High Temperature Stable Separator for Lithium Batteries Based on SiO2 and Hydroxypropyl Guar Gum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Membrane Characterization

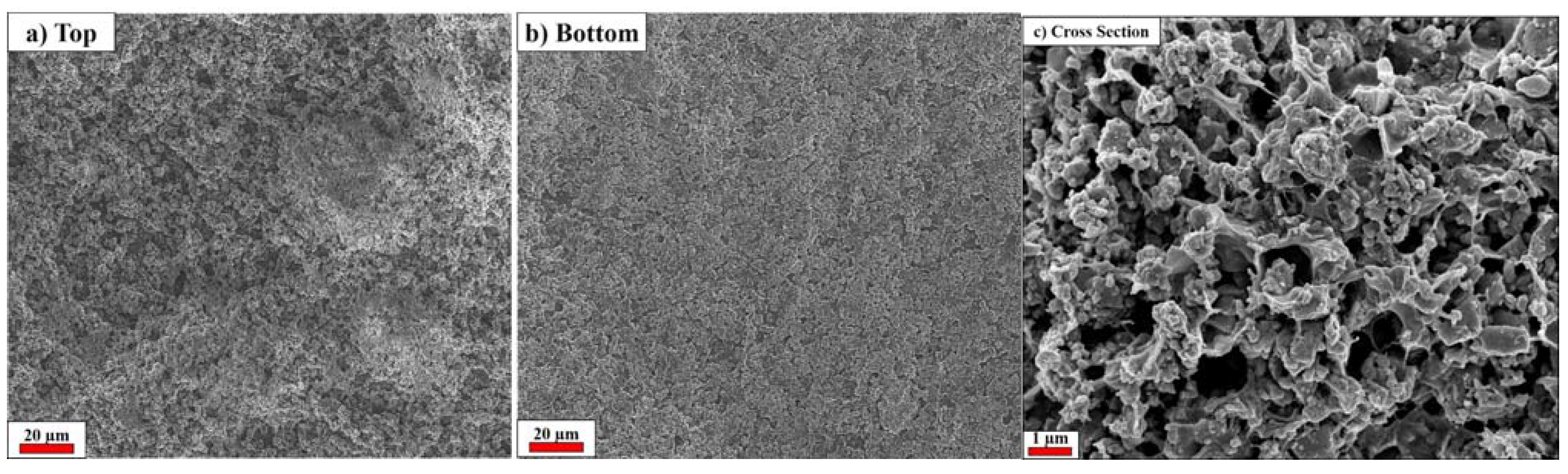
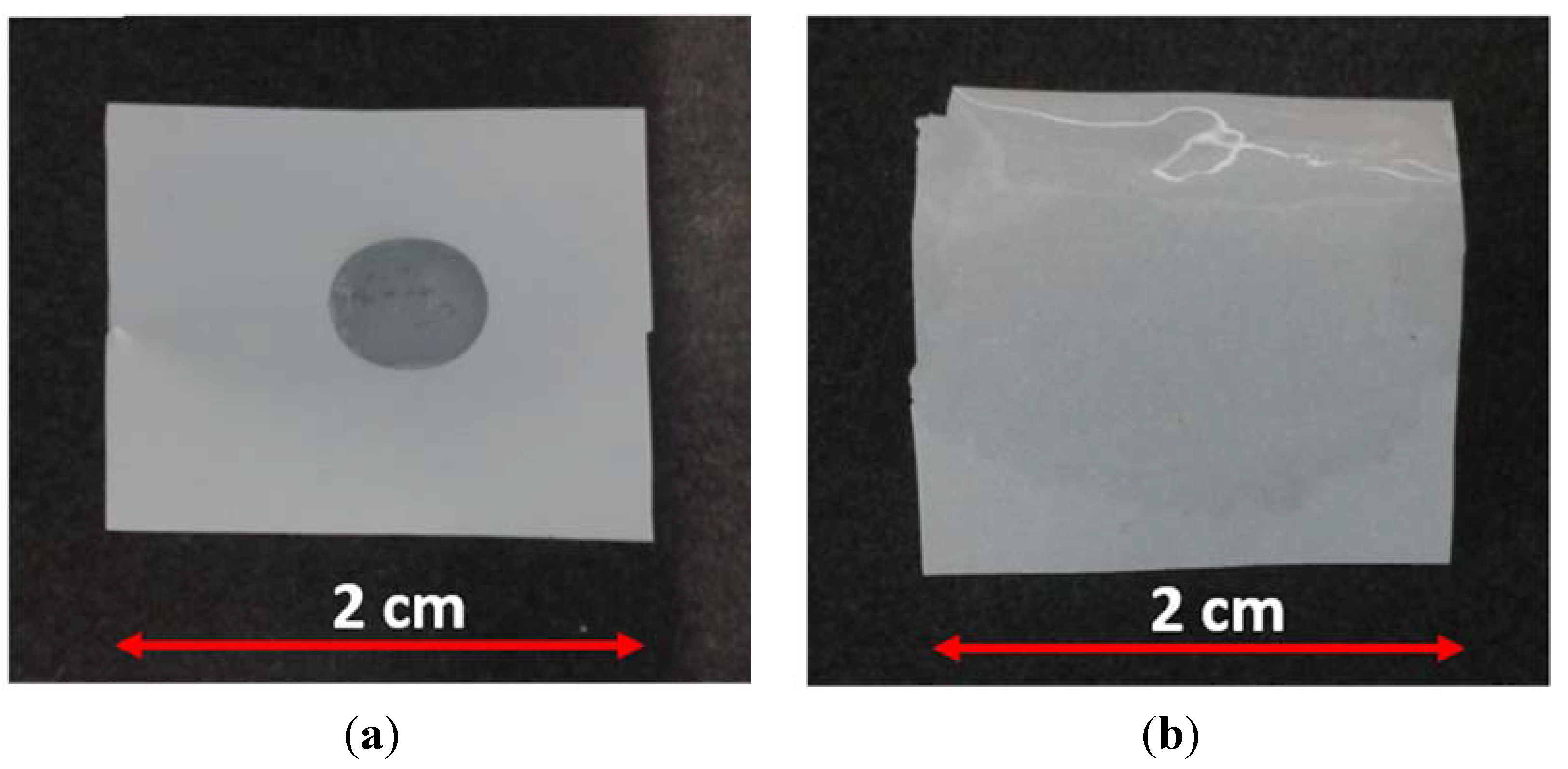
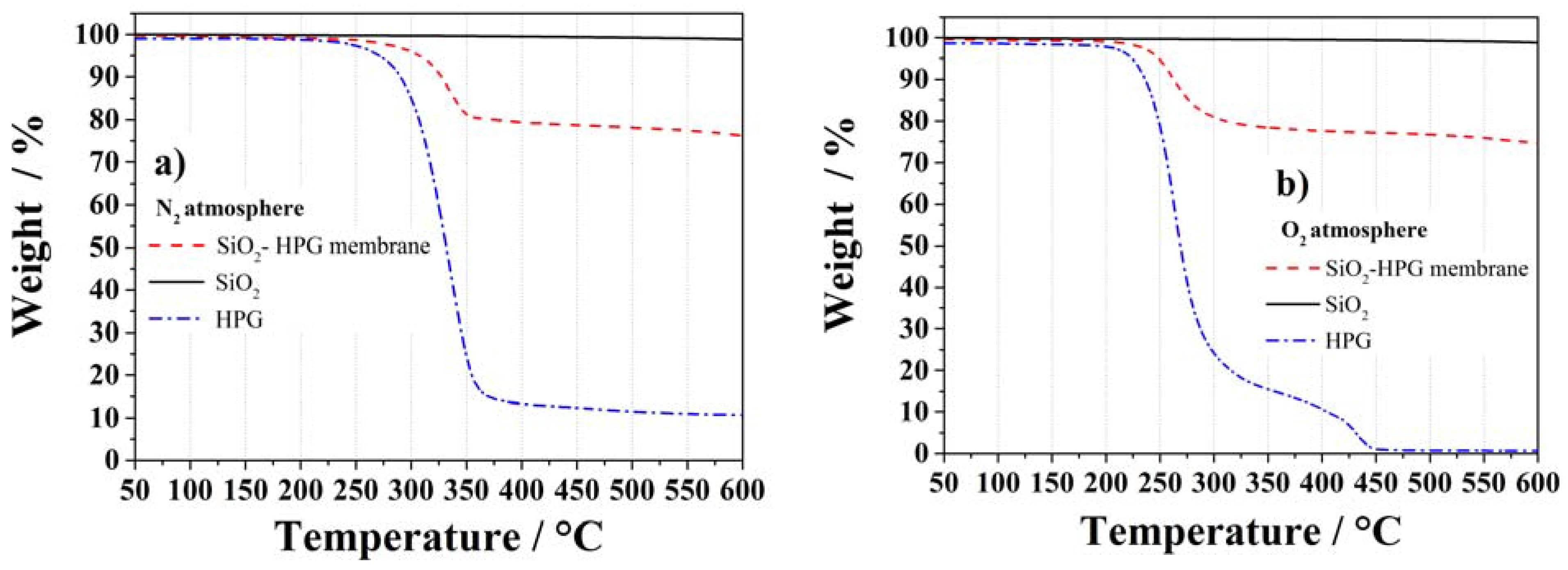
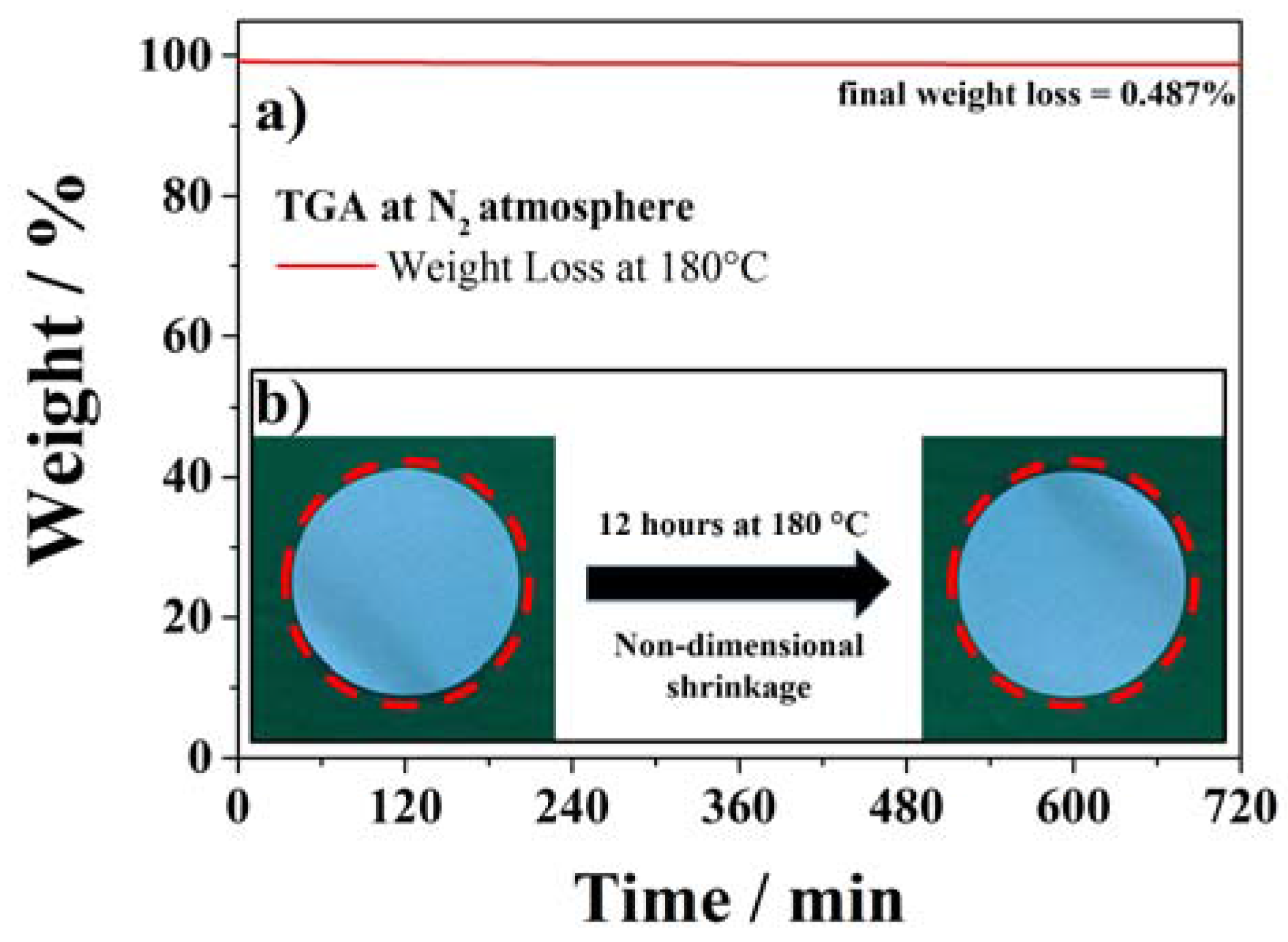
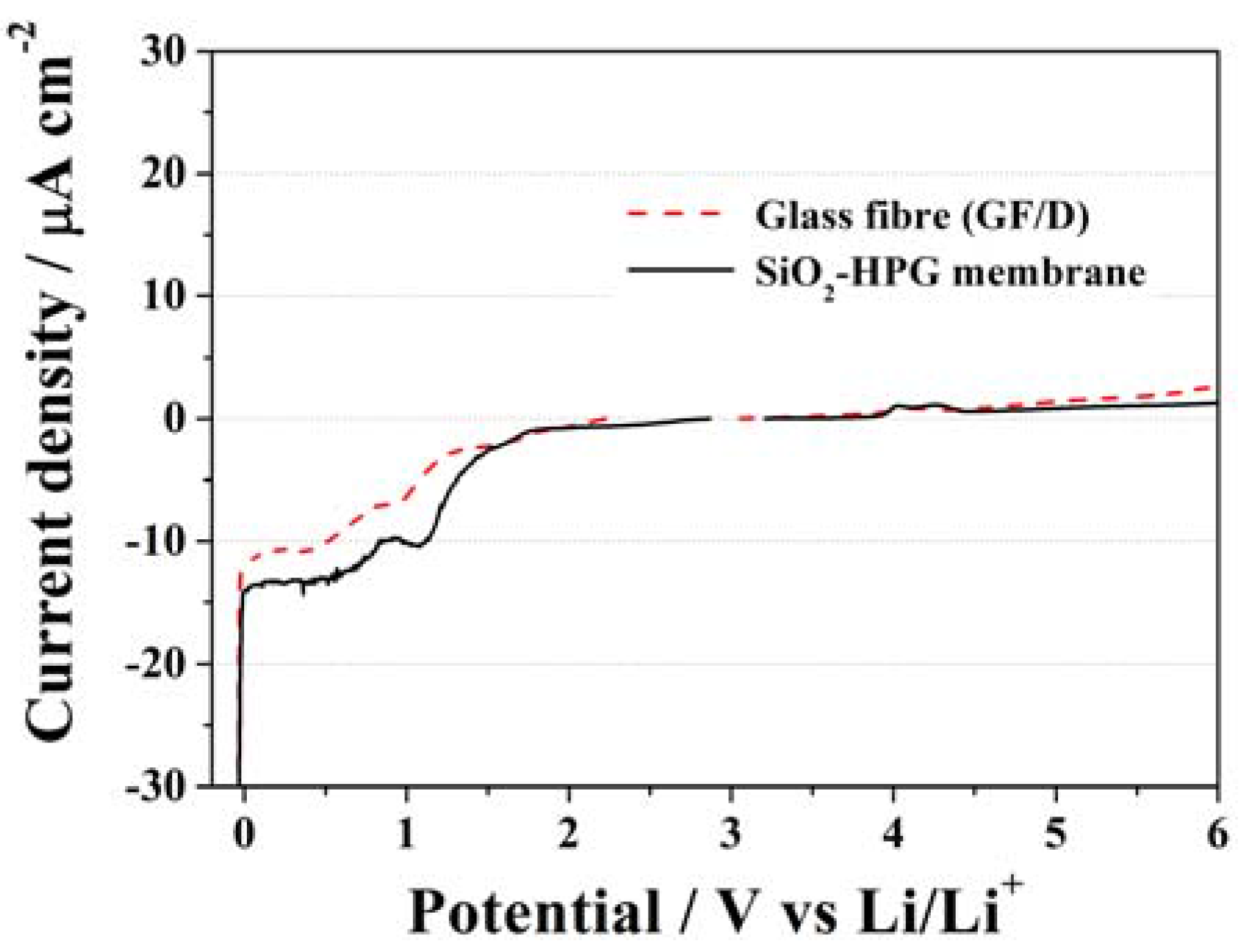
2.2. Electrochemical Tests


3. Experimental Section
3.1. Membrane Preparation
3.2. Membrane Characterization
3.3. Electrochemical Tests
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Prosini, P.P.; Villano, P.; Carewska, M. A novel intrinsically porous separator for self-standing lithium-ion batteries. Electrochim. Acta 2002, 48, 227–233. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Zhang, Z.M. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Yann Liaw, B.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Weber, C.J.; Geiger, S.; Falusi, S.; Roth, M. Material review of Li ion battery separators. AIP Conf. Proc. 2014, 66, 66–81. [Google Scholar]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Choi, E.S.; Yu, H.K.; Kim, J.H.; Wu, Q.; Chun, S.J.; Lee, S.Y.; Lee, S.Y. Colloidal silica nanoparticle-assisted structural control of cellulose nanofiber paper separators for lithium-ion batteries. J. Power Sources 2013, 242, 533–540. [Google Scholar] [CrossRef]
- Shin, W.K.; Kim, D.W. High performance ceramic-coated separators prepared with lithium ion-containing SiO2 particles for lithium-ion batteries. J. Power Sources 2013, 226, 54–60. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Cai, D.; Wang, H. Porous SiO2 as a separator to improve the electrochemical performance of spinel LiMn2O4 cathode. J. Membr. Sci. 2013, 449, 169–175. [Google Scholar] [CrossRef]
- Cho, J.-H.; Park, J.-H.; Lee, M.-H.; Song, H.-K.; Lee, S.-Y. A polymer electrolyte-skinned active material strategy toward high-voltage lithium ion batteries: A polyimide-coated LiNi0.5Mn1.5O4 spinel cathode material case. Energy Environ. Sci. 2012, 5, 7124–7131. [Google Scholar] [CrossRef]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Tu, J.; Yuan, Y.; Zhan, P.; Jiao, H.; Wang, X.; Zhu, H.; Jiao, S. Straightforward Approach toward SiO2 Nanospheres and Their Superior Lithium Storage Performance. J. Phys. Chem. C 2014, 118, 7357–7362. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kim, S.-J.; Oh, E.-S. Bio-Derivative Galactomannan Gum Binders for Li4Ti5O12 Negative Electrodes in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A2128–A2132. [Google Scholar] [CrossRef]
- Dufficy, M.K.; Khan, S.A.; Fedkiw, P.S. Galactomannan binding agents for silicon anodes in Li-ion batteries. J. Mater. Chem. A 2015, 3, 12023–12030. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A Robust Ion-Conductive Biopolymer as a Binder for Si Anodes of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Kuruba, R.; Kanchan, M.; Damodaran, K.; Jampani, P.H.; Gattu, B.; Patel, P.P.; Shanthi, P.M.; Damle, S.; Kumta, P.N. Guar gum: Structural and electrochemical characterization of natural polymer based binder for silicon e carbon composite rechargeable Li-ion battery anodes. J. Power Sources 2015, 298, 331–340. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Tubular array, dielectric, conductivity and electrochemical properties of biodegradable gel polymer electrolyte. Mater. Sci. Eng. B Solid -State Mater. Adv. Technol. 2014, 180, 12–19. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Miao, X.; Luo, J.; Jiang, B. Determination of the degree of substitution of hydroxypropyl guar gum at C-6 by Pyrolysis-Gas Chromatography spectrometry. Carbohydr. Polym. 2010, 82, 829–832. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, M.K. Guar Gum: Present Status and Applications. J. Pharm. Sci. Innov. 2013, 4, 24–28. [Google Scholar] [CrossRef]
- Hotza, D.; Greil, P. Review: Aqueous tape casting of ceramic powders. Mater. Sci. Eng. A 1995, 202, 206–217. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/Graphite Composite Electrodes for High-Capacity Anodes: Influence of Binder Chemistry on Cycling Stability. Electrochem. Solid -State Lett. 2008, 11, A76. [Google Scholar] [CrossRef]
- Huang, X.; Hitt, J. Lithium ion battery separators: Development and performance characterization of a composite membrane. J. Membr. Sci. 2013, 425–426, 163–168. [Google Scholar] [CrossRef]
- Nayak, B.R.; Singh, R.P. Development of graft copolymer flocculating agents based on hydroxypropyl guar gum and acrylamide. J. Appl. Polym. Sci. 2001, 81, 1776–1785. [Google Scholar] [CrossRef]
- Soppirnath, K.S.; Aminabhavi, T.M. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur. J. Pharm. Biopharm. 2002, 53, 87–98. [Google Scholar] [CrossRef]
- Beyler, C.L.; Hirschler, M.M. Chapter 7—Thermal Decomposition of Polymers. In SFPE Handbook of Fire Protection Engineering, Third Edition; National Fire Protection Association: Quincy, MA, USA, 2005; pp. 110–131. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Fan, J.; Fedkiw, P.S. Composite Electrolytes Prepared from Fumed Silica, Polyethylene Oxide Oligomers, and Lithium Salts. J. Electrochem. Soc. 1997, 144, 399–408. [Google Scholar] [CrossRef]
- Chun, S.-J.; Choi, E.-S.; Lee, E.-H.; Kim, J.H.; Lee, S.-Y.; Lee, S.-Y. Eco-friendly cellulose nanofiber paper-derived separator membranes featuring tunable nanoporous network channels for lithium-ion batteries. J. Mater. Chem. 2012, 22, 16618–16626. [Google Scholar] [CrossRef]
- Loeffler, N.; Von Zamory, J.; Laszczynski, N.; Doberdo, I.; Kim, G.T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder. J. Power Sources 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Zhu, X.P.; Mauger, A.; Qi, L.; Julien, C.M. Aging of LiNi1/3Mn1/3Co1/3O2 cathode material upon exposure to H2O. J. Power Sources 2011, 196, 5102–5108. [Google Scholar] [CrossRef]
- Moretti, A.; Kim, G.T.; Bresser, D.; Renger, K.; Paillard, E.; Marassi, R.; Winter, M.; Passerini, S. Investigation of different binding agents for nanocrystalline anatase TiO2 anodes and its application in a novel, green lithium-ion battery. J. Power Sources 2013, 221, 419–426. [Google Scholar] [CrossRef]
- Kim, G.-T.; Löffler, N.; Doberdo, I.; Laszczynski, N.; Bresser, D.; Passerini, S. Method for Producing an Electrode for a Lithium-Ion Battery. Patent No. WO 2015/062985 A1, 7 May 2015. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.V.; Loeffler, N.; Kim, G.-T.; Passerini, S. High Temperature Stable Separator for Lithium Batteries Based on SiO2 and Hydroxypropyl Guar Gum. Membranes 2015, 5, 632-645. https://doi.org/10.3390/membranes5040632
Carvalho DV, Loeffler N, Kim G-T, Passerini S. High Temperature Stable Separator for Lithium Batteries Based on SiO2 and Hydroxypropyl Guar Gum. Membranes. 2015; 5(4):632-645. https://doi.org/10.3390/membranes5040632
Chicago/Turabian StyleCarvalho, Diogo Vieira, Nicholas Loeffler, Guk-Tae Kim, and Stefano Passerini. 2015. "High Temperature Stable Separator for Lithium Batteries Based on SiO2 and Hydroxypropyl Guar Gum" Membranes 5, no. 4: 632-645. https://doi.org/10.3390/membranes5040632





