Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process
Abstract
:1. Introduction
2. Results and Discussion
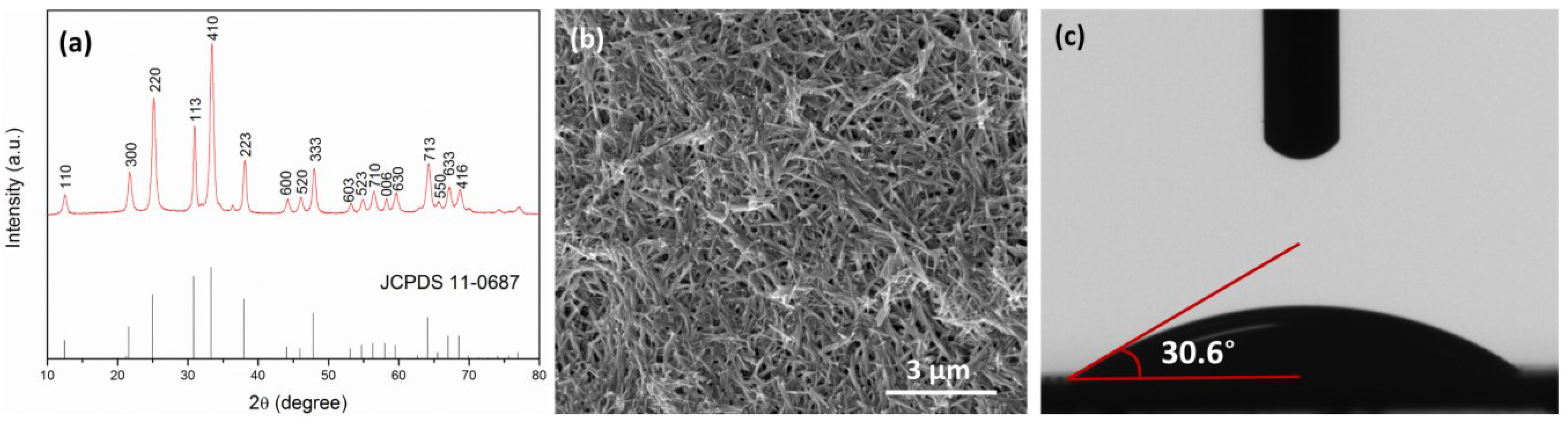
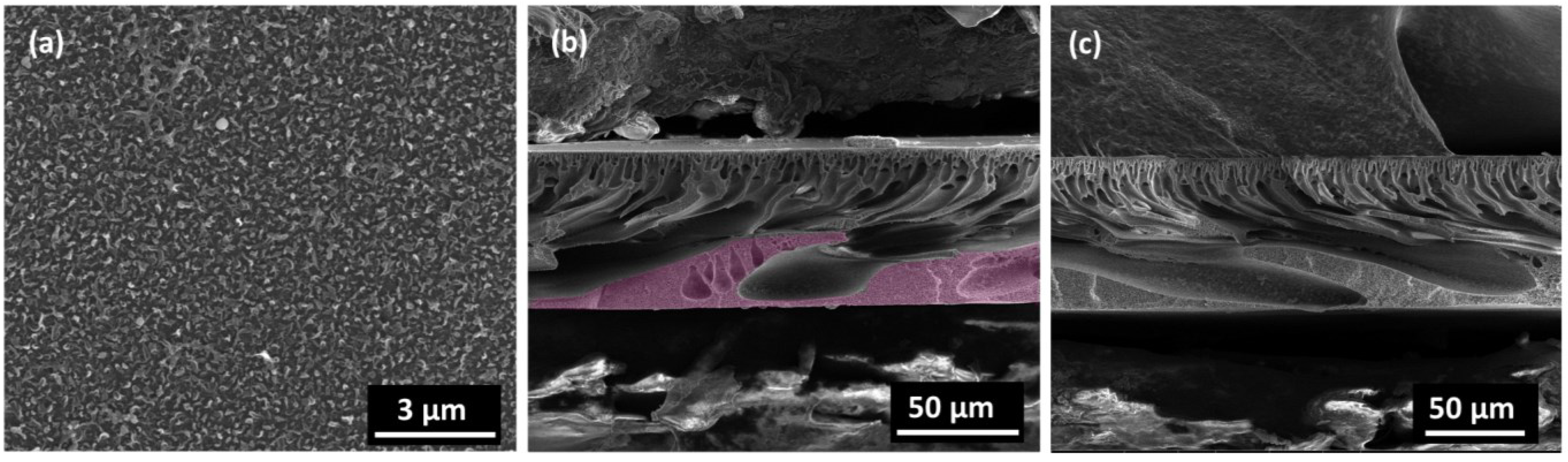
2.1. Characterization of Nanowire, Substrates and Membranes
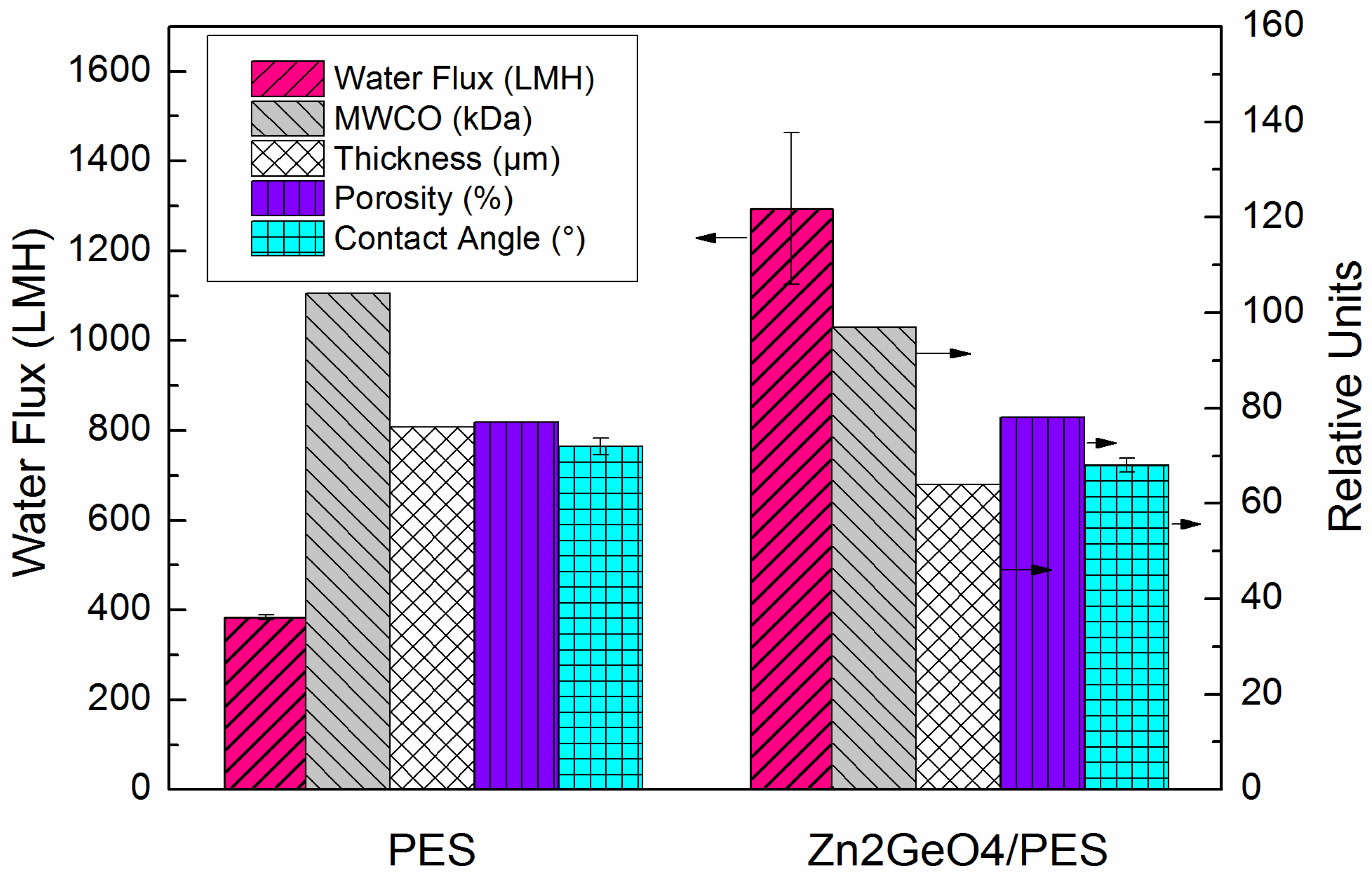
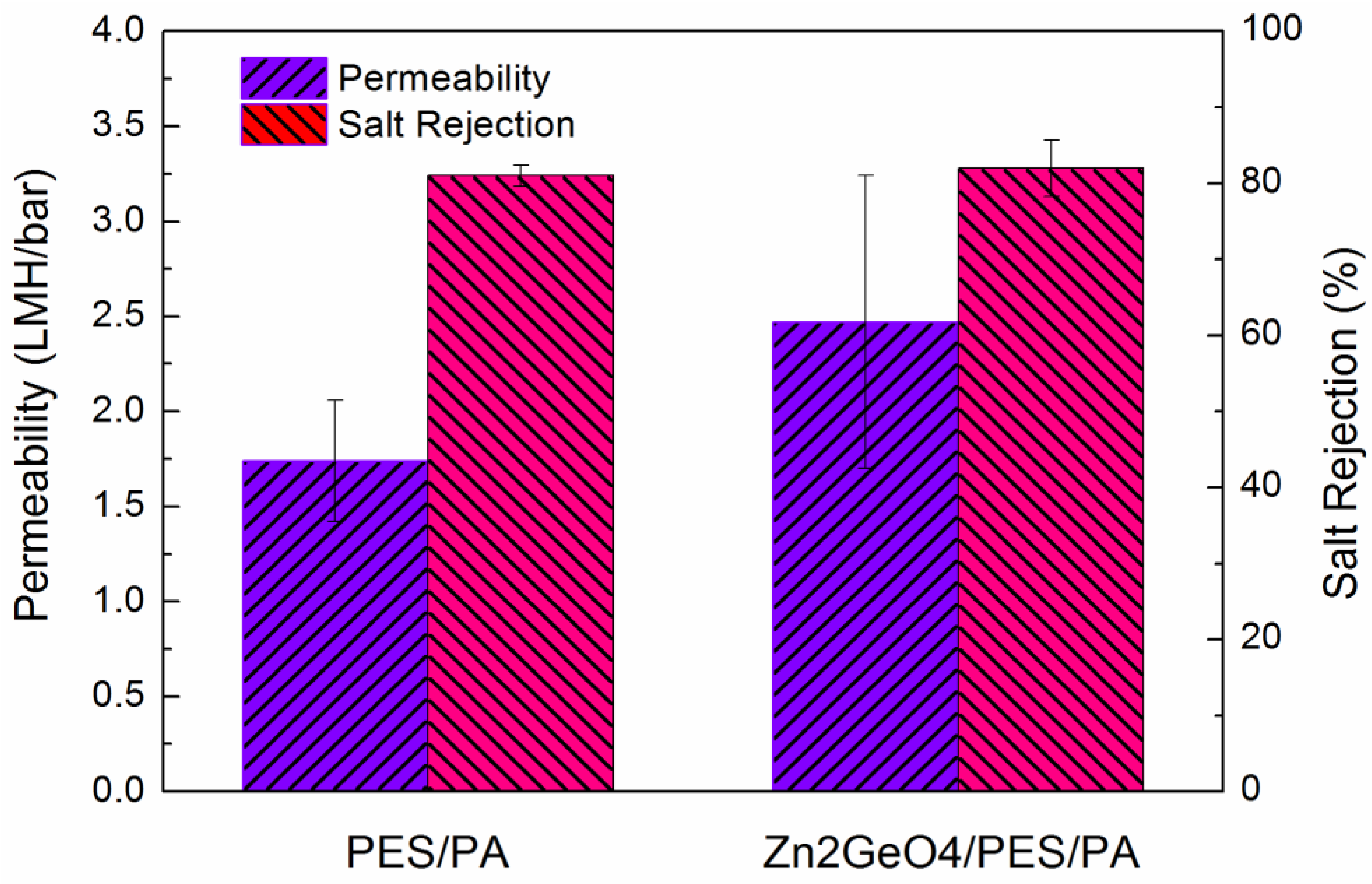
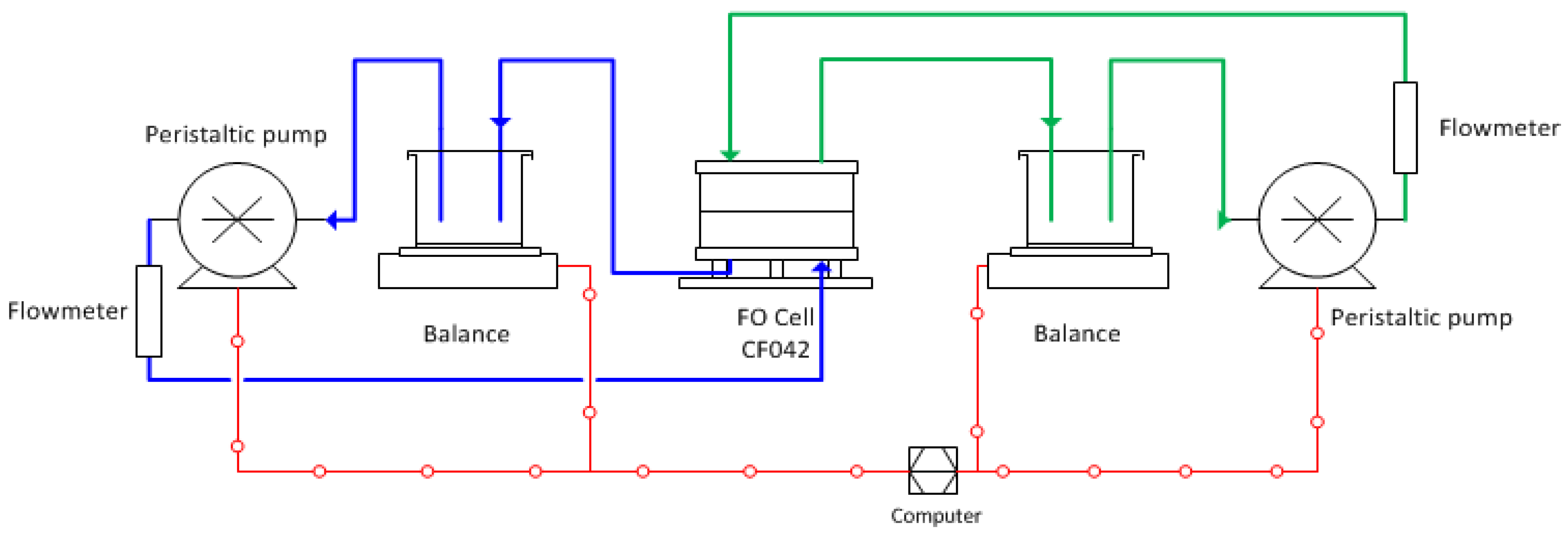
2.2. FO Membrane Performance
| Sample | Water permeability (A; LMH/bar) | NaCl Rejection (%) | Salt permeability (B; LMH) | B/A (bar) | Ref. |
|---|---|---|---|---|---|
| PESa | 1.74 ± 0.32 | 81.0 | 6.3 | 3.62 | current work |
| Zn2GeO4/PESa | 2.47 ± 0.77 | 82.0 | 8.4 | 3.40 | current work |
| CTA-HWb | 1.19 ± 0.19 | 78.5 | 0.09 | 0.08 | [7] |
| CTA-Wb | 0.33 ± 0.04 | 81.9 | 0.01 | 0.03 | [7] |
| CTA-NWb | 0.46 ± 0.07 | 92.4 | 0.01 | 0.02 | [7] |
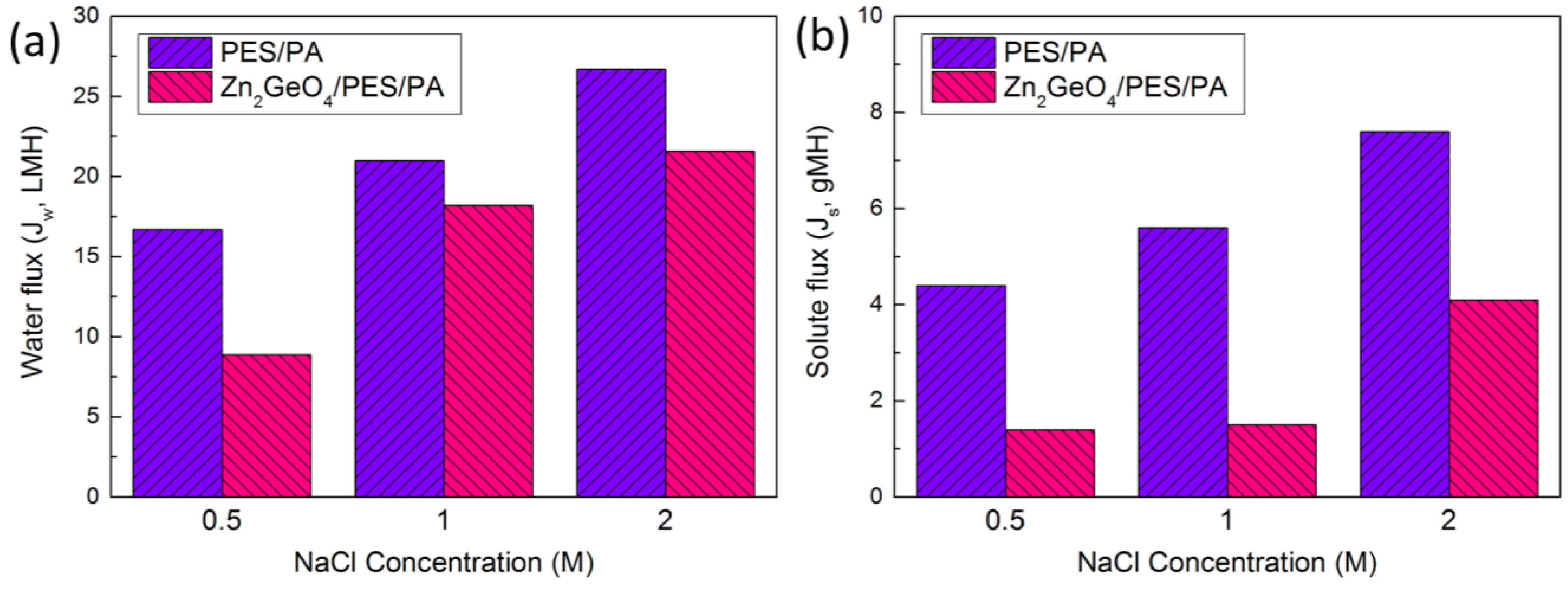
| Sample/ DS Concentration (M) | 0.5 | 1.0 | 2.0 | Km (105 s/m) | S (µm) | τ |
|---|---|---|---|---|---|---|
| Control | 3.8 | 3.8 | 3.5 | 2.2 | 352 | 3.56 |
| Zn2GeO4/PES/PA | 6.4 | 12.1 | 5.3 | 3.4 | 540 | 6.58 |
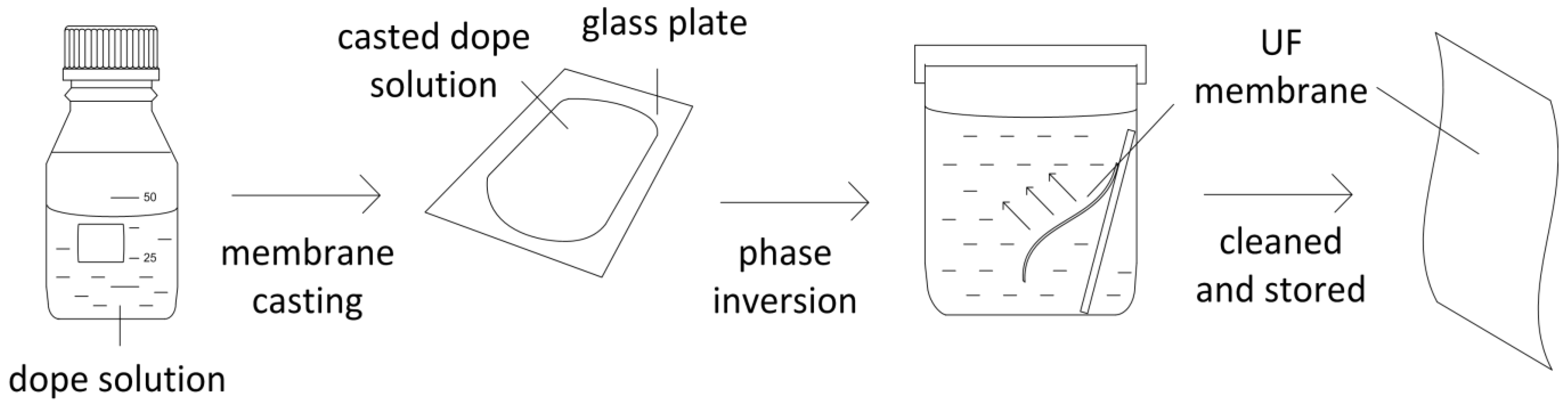
3. Experimental Section
| Sample | PES (wt %) | Zn2GeO4 (wt %) | NMP (wt %) |
|---|---|---|---|
| Control | 15.00 | 0.00 | 85.00 |
| Zn2GeO4/PES | 14.99 | 0.05 | 84.96 |
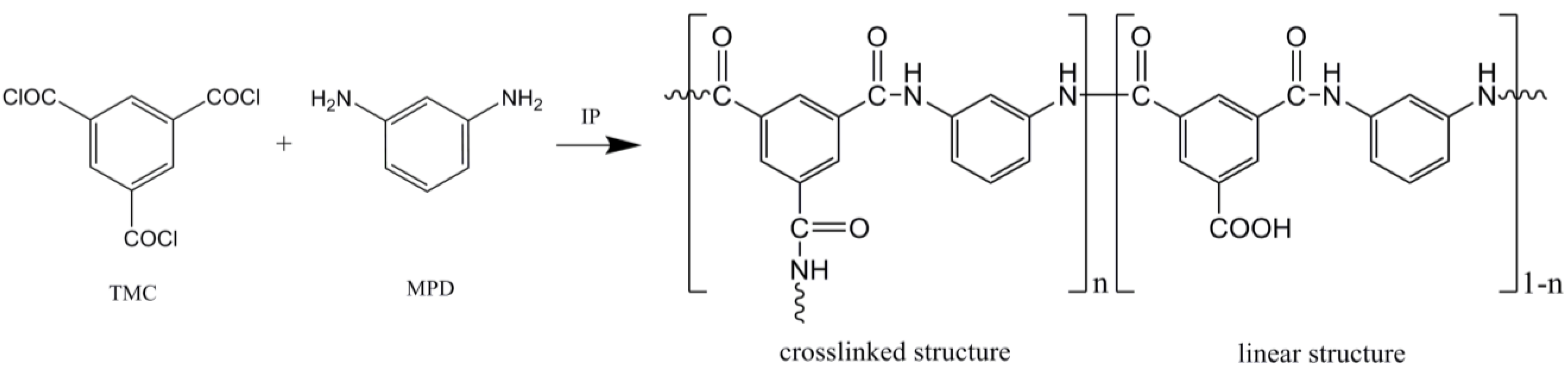
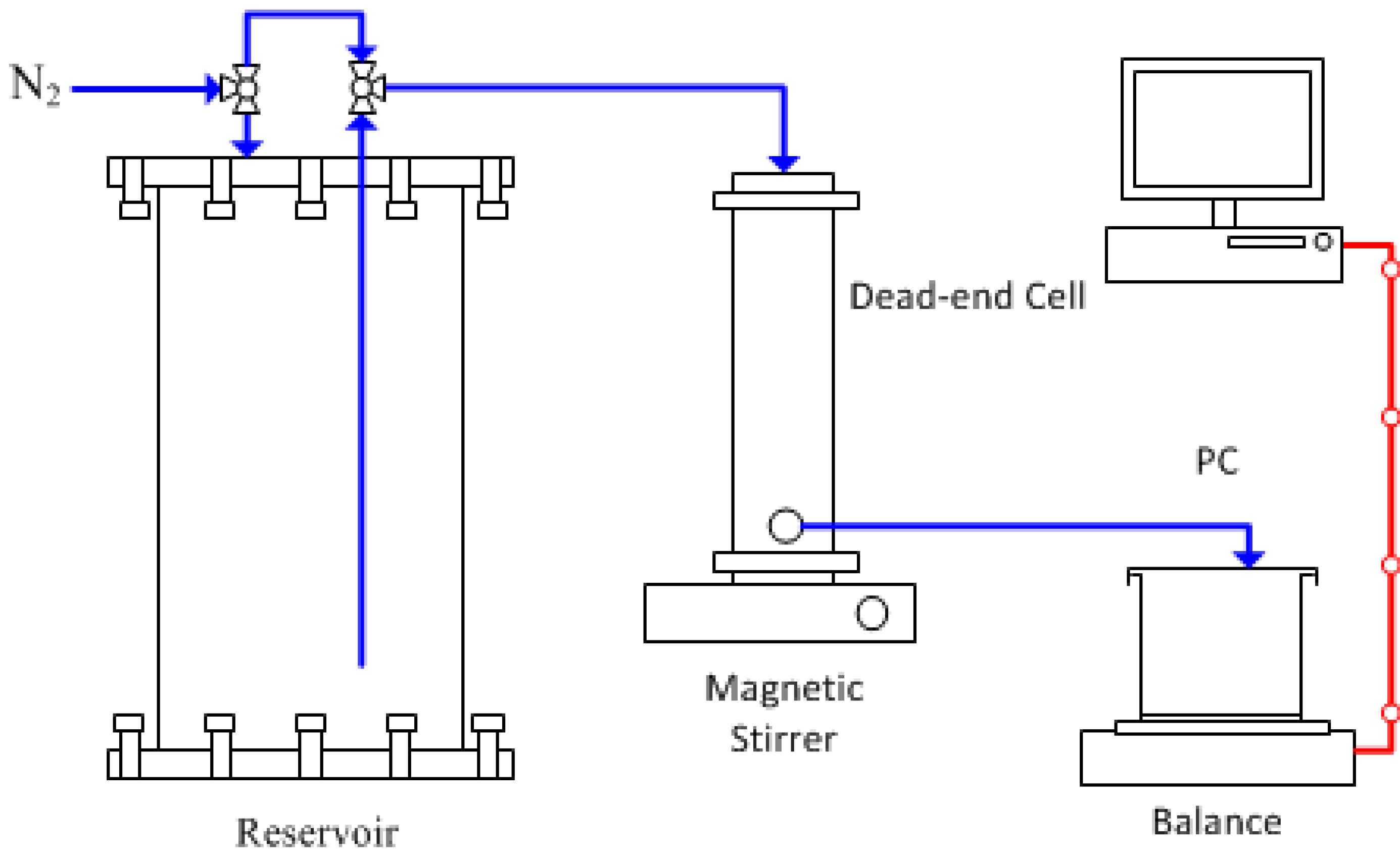
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chekli, L.; Phuntsho, S.; Shon, H.K.; Vigneswaran, S.; Kandasamy, J.; Chanan, A. A review of draw solutes in forward osmosis process and their use in modern applications. Desalination and Water Treat. 2012, 43, 167–184. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.E.; Dennett, K.E.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The role of physical and chemical parameters on forward osmosis membrane fouling during algae separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, L.; Nie, S.; Yan, T.; Tang, X.; Pan, W. A novel gastric-resident osmotic pump tablet: In vitro and in vivo evaluation. Int. J. Pharm. 2010, 383, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a loeb-sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High performance thin-film composite forward osmosis membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.-S.; Weber, M.; Maletzko, C.; Warzelhan, V. A sulfonated polyphenylenesulfone (sppsu) as the supporting substrate in thin film composite (tfc) membranes with enhanced performance for forward osmosis (fo). Chem. Eng. J. 2013, 220, 15–23. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.Y.; Chung, T.S.; Chen, H.; Jean, Y.C.; Amy, G. Well-constructed cellulose acetate membranes for forward osmosis: Minimized internal concentration polarization with an ultra-thin selective layer. J. Membr. Sci. 2010, 360, 522–535. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S. High performance thin-film composite forward osmosis hollow fiber membranes with macrovoid-free and highly porous structure for sustainable water production. Environ. Sci. Technol. 2012, 46, 7358–7365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-X.; Vanneste, J.; Poelmans, L.; Sotto, A.; Wang, X.-L.; Van der Bruggen, B. Effect of the manufacturing conditions on the structure and performance of thin-film composite membranes. J. Appl. Polym. Sci. 2012, 125, 3755–3769. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Saljoughi, E.; Ghasemipour, Z.; Hosseini, S.A. Preparation and characterization of modified polysulfone membranes with high hydrophilic property using variation in coagulation bath temperature and addition of surfactant. Polym. Eng. Sci. 2012, 52, 2196–2205. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Widjojo, N.; Chung, T.-S. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Sotto, A.; Rashed, A.; Zhang, R.X.; Martínez, A.; Braken, L.; Luis, P.; Van der Bruggen, B. Improved membrane structures for seawater desalination by studying the influence of sublayers. Desalination 2012, 287, 317–325. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.; Zhao, Y.; Gao, Y.; Tang, C.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Ge, Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Ismail, A.F. Synthesis of thin film nanocomposite forward osmosis membrane with enhancement in water flux without sacrificing salt rejection. Desalination 2013, 330, 90–99. [Google Scholar] [CrossRef]
- Dumée, L.; Lee, J.; Sears, K.; Tardy, B.; Duke, M.; Gray, S. Fabrication of thin film composite poly(amide)-carbon-nanotube supported membranes for enhanced performance in osmotically driven desalination systems. J. Membr. Sci. 2013, 427, 422–430. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6,6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Wang, X.; Yeh, T.-M.; Wang, Z.; Yang, R.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 2014, 55, 1358–1366. [Google Scholar] [CrossRef]
- Hoover, L.A.; Schiffman, J.D.; Elimelech, M. Nanofibers in thin-film composite membrane support layers: Enabling expanded application of forward and pressure retarded osmosis. Desalination 2013, 308, 73–81. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (pvdf) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Kim, H.-S.; Lee, K.-J.; Kim, H. Low internal concentration polarization in forward osmosis membranes with hydrophilic crosslinked pva nanofibers as porous support layer. Desalination 2014, 336, 24–31. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Double-blade casting technique for optimizing substrate membrane in thin-film composite forward osmosis membrane fabrication. J. Membr. Sci. 2014, 469, 112–126. [Google Scholar] [CrossRef]
- Wang, K.Y.; Ong, R.C.; Chung, T.-S. Double-skinned forward osmosis membranes for reducing internal concentration polarization within the porous sublayer. Ind. Eng. Chem. Res. 2010, 49, 4824–4831. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, K.Y.; Chung, T.-S. Dual-layer hollow fibers with enhanced flux as novel forward osmosis membranes for water production. Environ. Sci. Technol. 2009, 43, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Q.; Lin, X.; Liu, J.Z.; Wang, H. Hydrophilic nanowire modified polymer ultrafiltration membranes with high water flux. Acs Appl. Mater. Inter. 2014, 6, 19161–19167. [Google Scholar] [CrossRef]
- Kulkarni, A.; Mukherjee, D.; Gill, W.N. Flux enhancement by hydrophilization of thin film composite reverse osmosis membranes. J. Membr. Sci. 1996, 114, 39–50. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, S.S. Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane. J. Membr. Sci. 2006, 286, 193–201. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. 2012, 423–424, 362–370. [Google Scholar] [CrossRef]
- Low, Z.-X.; Razmjou, A.; Wang, K.; Gray, S.; Duke, M.; Wang, H. Effect of addition of two-dimensional zif-l nanoflakes on the properties of polyethersulfone ultrafiltration membrane. J. Membr. Sci. 2014, 460, 9–17. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, Z.-X.; Liu, Q.; Shamsaei, E.; Zhang, X.; Wang, H. Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process. Membranes 2015, 5, 136-149. https://doi.org/10.3390/membranes5010136
Low Z-X, Liu Q, Shamsaei E, Zhang X, Wang H. Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process. Membranes. 2015; 5(1):136-149. https://doi.org/10.3390/membranes5010136
Chicago/Turabian StyleLow, Ze-Xian, Qi Liu, Ezzatollah Shamsaei, Xiwang Zhang, and Huanting Wang. 2015. "Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process" Membranes 5, no. 1: 136-149. https://doi.org/10.3390/membranes5010136







