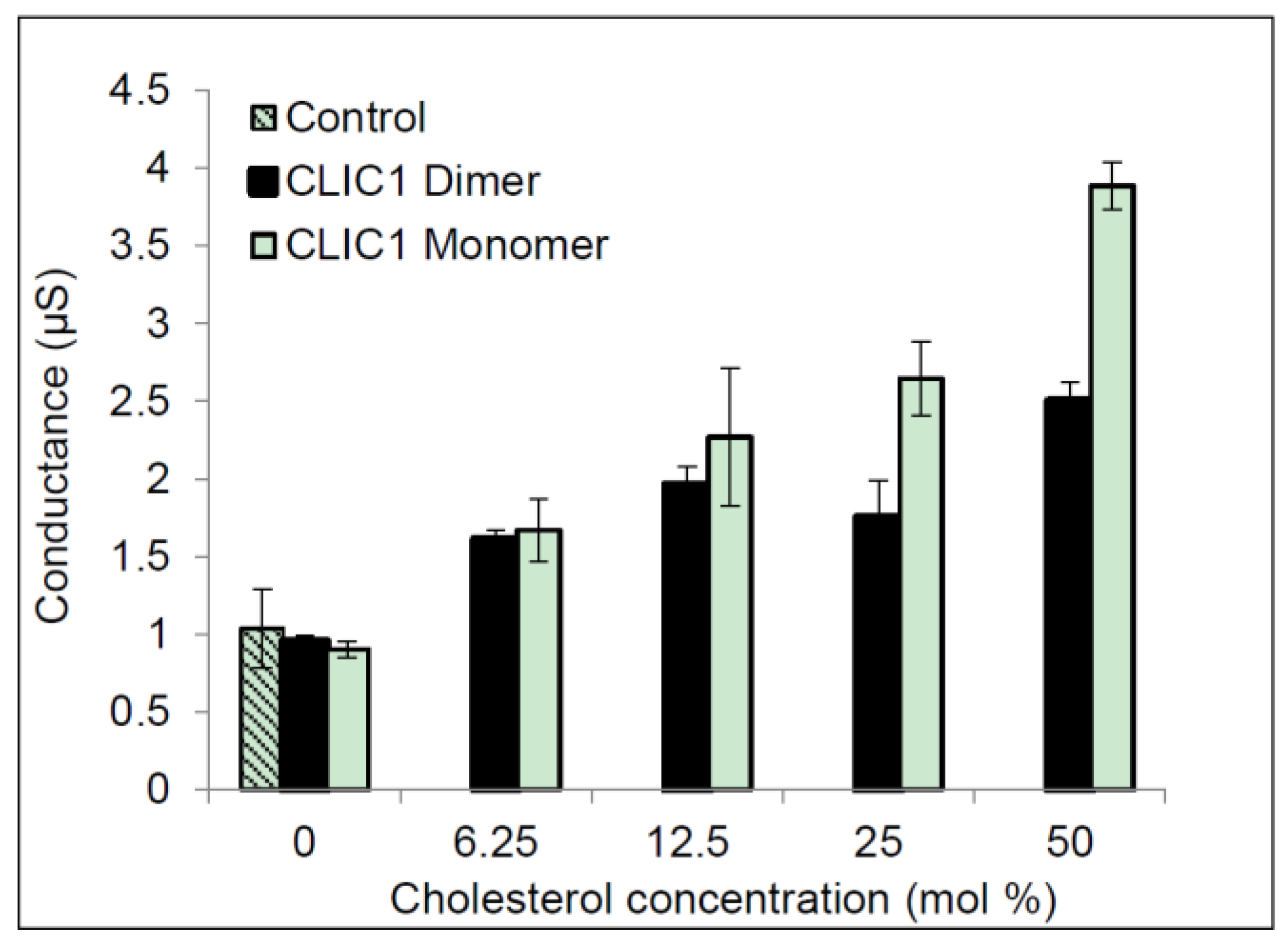
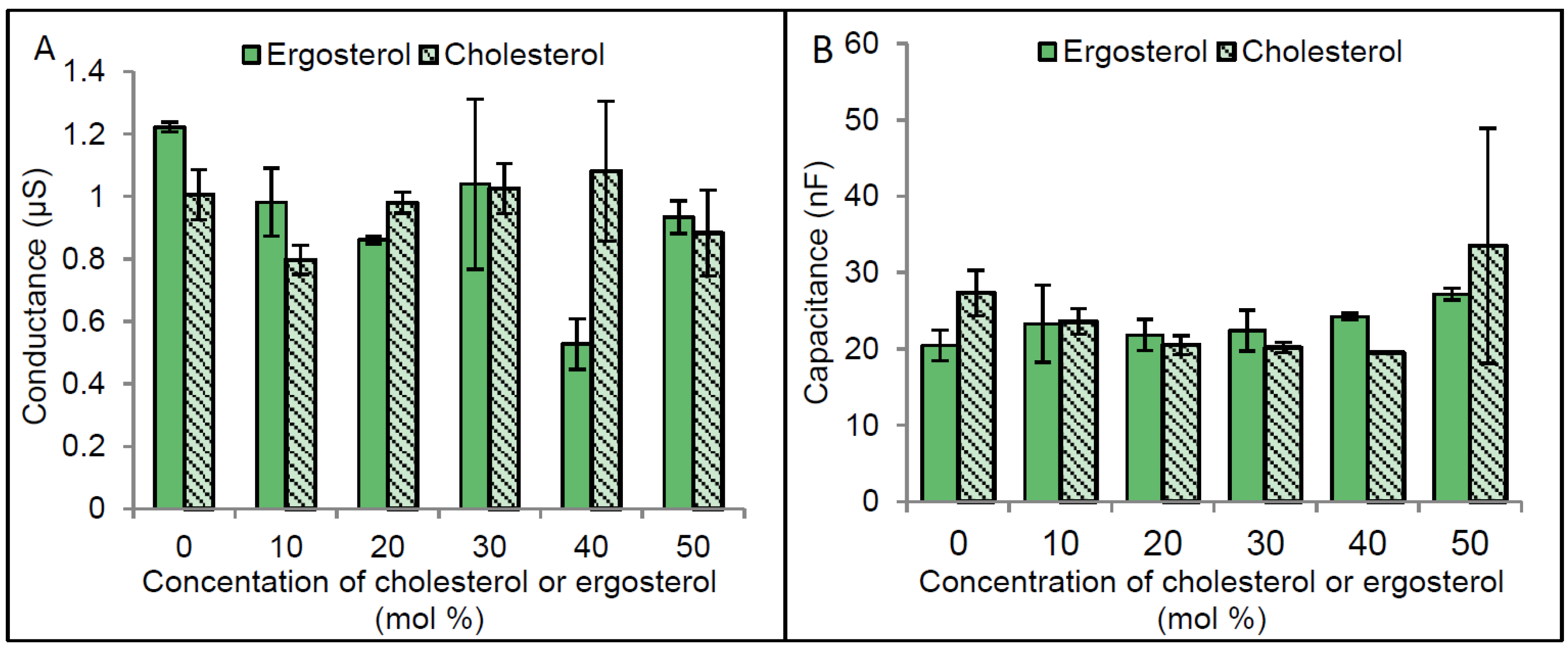
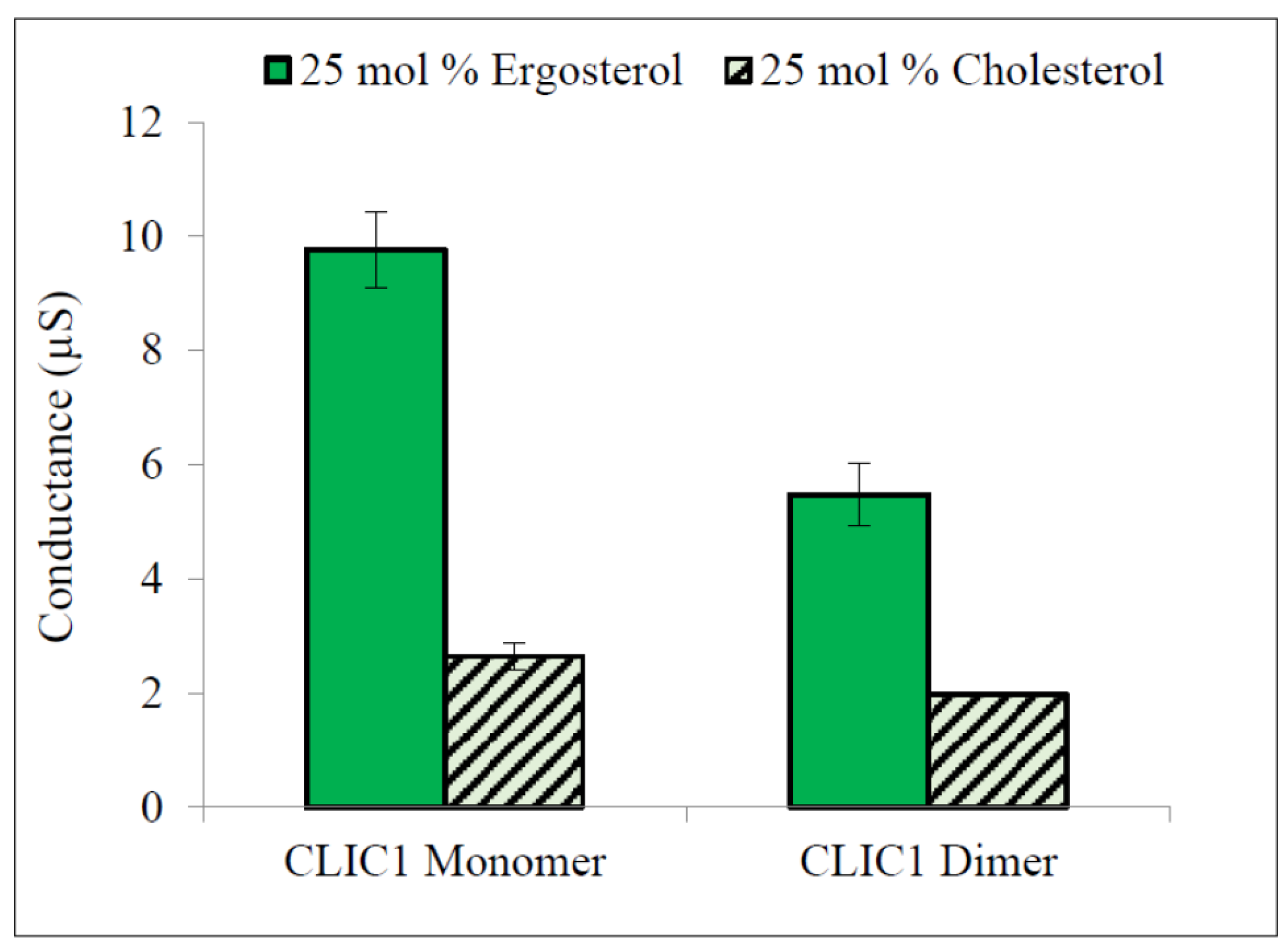
2.3. Regulation of CLIC1 Conductance by Sterols in tBLMs
3. Materials and Methods
3.1. Preparation of His-Tagged Recombinant CLIC1 WT, CLIC1-C24A and C59A Proteins
3.2. Recombinant CLIC1 Dimeric Protein
3.3. Preparation of Recombinant EXC-4 and CLIC1-C24S by GST Gene Fusion System
3.4. Formation of Tethered Bilayer Lipid Membranes (tBLM)
3.5. Formation of tBLM Using Yeast and Bacterial Lipids
3.6. Incorporation of CLIC1 WT, Mutants and EXC-4 into tBLMs Containing Cholesterol
3.7. Pre-Incubation of CLIC1 with Cholesterol or Ergosterol
3.8. Pre-Incubation of Listeriolysin-O with Cholesterol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valenzuela, S.M.; Martin, D.K.; Por, S.B.; Robbins, J.M.; Warton, K.; Bootcov, M.R.; Schofield, P.R.; Campbell, T.J.; Breit, S.N. Molecular cloning and expression of a chloride ion channel of cell nuclei. J. Biol. Chem. 1997, 272, 12575–12582. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Harrop, S.J.; Goodchild, S.C.; Phang, J.M.; Mynott, A.V.; Jiang, L.; Valenzuela, S.M.; Mazzanti, M.; Brown, L.J.; Breit, S.N.; et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010, 584, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, S.M.; Alkhamici, H.; Brown, L.J.; Almond, O.C.; Goodchild, S.C.; Carne, S.; Curmi, P.M.; Holt, S.A.; Cornell, B.A. Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Warton, K.; Tonini, R.; Fairlie, W.D.; Matthews, J.M.; Valenzuela, S.M.; Qiu, M.R.; Wu, W.M.; Pankhurst, S.; Bauskin, A.R.; Harrop, S.J. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a ph-dependent two-state process to form chloride ion channels with identical characteristics to those observed in chinese hamster ovary cells expressing CLIC1. J. Biol. Chem. 2002, 277, 26003–26011. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Harrop, S.J.; Fairlie, W.D.; Brown, L.J.; Pankhurst, G.J.; Pankhurst, S.; DeMaere, M.Z.; Campbell, T.J.; Bauskin, A.R.; Tonini, R. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004, 279, 9298–9305. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Cousin, M.; Ashley, R. Functional reconstitution of mammalian ‘chloride intracellular channels’ CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007, 274, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Tulk, B.M.; Kapadia, S.; Edwards, J.C. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am. J. Physiol. Cell Physiol. 2002, 282, C1103–C1112. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Assaad, N.N.; Harrop, S.J.; Brown, L.J.; Pankhurst, G.J.; Luciani, P.; Aguilar, M.I.; Mazzanti, M.; Berryman, M.A.; Breit, S.N. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 2005, 272, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ashley, R.H. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol. Membr. Biol. 2007, 24, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Ashley, R.H. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys. J. 2006, 90, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.R.; Westwood, P.K.; Boyd, A.; Ashley, R.H. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J. Biol. Chem. 1997, 272, 23880–23886. [Google Scholar] [CrossRef] [PubMed]
- Proutski, I.; Karoulias, N.; Ashley, R. Overexpressed chloride intracellular channel protein CLIC4 (p64H1) is an essential component of novel plasma membrane anion channels. Biochem. Biophys. Res. Commun. 2002, 297, 317–322. [Google Scholar] [CrossRef]
- Luetterforst, R.; Stang, E.; Zorzi, N.; Carozzi, A.; Way, M.; Parton, R.G. Molecular characterization of caveolin association with the golgi complex: Identification of a Cis-Golgi targeting domain in the caveolin molecule. J. Cell Biol. 1999, 145, 1443–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, M. Cholesterol and the activity of bacterial toxins. FEMS Microbiol. Lett. 2004, 238, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Suginita, W.; Karoulias, N.; Aitken, A.; Ashley, R.H. Chloride intracellular channel protein CLIC4 (p64H1) binds directly to brain dynamin I in a complex containing actin, tubulin and 14-3-3 isoforms. Biochem. J. 2001, 359, 55–64. [Google Scholar] [CrossRef]
- Bretscher, M.S.; Munro, S. Cholesterol and the golgi apparatus. Science 1993, 261, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Kucerka, N.; Nieh, M.-P.; Pencer, J.; Sachs, J.N.; Katsaras, J. What determines the thickness of a biological membrane. Gen. Physiol. Biophys. 2009, 28, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.R.; Al Khamici, H.; Holt, S.A.; Valenzuela, S.M. Cholesterol promotes interaction of the protein CLIC1 with phospholipid monolayers at the air–water interface. Membranes 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.; Silva, L.; Fedorov, A.; Prieto, M. Cholesterol and ergosterol influence nystatin surface aggregation: Relation to pore formation. Biophys. J. 2004, 87, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Köper, I. Tethered and polymer supported bilayer lipid membranes: Structure and function. Membranes 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Knoll, W.; Cho, N.-J. Biotechnology applications of tethered lipid bilayer membranes. Materials 2012, 5, 2637–2657. [Google Scholar] [CrossRef]
- Cornell, B.A.; Krishna, G.; Osman, P.D.; Pace, R.; Wieczorek, L. Tethered-bilayer lipid membranes as a support for membrane-active peptides. Biochem. Soc. Trans. 2001, 29, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, S.C.; Howell, M.W.; Cordina, N.M.; Littler, D.R.; Breit, S.N.; Curmi, P.M.; Brown, L.J. Oxidation promotes insertion of the CLIC1 chloride intracellular channel into the membrane. Eur. Biophys. J. 2009, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Fernández, A.; Encinar, J.; Gonzalez-Ros, J. Protein-promoted membrane domains. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.F.; Thomas, A.; Brasseur, R.; Vishwanathan, S.A.; Hunter, E.; Epand, R.M. Juxtamembrane protein segments that contribute to recruitment of cholesterol into domains. Biochemistry 2006, 45, 6105–6114. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.A.; Thomas, A.; Brasseur, R.; Epand, R.F.; Hunter, E.; Epand, R.M. Hydrophobic substitutions in the first residue of the CRAC segment of the gp41 protein of HIV. Biochemistry 2008, 47, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Harrop, S.J.; DeMaere, M.Z.; Fairlie, W.D.; Reztsova, T.; Valenzuela, S.M.; Mazzanti, M.; Tonini, R.; Qiu, M.R.; Jankova, L.; Warton, K.; et al. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-Å resolution. J. Biol. Chem. 2001, 276, 44993–45000. [Google Scholar] [CrossRef] [PubMed]
- Al Khamici, H.; Brown, L.J.; Hossain, K.R.; Hudson, A.L.; Sinclair-Burton, A.A.; Ng, J.P.; Daniel, E.L.; Hare, J.E.; Cornell, B.A.; Curmi, P.M.; et al. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.P.; Wallach, D.F. Multiple thermotropic state transitions in erythrocyte membranes a laser-raman study of the CH-stretching and acoustical regions. Biochim. Biophys. Acta (BBA) Biomembr. 1976, 436, 307–318. [Google Scholar] [CrossRef]
- Alberts, B.; Bray, D.; Hopkin, K.; Johnson, A.D.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Essential Cell Biology, 3rd ed.; Garland Science: New York, NY, USA; London, UK, 2010. [Google Scholar]
- De Almeida, R.F.; Fedorov, A.; Prieto, M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys. J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef]
- Xu, X.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts) comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [PubMed]
- Baginski, M.; Resat, H.; Borowski, E. Comparative molecular dynamics simulations of amphotericin B-cholesterol/ergosterol membrane channels. Biochim. Biophys. Acta (BBA) Biomembr. 2002, 1567, 63–78. [Google Scholar] [CrossRef]
- Giddings, K.S.; Johnson, A.E.; Tweten, R.K. Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. USA 2003, 100, 11315–11320. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R.K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, S.C.; Angstmann, C.N.; Breit, S.N.; Curmi, P.M.; Brown, L.J. Transmembrane extension and oligomerization of the CLIC1 chloride intracellular channel protein upon membrane interaction. Biochemistry 2011, 50, 10887–10897. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.G.; Berry, T.; Holt, S.A.; Hossain, K.R.; Le Brun, A.P.; Carne, S.; Al Khamici, H.; Coster, H.; Valenzuela, S.M.; Cornell, B. Evidence of the key role of H3O+ in phospholipid membrane morphology. Langmuir 2016, 32, 10725–10734. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khamici, H.; Hossain, K.R.; Cornell, B.A.; Valenzuela, S.M. Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes. Membranes 2016, 6, 51. https://doi.org/10.3390/membranes6040051
Al Khamici H, Hossain KR, Cornell BA, Valenzuela SM. Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes. Membranes. 2016; 6(4):51. https://doi.org/10.3390/membranes6040051
Chicago/Turabian StyleAl Khamici, Heba, Khondker R. Hossain, Bruce A. Cornell, and Stella M. Valenzuela. 2016. "Investigating Sterol and Redox Regulation of the Ion Channel Activity of CLIC1 Using Tethered Bilayer Membranes" Membranes 6, no. 4: 51. https://doi.org/10.3390/membranes6040051





