A New Emulsion Liquid Membrane Based on a Palm Oil for the Extraction of Heavy Metals
Abstract
:1. Introduction
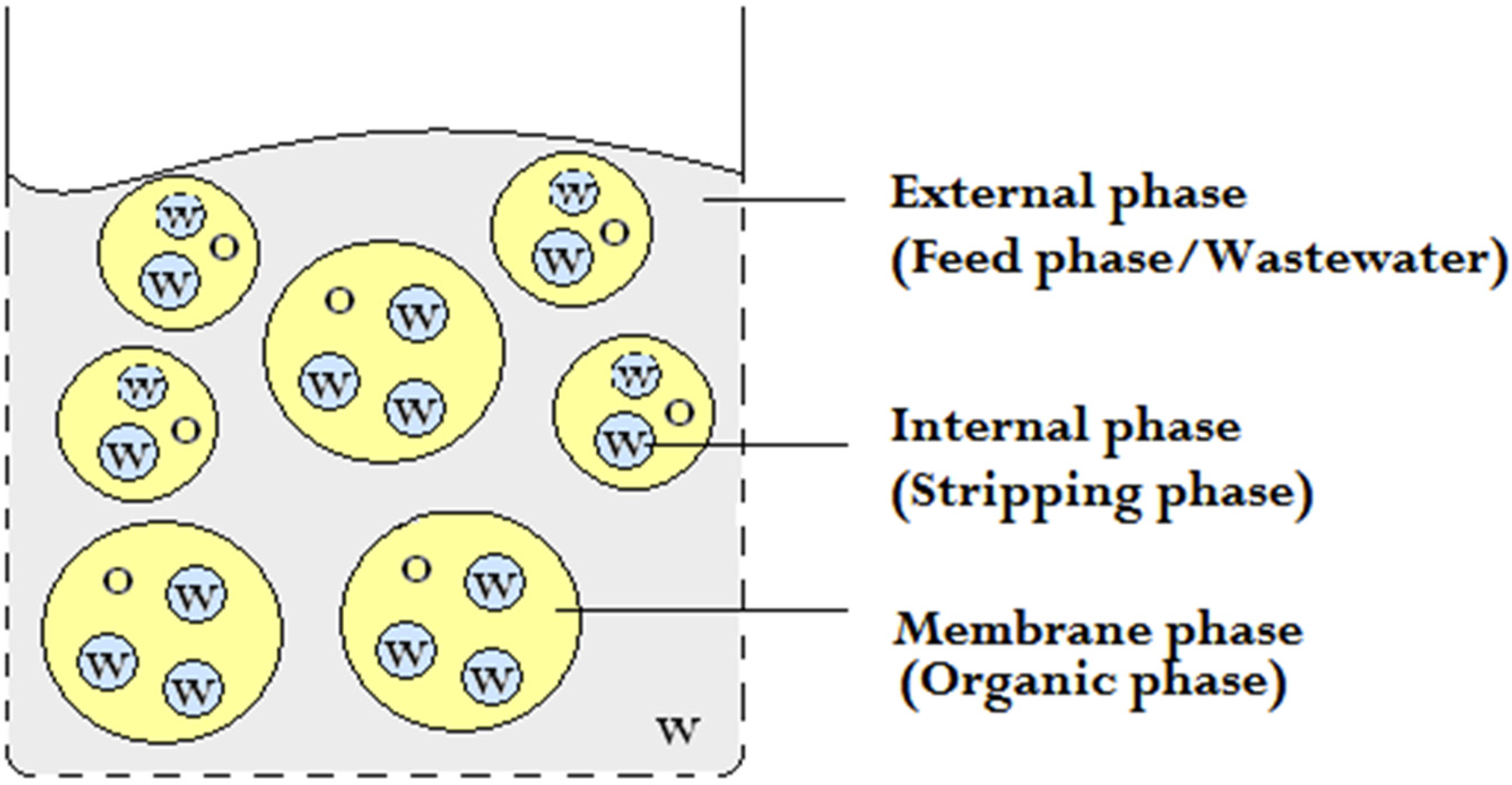
2. Experimental
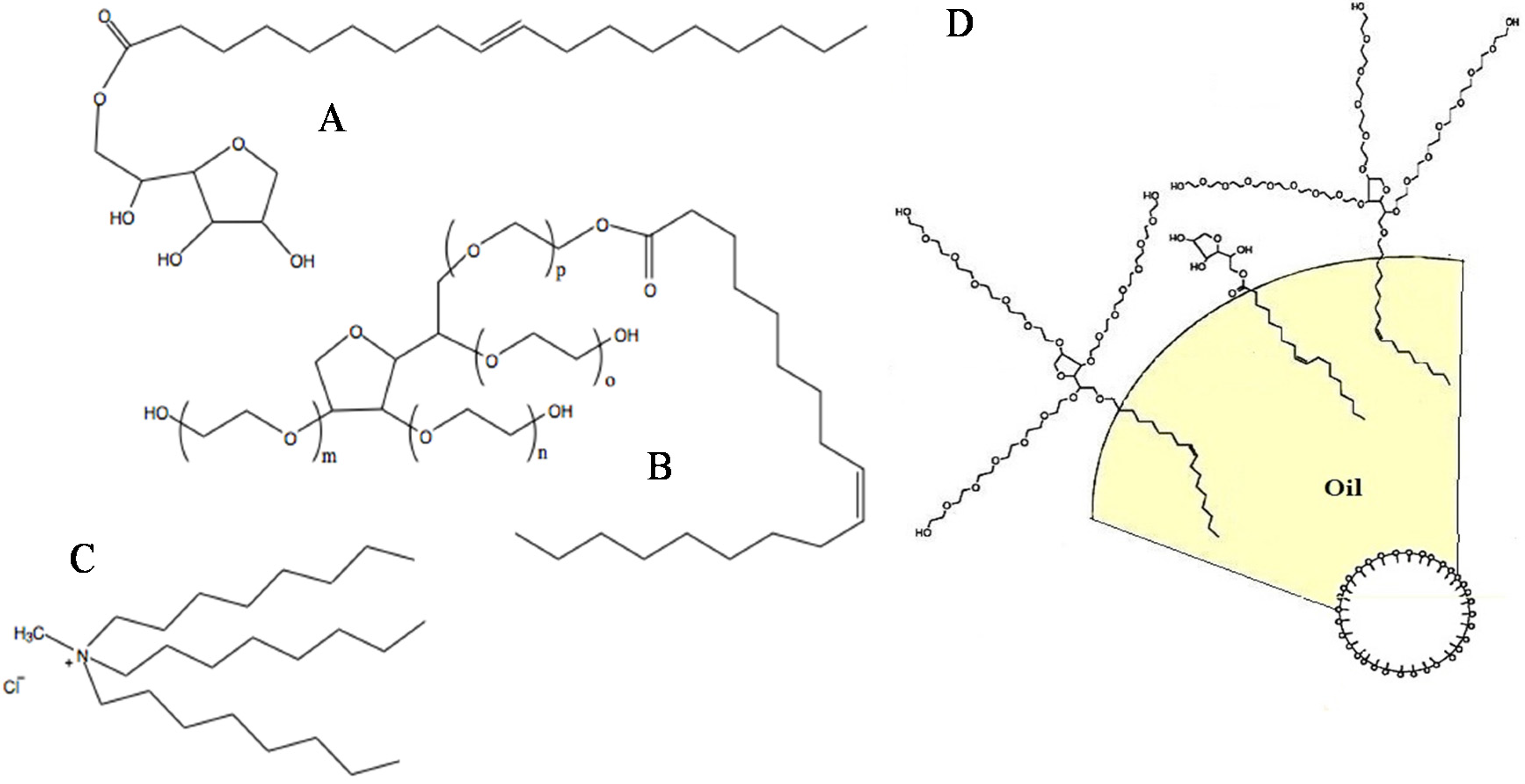
2.1. Materials
2.2. Analytical Measurements
2.3. Preparation and Evaluation of the ELM System

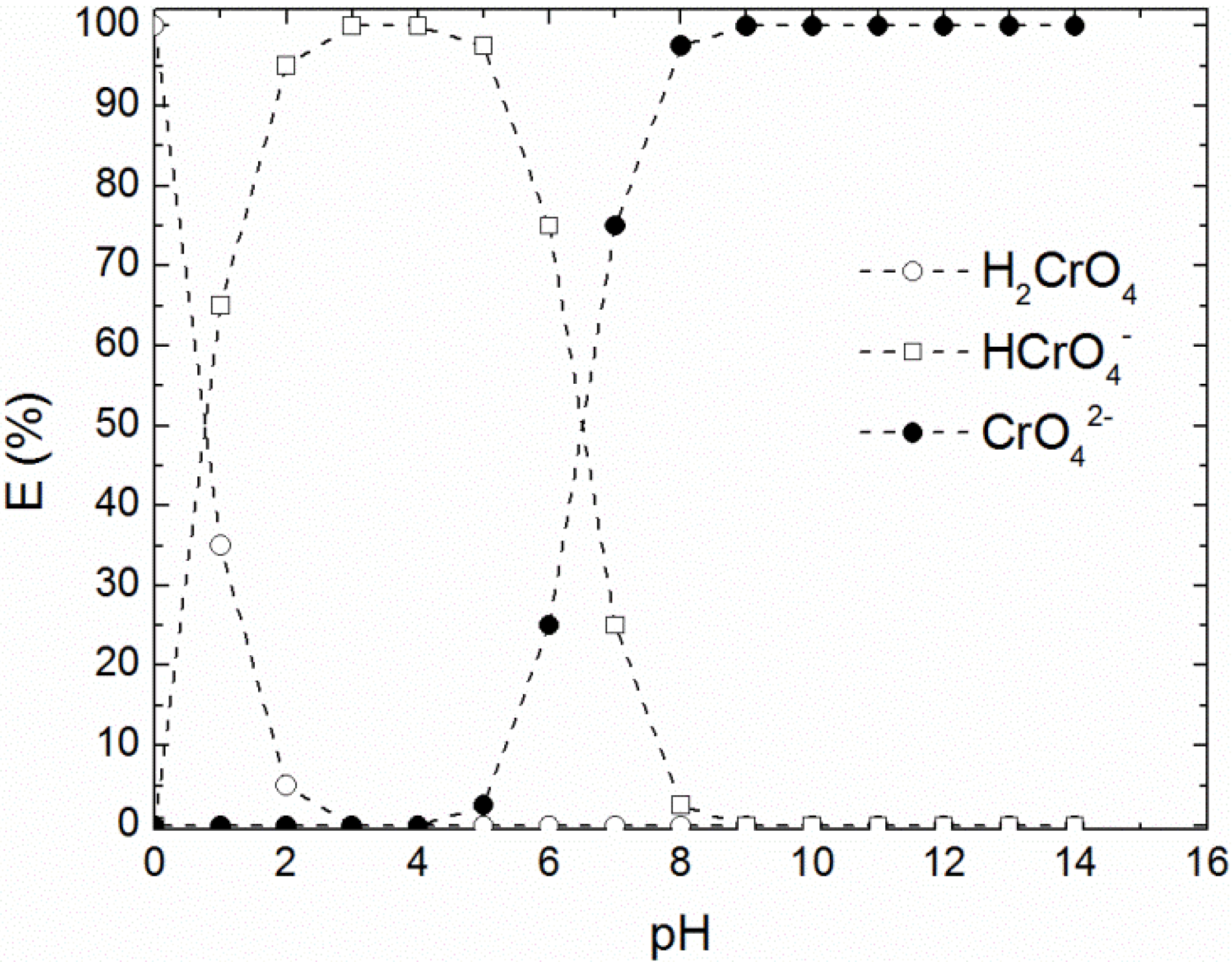
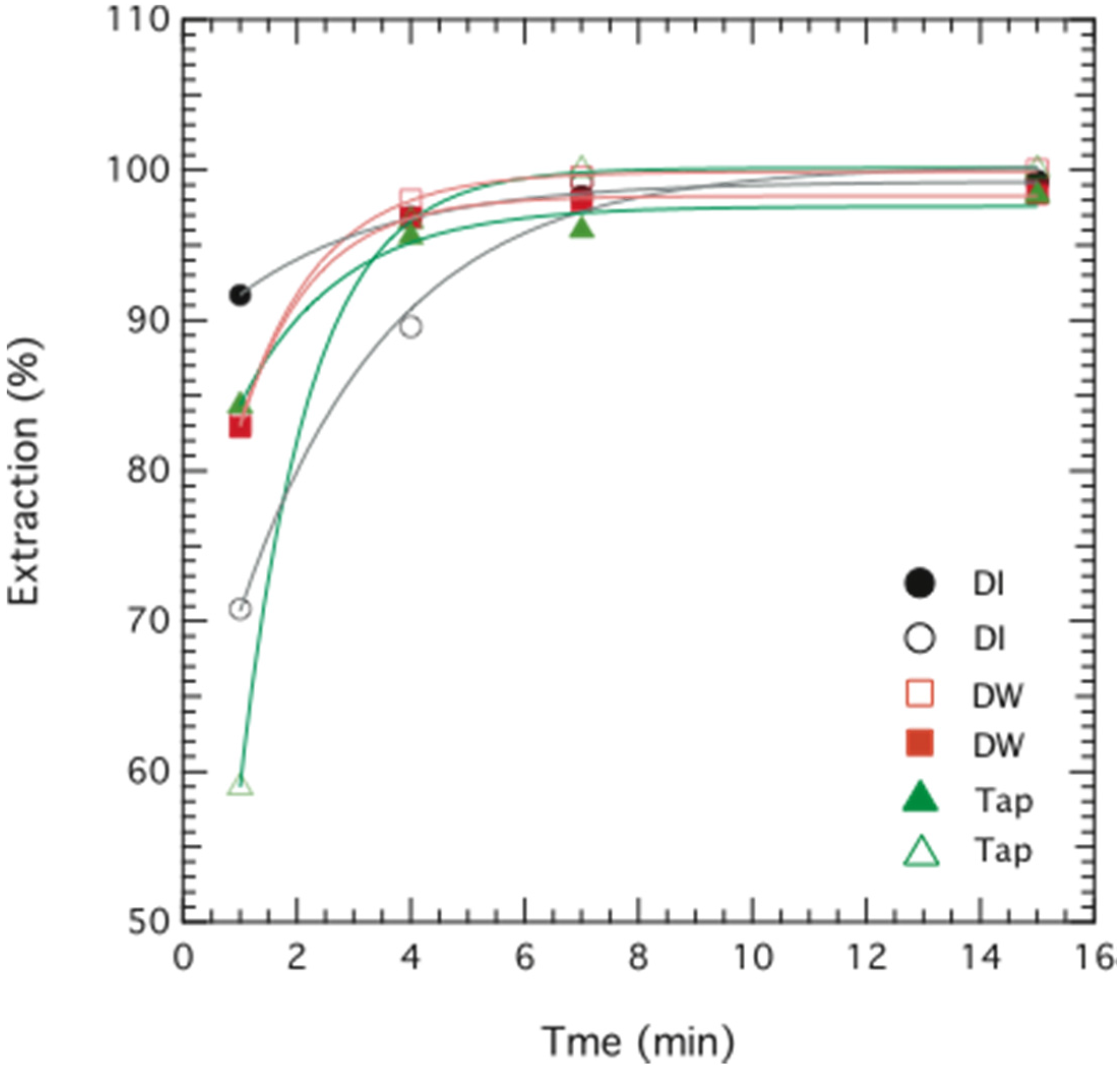
3. Results and Discussion
3.1. Chromium Extraction Using the Novel ELM System
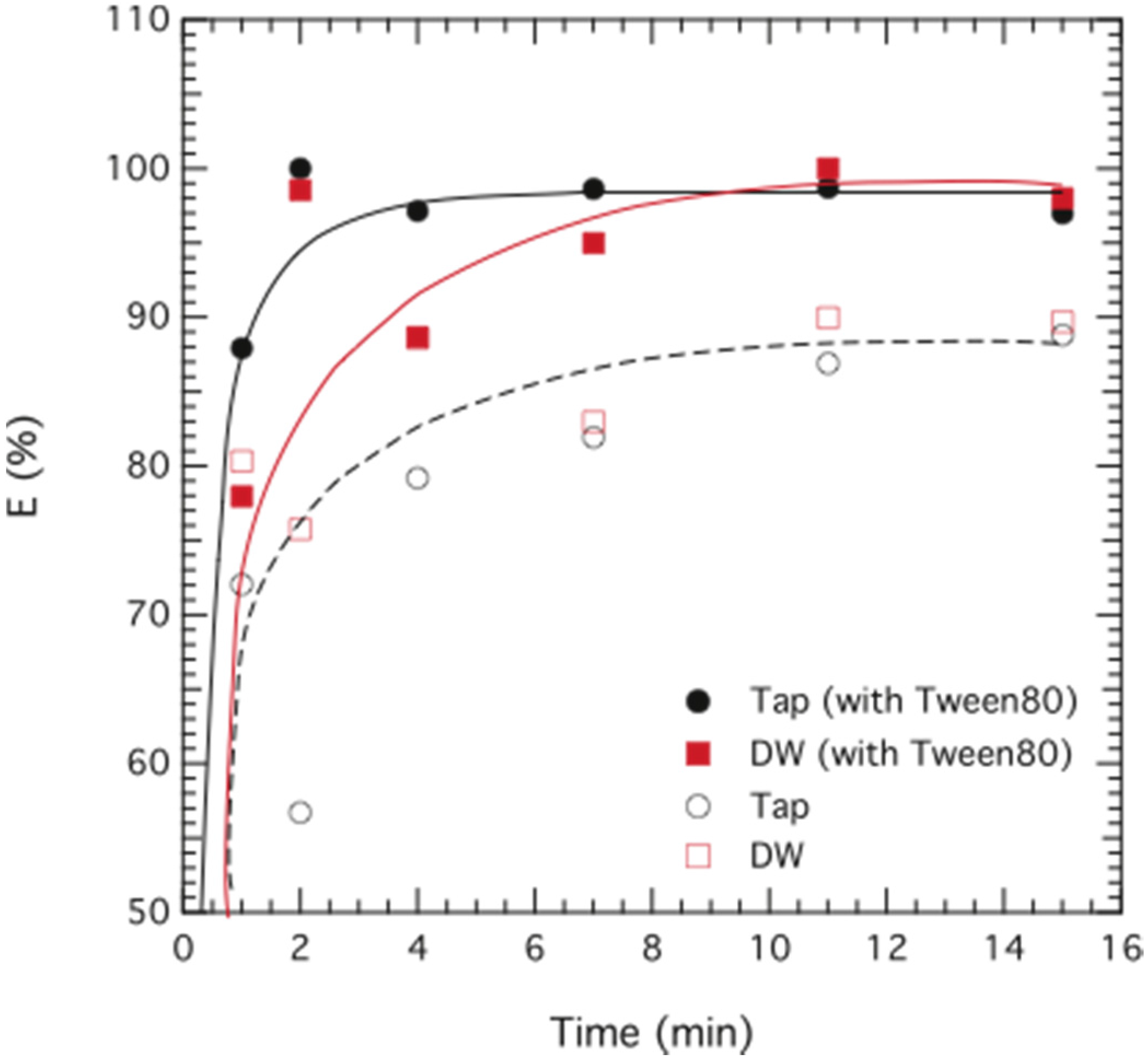
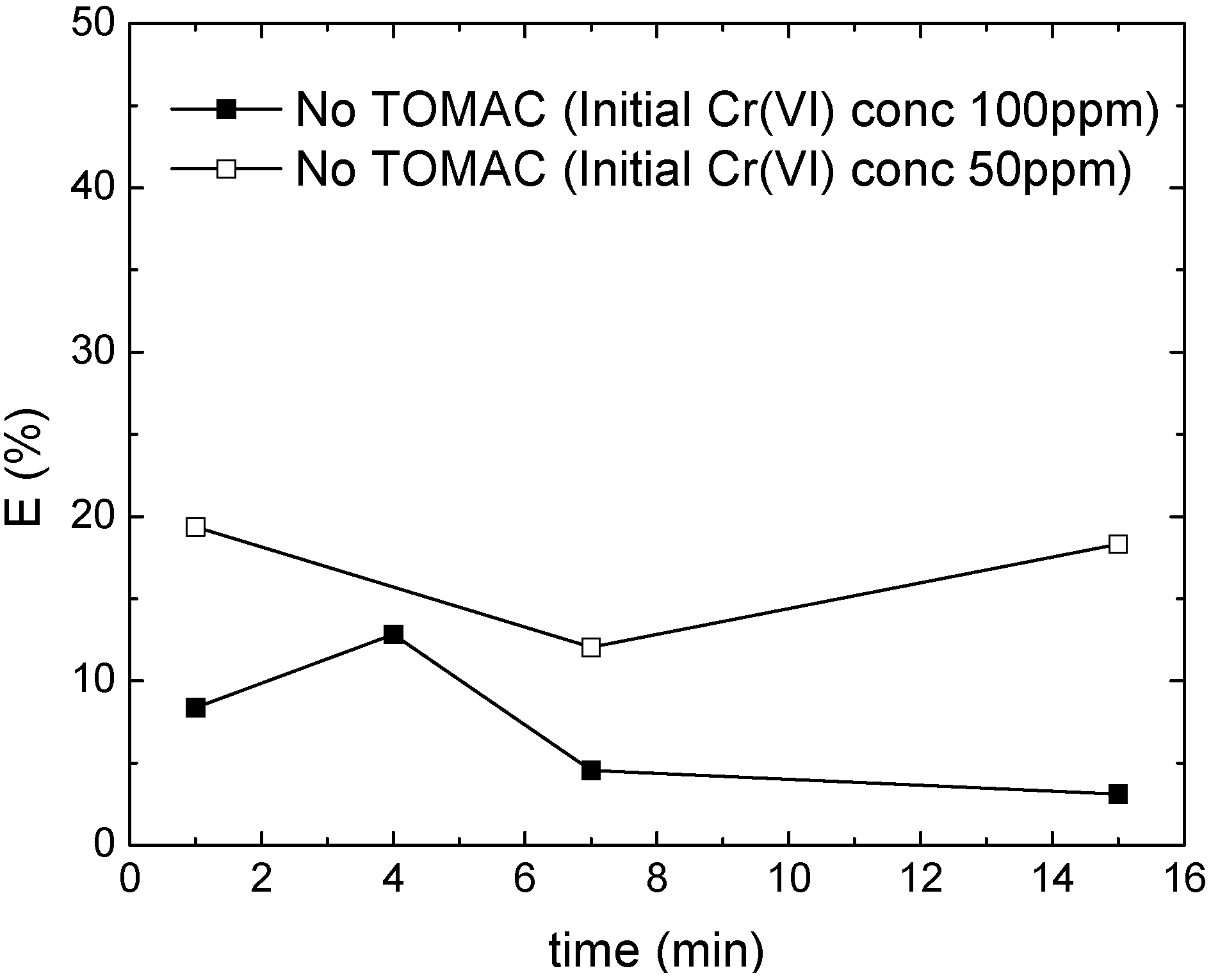

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, S.K.; Petrusevski, B.; Amy, G. Chromium removal from water: A review. J. Water Supply Res. Technol. AQUA 2008, 57, 541–553. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, Incorporating First and Second Addenda to Third Edition, 3rd ed.; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Jilska, P.M.; Geoff, S.W. Use of Emulsion Liquid Membrane Systems in Chemical and Biotechnological Separations. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; Taylor & Francis Group, LLC: Abingdon, UK, 2009. [Google Scholar]
- Li, N.N. Separating Hydrocarbons with Liquid Membranes. U.S. Patent 3,410,794, 12 November 1968. [Google Scholar]
- Goyal, R.K.; Jayakumar, N.S.; Hashim, M.A. Chromium removal by emulsion liquid membrane using [BMIM]+[NTf2]− as stabilizer and TOMAC as extractant. Desalination 2011, 278, 50–56. [Google Scholar] [CrossRef]
- García, M.G.; Acosta, A.O.; Marchese, J. Emulsion liquid membrane pertraction of Cr(III) from aqueous solutions using PC-88A as carrier. Desalination 2013, 318, 88–96. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Zhu, S. Flow and mass transfer characteristics in a falling-film extractor using hollow fiber as packing. Chem. Eng. J. 2005, 108, 161–168. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, W.; Liu, Y.; Dai, Y.; Cui, C. New liquid membrane technology for simultaneous extraction and stripping of copper(II) from wastewater. Chem. Eng. Sci. 2007, 62, 6090–6101. [Google Scholar] [CrossRef]
- Goyal, R.K.; Jayakumar, N.S.; Hasshim, M.A. A comparative study of experimental optimization and response surface optimization of Cr removal by emulsion ionic liquid membrane. J. Hazard. Mater. 2011, 195, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bart, H.-J.; Stevens, G.W. Reactive Solvent Extraction. In Ion Exchange and Solvent Extraction: A Series of Advances; Taylor & Francis Group: Boca Raton, FL, USA, 2004; Volume 17, pp. 38–83. [Google Scholar]
- Bin Mat, H.; Othman, N.B.; Hie, C.K. Selective Emulsion Liquid Membrane Extraction of Silver from Liquid Photographic Waste Indutries; University of Malaysia: Kuala Lumpur, Malaysia, 2006. [Google Scholar]
- Zhao, L.; Fei, D.; Dang, Y.; Zhou, X.; Xiao, J. Studies on the extraction of chromium(III) by emulsion liquid membrane. J. Hazard. Mater. 2010, 178, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kumbasar, R.A. Selective extraction of chromium (VI) from multicomponent acidic solutions by emulsion liquid membranes using tributhylphosphate as carrier. J. Hazard. Mater. 2010, 178, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.; Palanivelu, K. Recovery of phenol from aqueous solution by supported liquid membrane using vegetable oils as liquid membrane. J. Hazard. Mater. 2006, B131, 146–152. [Google Scholar] [CrossRef]
- Ehtash, M.; Fournier-Salaün, M.-C.; Dimitrov, K.; Salaün, P.; Saboni, A. Phenol removal from aqueous media by pertraction using vegetable oil as a liquid membrane. Chem. Eng. J. 2014, 250, 42–47. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Extraction of Cu(II) from aqueous solutions by vegetable oil-based organic solvents. J. Hazard. Mater. 2010, 181, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, G.; Teng, T.T. Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B. Desalination 2009, 249, 1062–1066. [Google Scholar] [CrossRef]
- Muthuraman, G.; Palanivelu, K. Transport of textile dye in vegetable oils based supported liquid membrane. Dye. Pigment. 2006, 70, 99–104. [Google Scholar] [CrossRef]
- Basiron, Y. Palm Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley&Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 333–400. [Google Scholar]
- Kotas, J.; Stasicka, Z. Chromium Occurrence in the Environment and Methods of its Speciation; Deparment of Inorganic Chemistry, Jagiellonian University: Kraków, Poland, 2000. [Google Scholar]
- Draxler, J.; Marr, R. Emulsion liquid membranes part I: Phenomenon and industrial application. Chem. Eng. Process. Process Intensif. 1986, 20, 319–329. [Google Scholar] [CrossRef]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2002. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Björkegren, S.; Karimi, R.F.; Martinelli, A.; Jayakumar, N.S.; Hashim, M.A. A New Emulsion Liquid Membrane Based on a Palm Oil for the Extraction of Heavy Metals. Membranes 2015, 5, 168-179. https://doi.org/10.3390/membranes5020168
Björkegren S, Karimi RF, Martinelli A, Jayakumar NS, Hashim MA. A New Emulsion Liquid Membrane Based on a Palm Oil for the Extraction of Heavy Metals. Membranes. 2015; 5(2):168-179. https://doi.org/10.3390/membranes5020168
Chicago/Turabian StyleBjörkegren, Sanna, Rose Fassihi Karimi, Anna Martinelli, Natesan Subramanian Jayakumar, and Mohd Ali Hashim. 2015. "A New Emulsion Liquid Membrane Based on a Palm Oil for the Extraction of Heavy Metals" Membranes 5, no. 2: 168-179. https://doi.org/10.3390/membranes5020168





