Membrane Bioprocesses for Pharmaceutical Micropollutant Removal from Waters
Abstract
:1. Introduction
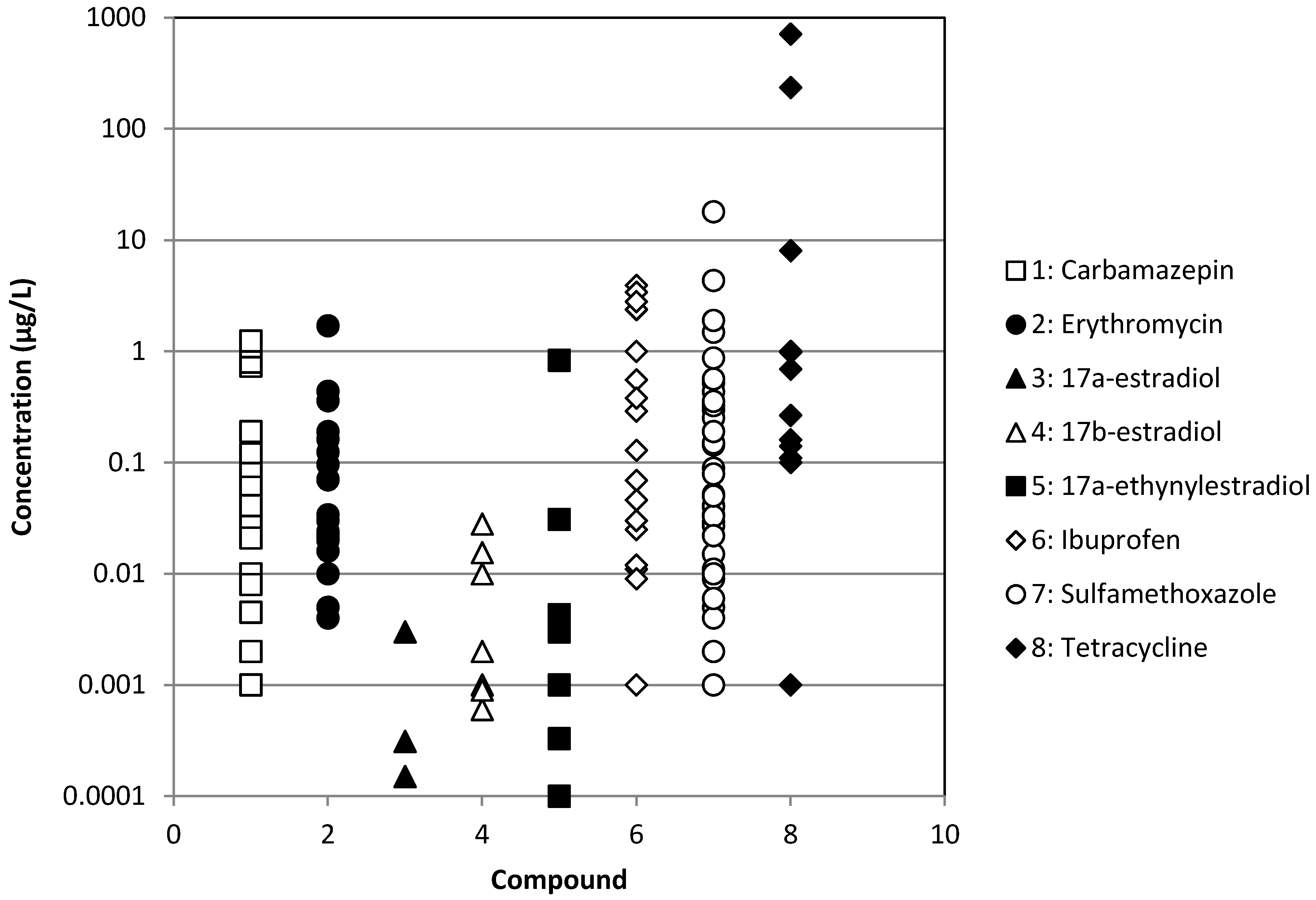

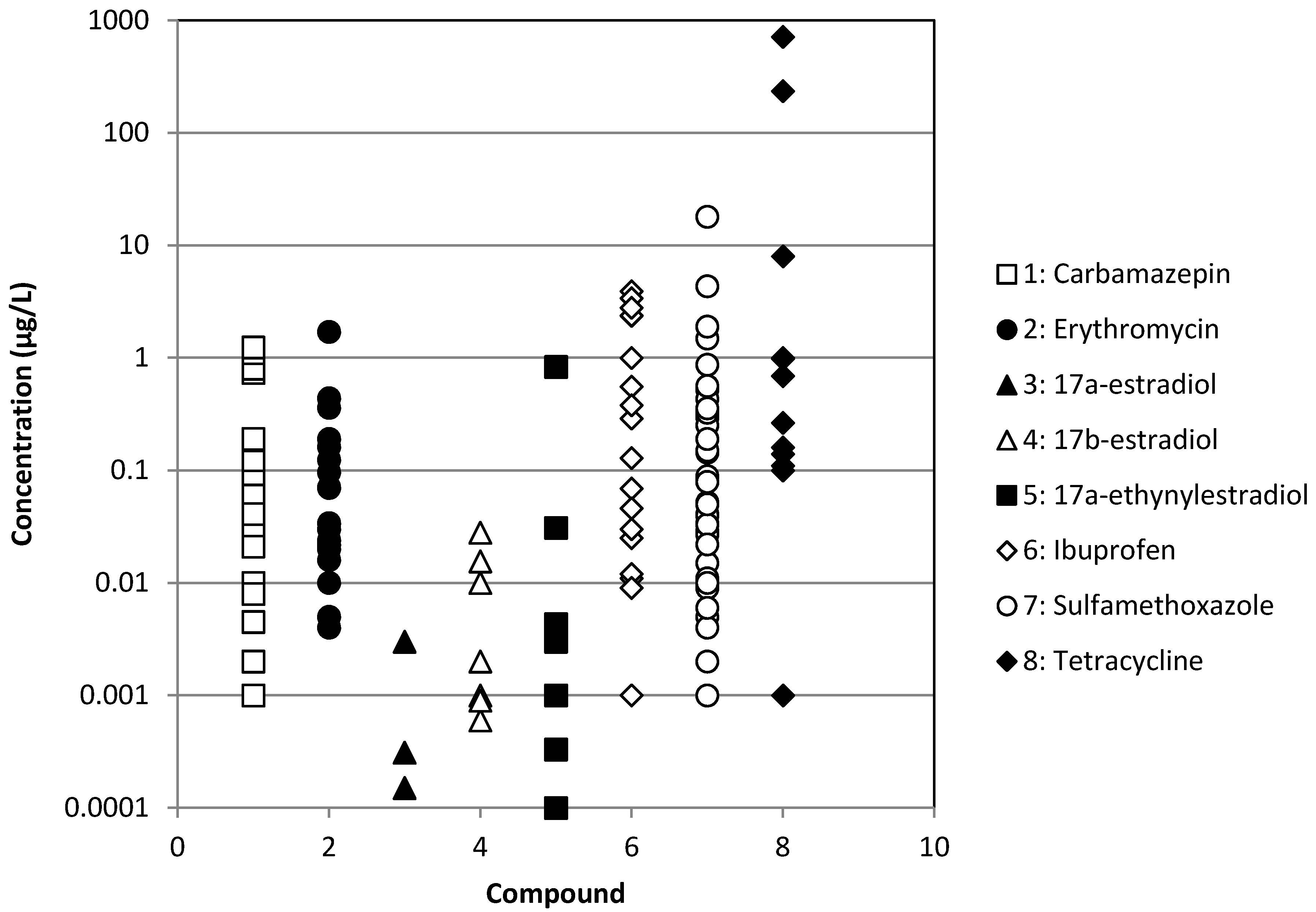
| Chemical | WWTP Effluents | Surface waters | ||
|---|---|---|---|---|
| Municipal | Hospital | Industrial | ||
| Ibuprofen | [3,8,12,15,26,27,28,29,30,31,32,33,34,35,36] | [33,34,36] | [37] | [3,6,8,28,30,38,39,40,41,42] |
| Erythromycin | [3,7,15,29,32,34,40,43,44,45,46] | [34] | [37] | [3,6,7,39,46,47,48,49] |
| Sulfamethoxazole | [2,3,7,8,15,26,27,28,29,30,32,33,34,40,43,46,50,51,52,53,54,55,56] | [33,34,50,55] | [37] | [2,3,6,7,8,28,30,38,39,40,41,46,47,48,49,50,57,58] |
| Tetracycline | [3,7,15,29,44,51,52,53,56,59,60,61] | [33,34,59,61] | [9,37,59] | [3,6,7,9,38,39,48,59,61] |
| Carbamazepine | [2,3,8,15,26,27,28,29,32,34,40,62] | [34] | [37] | [2,3,8,28,38,39,40,41,42,62] |
| 17α-estradiol | [3,8,29,36,63] | [36] | [8,63] | |
| 17α-ethinylestradiol | [3,8,29,32,63,64] | [37] | [8,39,63,64] | |
| 17β-estradiol | [3,8,29,32,45,63,64,65] | [33] | [37] | [8,63,64,65] |
2. Biological Treatments
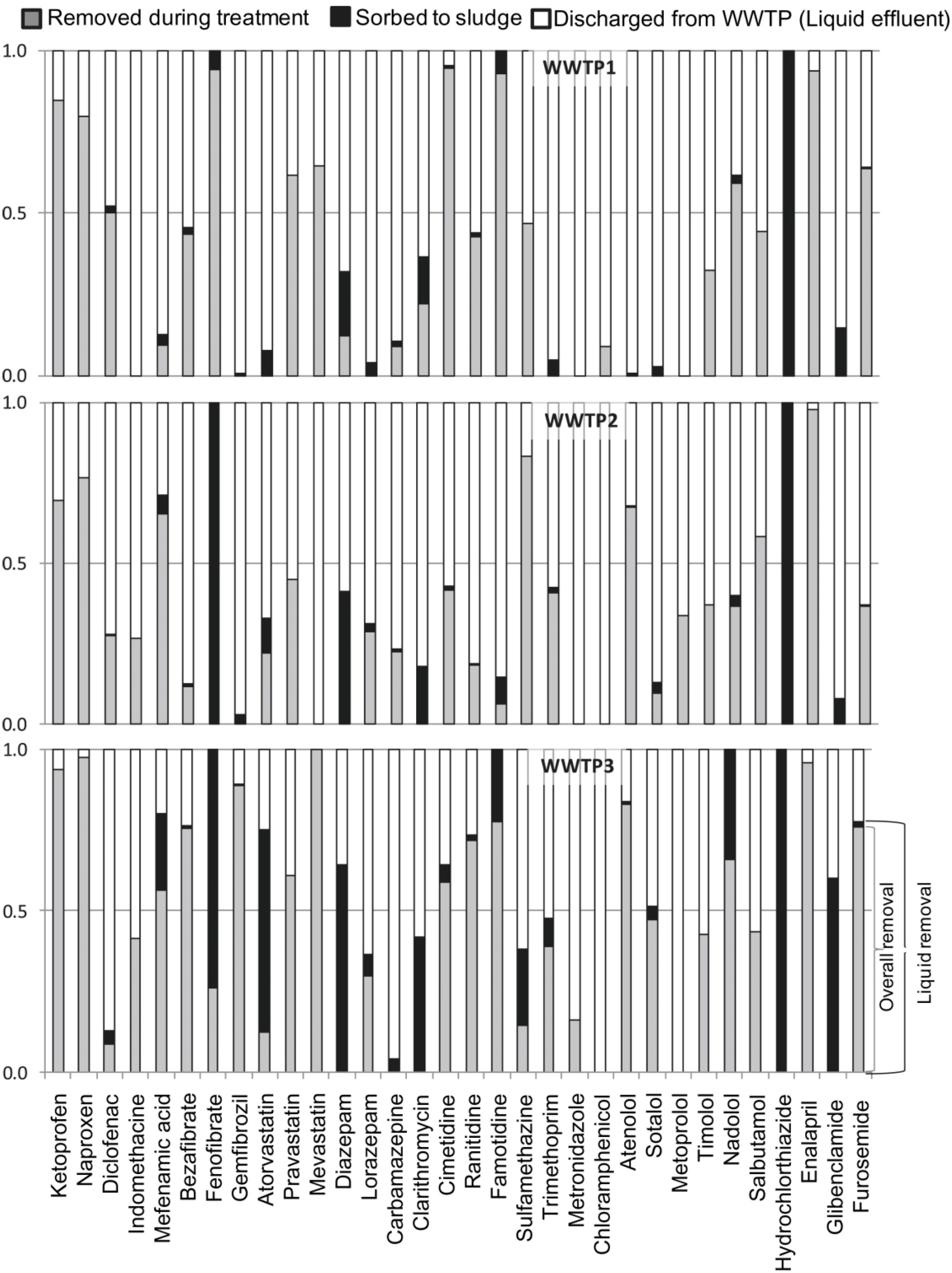
3. Membrane Bioreactors (MBR)
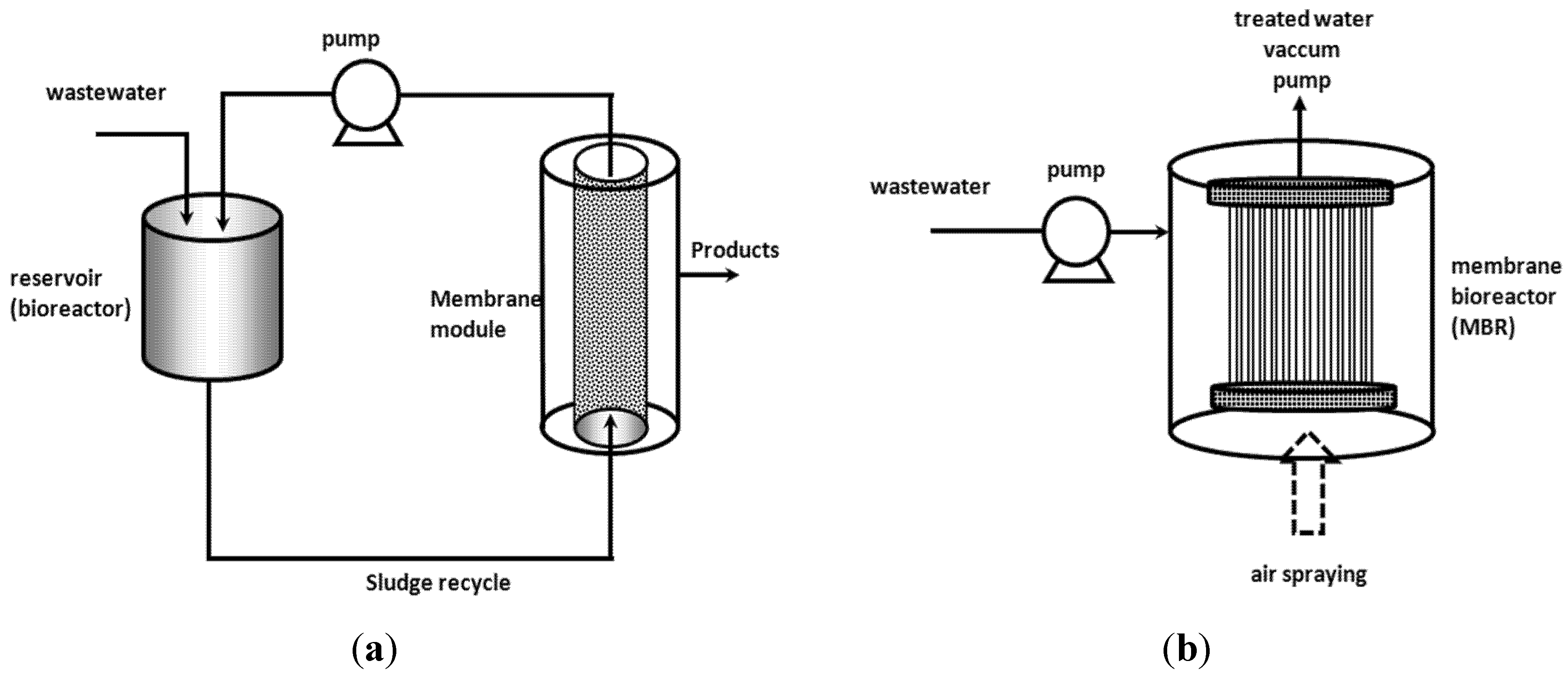
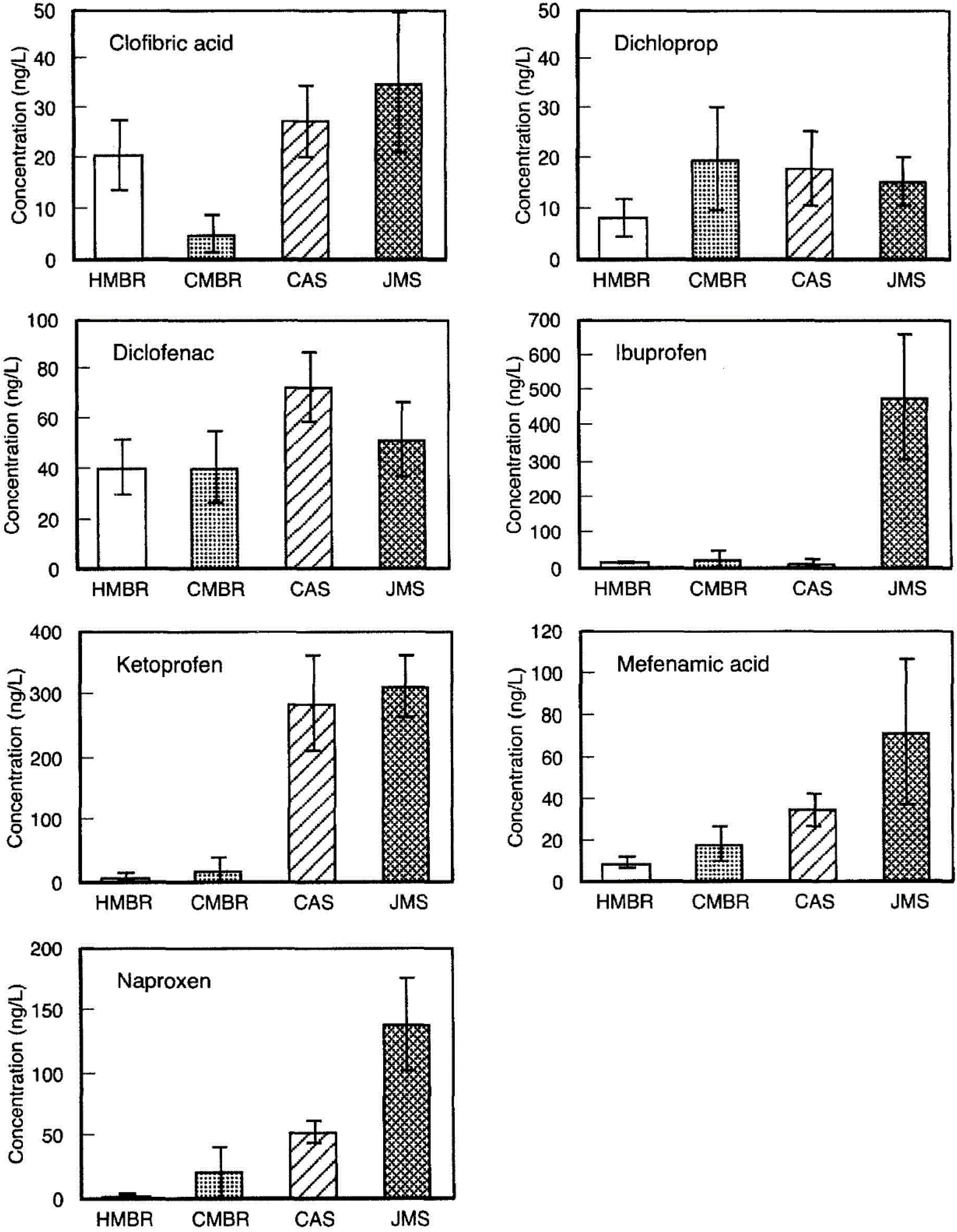
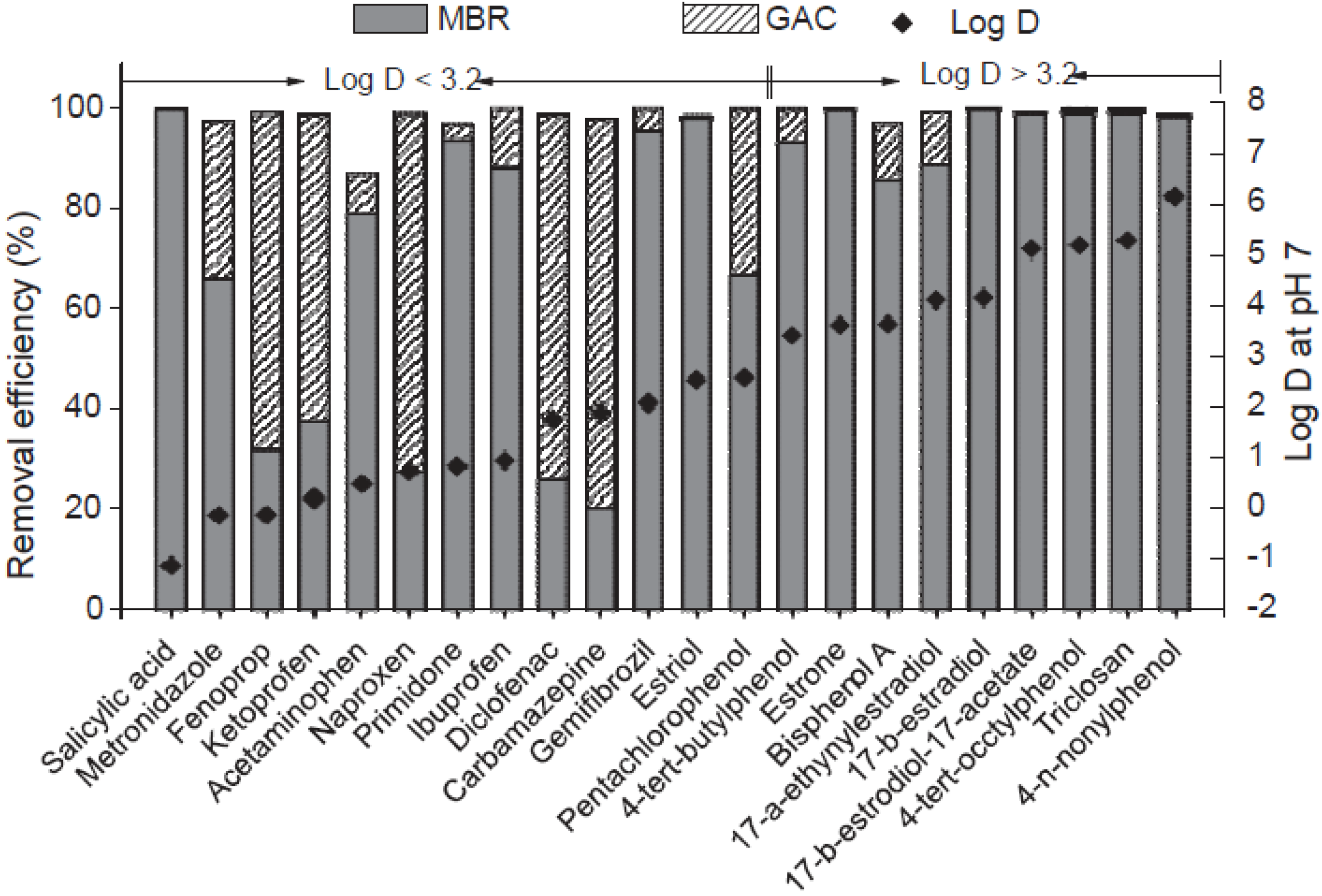
4. Enzymatic Treatments
4.1. Biocatalysts
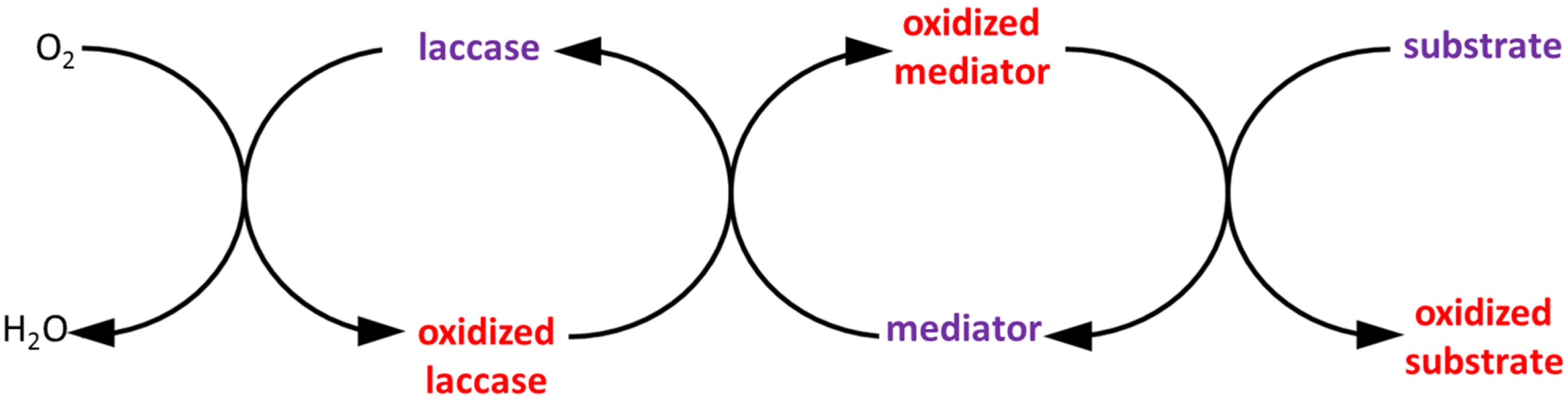
4.2. Enzymatic Reactors
5. Enzymatic Membrane Reactors (EMRs)
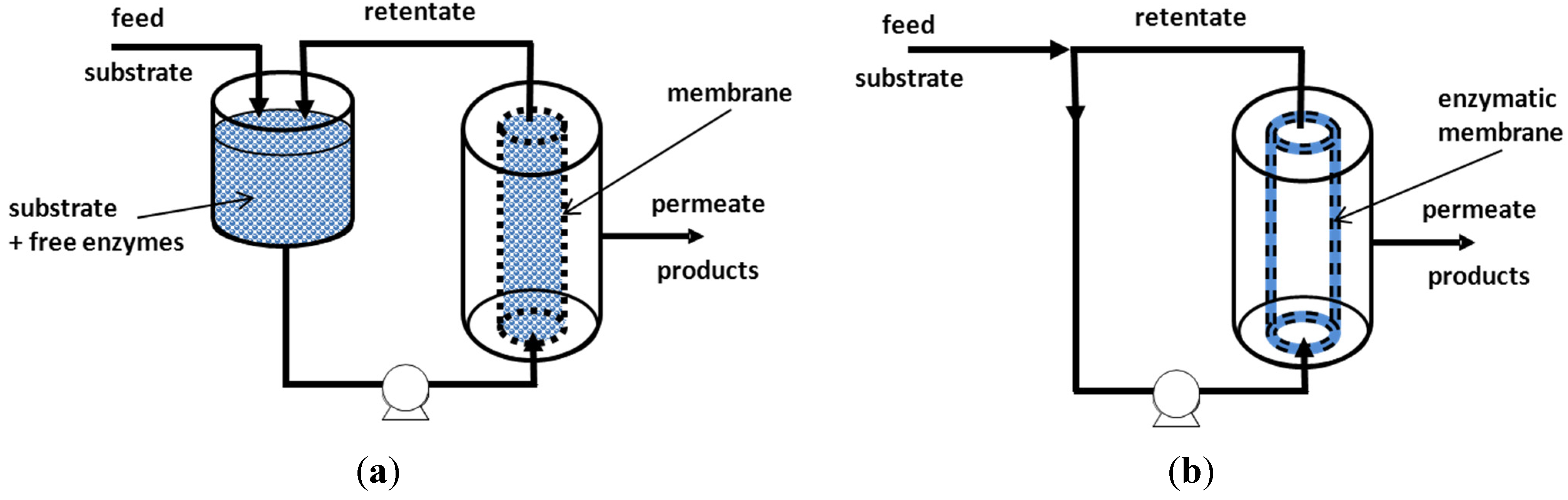
5.1. Enzymatic Reactor Coupled to a Membrane Unit
| Enzymes | Membrane Type | Reactor Type | Applications | Ref. |
|---|---|---|---|---|
| Laccase from C. bulleri | PAN UF membrane 20 kDa | CSTR | Degradation of triarylmethane dyes | [168] |
| Tyrosinase | PES UF membrane 30 kDa | CSTR | Degradation of polyphenols | [167] |
| Laccase from T. versicolor | PS UF Membrane 10 kDa | CSTR | Degradation of dyes | [172] |
| laccase from M. thermophila | PES UF membrane 10 kDa | CSTR | Degradation of estrogen | [157] |
| Laccase and HRP | Flat sheet polymeric NF membranes | CSTR | Degradation of BPA | [170] |
| Laccase from A. oryzae | 6 kDa polyacrylonitrile hollow fiber membrane | Membrane submerged in the reactor | Degradation of BPA and diclofenac | [171] |
| Membrane submerged in the reactor; GAC was added | Degradation of carbamazepine, diclofenac, sulfamethoxazole and atrazine | [162] |
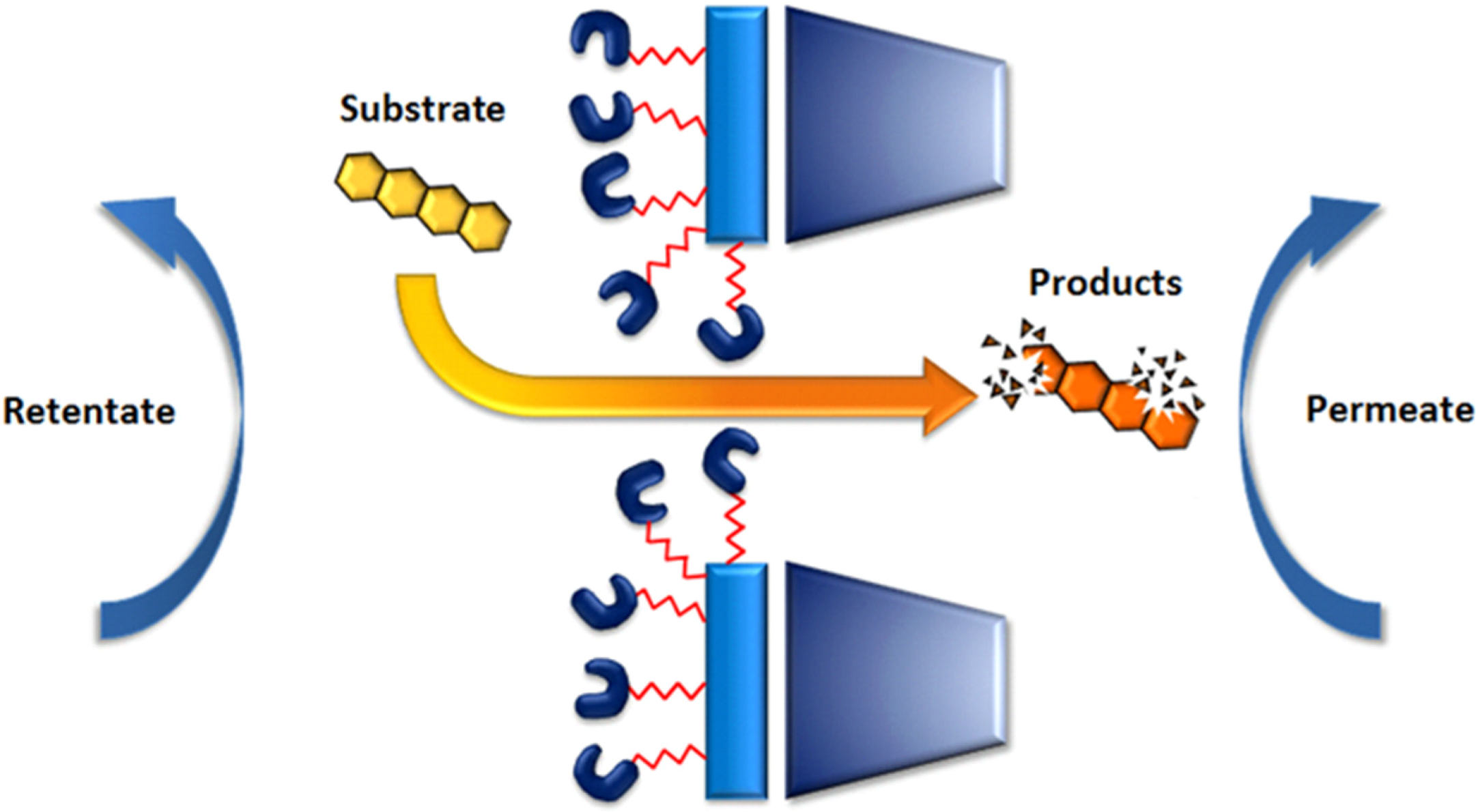
5.2. The Genuine Enzymatic Membrane Reactor
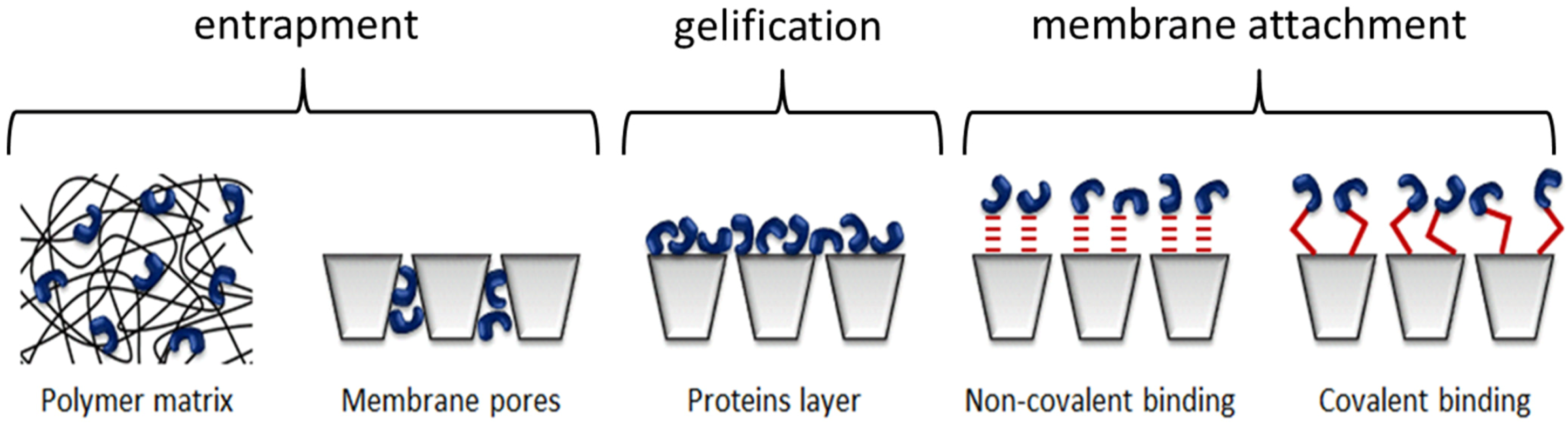
| Type of immobilization | Enzymes | Membrane Types | Immobilization Types | Applications (removal of) | Ref. |
|---|---|---|---|---|---|
| Entrapment | Crude enzyme extract of Pseudomonas sp. | Flat polyacrylonitrile (PAN) UF membrane | Entrapment within membrane by mixing the enzymes with casting solution | phenols | [198] |
| Laccase from P. oryzae | Spira-cel spiral wound module with a polyethersulfone membrane | Entrapment within membrane by filtration | phenols | [199] | |
| Laccase and horseradish peroxidase | Polypropylene hollow fiber membrane (0.2 µm) | Entrapment within membrane by filtration | hydroxylated aromatic compounds | [200] | |
| Laccase from Trametes versicolor | TiO2 blended polyethersulfone (PES) membranes and TiO2 sol-gel coated PVDF membranes (0.1 and 0.45 µm) | Adsorption or covalent bonding on TiO2 nanoparticle | Bisphenol A (BPA) | [192,201] | |
| Membrane attachment | Polyphenol oxidase | 0.45 µm flat nylon membrane and polysulfone capillary membrane | Adsorption with glutaraldehyde cross-linking | phenols | [202] |
| Polyethersulfone and polysulfone capillary membranes | Adsorption | phenols | [203,204] | ||
| Polyethersulfone capillary membranes and hydrophilic nylon flat-sheet membranes | Adsorption or adsorption with glutaraldehyde cross-linking | p-cresol | [205] | ||
| Crude enzyme extract of Pseudomonas syringae | Flat polyamide membrane (0.2 µm) | Covalent bonding | phenol and catechol | [193] | |
| Horseradish peroxidase | Flat polyacrylonitrile (PAN) UF membrane | Adsorption and covalent bonding | phenol | [206] | |
| Laccase from Trametes versicolor | Flat modified PVDF microfiltration membrane | Covalent bonding | phenols | [207] | |
| Chitosan/poly(vinyl alcohol) composite nanofibrous membranes | Covalent bonding | 2,4-dichlorophenol | [156] | ||
| α-alumina membrane (0.2 and 1.4 µm) | Covalent bonding | phenols | [128] | ||
| tetracycline | [197] |
| Biological Treatment | Advantages | Drawbacks |
|---|---|---|
| Membrane reactor with free enzymes | Homogeneous mixing In some cases higher enzymatic activity than grafted enzymes | Less stability than with grafted enzymes |
| Packed-bed reactor with grafted enzymes | Biocatalyst recycling | Pressure drop Preferential pathways In some cases lower enzymatic activity than free enzymes Possibly diffusion limitations (internal and external) |
| Fluidized-bed reactor with grafted enzymes | Biocatalyst recycling Pressure drops reduced Better homogeneity | Additional energy cost (gas) In some cases lower enzymatic activity than free enzymes Possible diffusion limitations (internal) |
| Enzymatic membrane reactor | Biocatalyst recycling Decrease of diffusion limitations Separation and reaction take place simultaneously | Membrane clogging In some lower enzymatic activity than free enzymes In some cases lower enzymatic activity than free enzymes Limited loading of enzymes |
6. Conclusions
Acknowledgements
Authors Contributions
Conflicts of Interest
References
- Patrick, D.M.; Marra, F.; Hutchinson, J.; Monnet, D.L.; Ng, H.; Bowie, W.R. Per capita antibiotic consumption: How does a north american jurisdiction compare with Europe? Clin. Infect. Dis. 2004, 39, 11–17. [Google Scholar]
- Al Aukidy, M.; Verlicchi, P.; Jelic, A.; Petrovic, M.; Barcelo, D. Monitoring release of pharmaceutical compounds: Occurrence and environmental risk assessment of two wwtp effluents and their receiving bodies in the Po valley, Italy. Sci. Total Environ. 2012, 438, 15–25. [Google Scholar]
- Tambosi, J.L.; Yamanaka, L.Y.; Jose, H.J.; Moreira, R.D.P.M.; Schroder, H.F. Recent research data on the removal of pharmaceuticals from sewage treatment plants (stp). Quim. Nova 2010, 33, 411–420. [Google Scholar]
- Wirtz, V.J.; Dreser, A.; Gonzales, R. Trends in antibiotic utilization in eight latin American countries, 1997–2007. Rev. Panam Salud Publ. 2010, 27, 219–225. [Google Scholar]
- Schwab, B.W.; Hayes, E.P.; Fiori, J.M.; Mastrocco, F.J.; Roden, N.M.; Cragin, D.; Meyerhoff, R.D.; D'Aco, V.J.; Anderson, P.D. Human pharmaceuticals in us surface waters: A human health risk assessment. Regul. Toxicol. Pharm. 2005, 42, 296–312. [Google Scholar]
- Mompelat, S.; le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar]
- Chen, F.; Ying, G.G.; Kong, L.X.; Wang, L.; Zhao, J.L.; Zhou, L.J.; Zhang, L.J. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ. Pollut. 2011, 159, 1490–1498. [Google Scholar]
- Siemens, J.; Huschek, G.; Siebe, C.; Kaupenjohann, M. Concentrations and mobility of human pharmaceuticals in the world's largest wastewater irrigation system, Mexico city-mezquital valley. Water Res. 2008, 42, 2124–2134. [Google Scholar]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Heal. 2011, 214, 442–448. [Google Scholar]
- Niu, J.F.; Li, Y.; Wang, W.L. Light-source-dependent role of nitrate and humic acid in tetracycline photolysis: Kinetics and mechanism. Chemosphere 2013, 92, 1423–1429. [Google Scholar]
- Martinovic, D.; Hogarth, W.T.; Jones, R.E.; Sorensen, P.W. Environmental estrogens suppress hormones, behavior, and reproductive fitness in male fathead minnows. Environ. Toxicol. Chem. 2007, 26, 271–278. [Google Scholar]
- Quinn, B.; Gagne, F.; Blaise, C. An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, hydra attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar]
- Liu, F.; Ying, G.G.; Tao, R.; Zhao, J.-L.; Yang, J.F.; Zhao, L.F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol a exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar]
- Hernando, M.D.; Mezcua, M.; Fernandez-Alba, A.R.; Barcelo, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar]
- Tacconelli, E.; de Angelis, G.; Cataldo, M.A.; Mantengoli, E.; Spanu, T.; Pan, A.; Corti, G.; Radice, A.; Stolzuoli, L.; Antinori, S.; et al. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: A hospital population-based study. Antimicrob. Agents Chemother. 2009, 53, 4264–4269. [Google Scholar]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar]
- Baquero, F.; Martinez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotech. 2008, 19, 260–265. [Google Scholar]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar]
- Gros, M.; Petrovic, M.; Barcelo, D. Tracing pharmaceutical residues of different therapeutic classes in environmental waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching. Anal. Chem. 2009, 81, 898–912. [Google Scholar]
- Miege, C.; Choubert, J.M.; Ribeiro, L.; Eusebe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants—Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar]
- Pailler, J.Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathway's and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (ppcps) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar]
- Thomas, K.V.; Dye, C.; Schlabach, M.; Langford, K.H. Source to sink tracking of selected human pharmaceuticals from two oslo city hospitals and a wastewater treatment works. J. Environ. Monitor 2007, 9, 1410–1418. [Google Scholar]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barcelo, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar]
- Yu, J.T.; Bouwer, E.J.; Coelhan, M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agr. Water Manag. 2006, 86, 72–80. [Google Scholar]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar]
- Cardoso, O.; Porcher, J.-M.; Sanchez, W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere 2014, 115. [Google Scholar] [CrossRef]
- Boxall, A.B.A. The environmental side effects of medication—How are human and veterinary medicines in soils and water bodies affecting human and environmental health? EMBO Rep. 2004, 5, 1110–1116. [Google Scholar]
- Deo, R.P. Pharmaceuticals in the surface water of the USA: A review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar]
- Vazquez-Roig, P.; Andreu, V.; Onghena, M.; Blasco, C.; Pico, Y. Assessment of the occurrence and distribution of pharmaceuticals in a Mediterranean wetland (L'Albufera, Valencia, Spain) by LC-MS/MS. Anal. Bioanal. Chem. 2011, 400, 1287–1301. [Google Scholar]
- Vieno, N.M.; Harkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar]
- Gracia-Lor, E.; Sancho, J.V.; Serrano, R.; Hernández, F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the spanish mediterranean area of valencia. Chemosphere 2012, 87, 453–462. [Google Scholar]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeunq, L.W.Y.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K.S. Removal of antibiotics from wastewater by sewage treatment facilities in hong kong and shenzhen, china. Water Res. 2008, 42, 395–403. [Google Scholar]
- Schlusener, M.P.; Bester, K. Determination of steroid hormones, hormone conjugates and macrolide antibiotics in influents and effluents of sewage treatment plants utilising high-performance liquid chromatography/tandem mass spectrometry with electrospray and atmospheric pressure chemical ionisation. Rapid Commun. Mass Spectrom. 2005, 19, 3269–3278. [Google Scholar]
- Senta, I.; Terzic, S.; Ahel, M. Simultaneous determination of sulfonamides, fluoroquinolones, macrolides and trimethoprim in wastewater and river water by LC-tandem-MS. Chromatographia 2008, 68, 747–758. [Google Scholar]
- Christian, T.; Schneider, R.J.; Farber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydroch. Hydrob. 2003, 31, 36–44. [Google Scholar]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.Q.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the haihe river basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar]
- Massey, L.B.; Haggard, B.E.; Galloway, J.M.; Loftin, K.A.; Meyer, M.T.; Green, W.R. Antibiotic fate and transport in three effluent-dominated ozark streams. Ecol. Eng. 2010, 36, 930–938. [Google Scholar]
- Baran, W.; Adamek, E.; Ziemianska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar]
- Batt, A.L.; Aga, D.S. Simultaneous analysis of multiple classes of antibiotics by ion trap LC/MS/MS for assessing surface water and groundwater contamination. Anal. Chem. 2005, 77, 2940–2947. [Google Scholar]
- Batt, A.L.; Bruce, I.B.; Aga, D.S. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environ. Pollut. 2006, 142, 295–302. [Google Scholar]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in four full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar]
- Botitsi, E.; Frosyni, C.; Tsipi, D. Determination of pharmaceuticals from different therapeutic classes in wastewaters by liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal. Bioanal. Chem. 2007, 387, 1317–1327. [Google Scholar]
- Gros, M.; Rodriguez-Mozaz, S.; Barcelo, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar]
- Yang, S.W.; Carlson, K. Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Res. 2003, 37, 4645–4656. [Google Scholar]
- Diaz-Cruz, M.S.; Garcia-Galan, M.J.; Barcelo, D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J. Chromatogr. A 2008, 1193, 50–59. [Google Scholar]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of sulfonamide residues along the ebro river basin: Removal in wastewater treatment plants and environmental impact assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar]
- Pena, A.; Paulo, M.; Silva, L.J.G.; Seifrtova, M.; Lino, C.M.; Solich, P. Tetracycline antibiotics in hospital and municipal wastewaters: A pilot study in portugal. Anal. Bioanal. Chem. 2010, 396, 2929–2936. [Google Scholar]
- Vieno, N.M.; Tuhkanen, T.; Kronberg, L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography-tandem mass spectrometry detection. J. Chromatogr. A 2006, 1134, 101–111. [Google Scholar]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (edcs) in wastewater treatment—Physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar]
- Manickum, T.; John, W. Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South africa). Sci. Total Environ. 2014, 468–469, 584–597. [Google Scholar]
- Gorga, M.; Petrovic, M.; Barcelo, D. Multi-residue analytical method for the determination of endocrine disruptors and related compounds in river and waste water using dual column liquid chromatography switching system coupled to mass spectrometry. J. Chromatogr. A 2013, 1295, 57–66. [Google Scholar]
- Silva, C.P.; Otero, M.; Esteves, V. Processes for the elimination of estrogenic steroid hormones from water: A review. Environ. Pollut. 2012, 165, 38–58. [Google Scholar]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar]
- Onesios, K.M.; Bouwer, E.J. Biological removal of pharmaceuticals and personal care products during laboratory soil aquifer treatment simulation with different primary substrate concentrations. Water Res. 2012, 46, 2365–2375. [Google Scholar]
- Muller, M.; Combalbert, S.; Delgenes, N.; Bergheaud, V.; Rocher, V.; Benoit, P.; Delgenes, J.P.; Patureau, D.; Hernandez-Raquet, G. Occurrence of estrogens in sewage sludge and their fate during plant-scale anaerobic digestion. Chemosphere 2010, 81, 65–71. [Google Scholar]
- Carballa, M.; Omil, F.; Ternes, T.; Lema, J.M. Fate of pharmaceutical and personal care products (ppcps) during anaerobic digestion of sewage sludge. Water Res. 2007, 41, 2139–2150. [Google Scholar]
- Czajka, C.P.; Londry, K.L. Anaerobic biotransformation of estrogens. Sci. Total Environ. 2006, 367, 932–941. [Google Scholar]
- Andersen, H.; Siegrist, H.; Halling-Sorensen, B.; Ternes, T.A. Fate of estrogens in a municipal sewage treatment plant. Environ. Sci. Technol. 2003, 37, 4021–4026. [Google Scholar]
- Ren, Y.X.; Nakano, K.; Nomura, M.; Chiba, N.; Nishimura, O. Effects of bacterial activity on estrogen removal in nitrifying activated sludge. Water Res. 2007, 41, 3089–3096. [Google Scholar]
- Servos, M.R.; Bennie, D.T.; Burnison, B.K.; Jurkovic, A.; McInnis, R.; Neheli, T.; Schnell, A.; Seto, P.; Smyth, S.A.; Ternes, T.A. Distribution of estrogens, 17 beta-estradiol and estrone, in canadian municipal wastewater treatment plants. Sci. Total Environ. 2005, 336, 155–170. [Google Scholar]
- De Graaff, M.S.; Vieno, N.M.; Kujawa-Roeleveld, K.; Zeeman, G.; Temmink, H.; Buisman, C.J.N. Fate of hormones and pharmaceuticals during combined anaerobic treatment and nitrogen removal by partial nitritation-anammox in vacuum collected black water. Water Res. 2011, 45, 375–383. [Google Scholar]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sanchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar]
- Gao, P.; Ding, Y.J.; Li, H.; Xagoraraki, I. Occurrence of pharmaceuticals in a municipal wastewater treatment plant: Mass balance and removal processes. Chemosphere 2012, 88, 17–24. [Google Scholar]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar]
- Schlüsener, M.P.; Bester, K. Behavior of steroid hormones and conjugates during wastewater treatment—A comparison of three sewage treatment plants. Clean-Soil Air Water 2008, 36, 25–33. [Google Scholar]
- Martinez, F.; Lopez-Munoz, M.J.; Aguado, J.; Melero, J.A.; Arsuaga, J.; Sotto, A.; Molina, R.; Segura, Y.; Pariente, M.I.; Revilla, A.; et al. Coupling membrane separation and photocatalytic oxidation processes for the degradation of pharmaceutical pollutants. Water Res. 2013, 47, 5647–5658. [Google Scholar]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chevre, N.; Scharer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461, 480–498. [Google Scholar]
- Fernandez, R.L.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of pharmaceuticals and endocrine disrupting chemicals by a submerged membrane photocatalysis reactor (mpr). Sep. Purif. Technol. 2014, 127, 131–139. [Google Scholar]
- Miralles-Cuevas, S.; Audino, F.; Oller, I.; Sanchez-Moreno, R.; Perez, J.A.S.; Malato, S. Pharmaceuticals removal from natural water by nanofiltration combined with advanced tertiary treatments (solar photo-fenton, photo-fenton-like fe(iii)-edds complex and ozonation). Sep. Purif. Technol. 2014, 122, 515–522. [Google Scholar]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar]
- Pollice, A.; Laera, G.; Saturno, D.; Giordano, C. Effects of sludge retention time on the performance of a membrane bioreactor treating municipal sewage. J. Membrane Sci. 2008, 317, 65–70. [Google Scholar]
- Kaya, Y.; Ersan, G.; Vergili, I.; Gonder, Z.B.; Yilmaz, G.; Dizge, N.; Aydiner, C. The treatment of pharmaceutical wastewater using in a submerged membrane bioreactor under different sludge retention times. J. Membrane Sci. 2013, 442, 72–82. [Google Scholar]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar]
- Bernhard, M.; Muller, J.; Knepper, T.R. Biodegradation of persistent polar pollutants in wastewater: Comparison of an optimised lab-scale membrane bioreactor and activated sludge treatment. Water Res. 2006, 40, 3419–3428. [Google Scholar]
- Kimura, K.; Hara, H.; Watanabe, Y. Removal of pharmaceutical compounds by submerged membrane bioreactors (mbrs). Desalination 2005, 178, 135–140. [Google Scholar]
- Cases, V.; Alonso, V.; Argandona, V.; Rodriguez, M.; Prats, D. Endocrine disrupting compounds: A comparison of removal between conventional activated sludge and membrane bioreactors. Desalination 2011, 272, 240–245. [Google Scholar]
- Sipma, J.; Osuna, B.; Collado, N.; Monclus, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in mbr and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar]
- Clouzot, L.; Doumenq, P.; Vanloot, P.; Roche, N.; Marrot, B. Membrane bioreactors for 17 alpha-ethinylestradiol removal. J. Membrane Sci. 2010, 362, 81–85. [Google Scholar]
- Radjenovic, J.; Petrovic, M.; Barcelo, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar]
- Radjenovic, J.; Petrovic, M.; Barcelo, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (cas) and advanced membrane bioreactor (mbr) treatment. Water Res. 2009, 43, 831–841. [Google Scholar]
- Fan, H.; Li, J.; Zhang, L.; Feng, L. Contribution of sludge adsorption and biodegradation to the removal of five pharmaceuticals in a submerged membrane bioreactor. Biochem. Eng. J. 2014, 88, 101–107. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Kang, J.G.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by a membrane bioreactor-granular activated carbon (mbr-gac) system. Bioresour. Technol. 2012, 113, 169–173. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Yang, S.F.; Kang, J.G.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by an mbr comprising a mixed culture of bacteria and white-rot fungi. Bioresour. Technol. 2013, 148, 234–241. [Google Scholar]
- Remy, M.; van der Marel, P.; Zwijnenburg, A.; Rulkens, W.; Temmink, H. Low dose powdered activated carbon addition at high sludge retention times to reduce fouling in membrane bioreactors. Water Res. 2009, 43, 345–350. [Google Scholar]
- Chen, Z.-B.; He, Z.-W.; Tang, C.-C.; Hu, D.-X.; Cui, Y.-B.; Wang, A.-J.; Zhang, Y.; Yan, L.-L.; Ren, N.-Q. Performance and model of a novel multi-sparger multi-stage airlift loop membrane bioreactor to treat high-strength 7-aca pharmaceutical wastewater: Effect of hydraulic retention time, temperature and ph. Bioresour. Technol. 2014, 167, 241–250. [Google Scholar]
- Dutta, K.; Lee, M.-Y.; Lai, W.W.-P.; Lee, C.H.; Lin, A.Y.-C.; Lin, C.-F.; Lin, J.-G. Removal of pharmaceuticals and organic matter from municipal wastewater using two-stage anaerobic fluidized membrane bioreactor. Bioresour. Technol. 2014, 165, 42–49. [Google Scholar]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliver. Rev. 2005, 57, 1451–1470. [Google Scholar]
- Fan, C.A.; He, J.Z. Proliferation of antibiotic resistance genes in microbial consortia of sequencing batch reactors (sbrs) upon exposure to trace erythromycin or erythromycin-H2O. Water Res. 2011, 45, 3098–3106. [Google Scholar]
- Kim, Y.H.; Cha, C.J.; Cerniglia, C.E. Purification and characterization of an erythromycin esterase from an erythromycin-resistant pseudomonas sp. FEMS Microbiol. Lett. 2002, 210, 239–244. [Google Scholar]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar]
- Demarche, P.; Junghanns, C.; Nair, R.R.; Agathos, S.N. Harnessing the power of enzymes for environmental stewardship. Biotechnol. Adv. 2012, 30, 933–953. [Google Scholar]
- Durán, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B Environ. 2000, 28, 83–99. [Google Scholar]
- Aitken, M.D. Waste treatment applications of enzymes: Opportunities and obstacles. Chem. Eng. J. 1993, 52, B49–B58. [Google Scholar]
- Karam, J.; Nicell, J.A. Potential applications of enzymes in waste treatment. J. Chem. Technol. Biot. 1997, 69, 141–153. [Google Scholar]
- Nyanhongo, G.S.; Gubitz, G.; Sukyai, P.; Leitner, C.; Haltrich, D.; Ludwig, R. Oxidoreductases from trametes spp. In biotechnology: A wealth of catalytic activity. Food Technol. Biotech. 2007, 45, 250–268. [Google Scholar]
- De Gunzburg, J.; Bensoussan, C. Methods for the Inactivation of Antibiotics. WO 2012007536 A1, 19 January 2012. [Google Scholar]
- Cajthaml, T.; Kresinova, Z.; Svobodova, K.; Moder, M. Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 2009, 75, 745–750. [Google Scholar]
- Auriol, M.; Filali-Meknassi, Y.; Adams, C.D.; Tyagi, R.D. Natural and synthetic hormone removal using the horseradish peroxidase enzyme: Temperature and ph effects. Water Res. 2006, 40, 2847–2856. [Google Scholar]
- Suzuki, K.; Hirai, H.; Murata, H.; Nishida, T. Removal of estrogenic activities of 17 beta-estradiol and ethinylestradiol by ligninolytic enzymes from white rot fungi. Water Res. 2003, 37, 1972–1975. [Google Scholar]
- Tamagawa, Y.; Yamaki, R.; Hirai, H.; Kawai, S.; Nishida, T. Removal of estrogenic activity of natural steroidal hormone estrone by ligninolytic enzymes from white rot fungi. Chemosphere 2006, 65, 97–101. [Google Scholar]
- Auriol, M.; Filali-Meknassi, Y.; Tyagi, R.D.; Adams, C.D. Oxidation of natural and synthetic hormones by the horseradish peroxidase enzyme in wastewater. Chemosphere 2007, 68, 1830–1837. [Google Scholar]
- Melo, C.F.; Dezotti, M. Evaluation of a horseradish peroxidase-catalyzed process for triclosan removal and antibacterial activity reduction. J. Chem. Technol. Biot. 2013, 88, 930–936. [Google Scholar]
- Zhang, Y.J.; Geissen, S.U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar]
- Wen, X.; Jia, Y.; Li, J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from phanerochaete chrysosporium—A white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar]
- Suda, T.; Hata, T.; Kawai, S.; Okamura, H.; Nishida, T. Treatment of tetracycline antibiotics by laccase in the presence of 1-hydroxybenzotriazole. Bioresour. Technol. 2012, 103, 498–501. [Google Scholar]
- Hata, T.; Shintate, H.; Kawai, S.; Okamura, H.; Nishida, T. Elimination of carbamazepine by repeated treatment with laccase in the presence of 1-hydroxybenzotriazole. J. Hazard. Mater. 2010, 181, 1175–1178. [Google Scholar]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar]
- Majeau, J.-A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar]
- Desai, S.S.; Nityanand, C. Microbial laccases and their applications: A review. J. Biotechnol. 2011, 3, 98–124. [Google Scholar]
- Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Burton, S. Potential applications of laccase-mediated coupling and grafting reactions: A review. Enzyme Microb Tech. 2011, 48, 195–208. [Google Scholar]
- Rodríguez-Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar]
- Chea, V.; Paolucci-Jeanjean, D.; Belleville, M.P.; Sanchez, J. Optimization and characterization of an enzymatic membrane for the degradation of phenolic compounds. Catal. Today 2012, 193, 49–56. [Google Scholar]
- Widsten, P.; Kandelbauer, A. Laccase applications in the forest products industry: A review. Enzyme Microb. Tech. 2008, 42, 293–307. [Google Scholar]
- Auriol, M.; Filali-Meknassi, Y.; Tyagi, R.D.; Adams, C.D. Laccase-catalyzed conversion of natural and synthetic hormones from a municipal wastewater. Water Res. 2007, 41, 3281–3288. [Google Scholar]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.A.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813. [Google Scholar]
- Auriol, M.; Filali-Meknassi, Y.; Adams, C.D.; Tyagi, R.D.; Noguerol, T.N.; Pina, B. Removal of estrogenic activity of natural and synthetic hormones from a municipal wastewater: Efficiency of horseradish peroxidase and laccase from trametes versicolor. Chemosphere 2008, 70, 445–452. [Google Scholar]
- Weng, S.-S.; Ku, K.-L.; Lai, H.-T. The implication of mediators for enhancement of laccase oxidation of sulfonamide antibiotics. Bioresour. Technol. 2012, 113, 259–264. [Google Scholar]
- Galli, C.; Gentili, P. Chemical messengers: Mediated oxidations with the enzyme laccase. J. Phys. Org. Chem. 2004, 17, 973–977. [Google Scholar]
- Canas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar]
- Kurniawati, S.; Nicell, J.A. Efficacy of mediators for enhancing the laccase-catalyzed oxidation of aqueous phenol. Enzyme Microb. Tech. 2007, 41, 353–361. [Google Scholar]
- Husain, M.; Husain, Q. Applications of redox mediators in the treatment of organic pollutants by using oxidoreductive enzymes: A review. Crit. Rev. Environ. Sci. Technol. 2008, 38, 1–42. [Google Scholar]
- Banci, L.; Ciofi-Baffoni, S.; Tien, M. Lignin and mn peroxidase-catalyzed oxidation of phenolic lignin oligomers. Biochemistry 1999, 38, 3205–3210. [Google Scholar]
- Garcia, H.A.; Hoffman, C.M.; Kinney, K.A.; Lawler, D.F. Laccase-catalyzed oxidation of oxybenzone in municipal wastewater primary effluent. Water Res. 2011, 45, 1921–1932. [Google Scholar]
- Inoue, Y.; Hata, T.; Kawai, S.; Okamura, H.; Nishida, T. Elimination and detoxification of triclosan by manganese peroxidase from white rot fungus. J. Hazard. Mater. 2010, 180, 764–767. [Google Scholar]
- Catapane, M.; Nicolucci, C.; Menale, C.; Mita, L.; Rossi, S.; Mita, D.G.; Diano, N. Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from trametes versicolor. J. Hazard. Mater. 2013, 248, 337–346. [Google Scholar]
- Nicolucci, C.; Rossi, S.; Menale, C.; Godjevargova, T.; Ivanov, Y.; Bianco, M.; Mita, L.; Bencivenga, U.; Mita, D.G.; Diano, N. Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation 2011, 22, 673–683. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Tech. 2007, 40, 1451–1463. [Google Scholar]
- Fernandez-Fernandez, M.; Sanroman, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar]
- Duran, N.; Rosa, M.A.; D'Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzyme Microb. Tech. 2002, 31, 907–931. [Google Scholar]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (bmrs). Green Chem. 2011, 13, 1609–1623. [Google Scholar]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar]
- Mori, M.; Garcia, R.G.; Belleville, M.P.; Paolucci-Jeanjean, D.; Sanchez, J.; Lozano, P.; Vaultier, M.; Rios, G. A new way to conduct enzymatic synthesis in an active membrane using ionic liquids as catalyst support. Catal. Today 2005, 104, 313–317. [Google Scholar]
- Cao, L.Q. Immobilised enzymes: Science or art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Tech. 2011, 49, 326–346. [Google Scholar]
- Plagemann, R.; Jonas, L.; Kragl, U. Ceramic honeycomb as support for covalent immobilization of laccase from trametes versicolor and transformation of nuclear fast red. Appl. Microbiol. Biot. 2011, 90, 313–320. [Google Scholar]
- Dai, Y.R.; Niu, J.F.; Yin, L.F.; Xu, J.J.; Xu, J.R. Laccase-carrying electrospun fibrous membrane for the removal of polycyclic aromatic hydrocarbons from contaminated water. Sep. Purif. Technol. 2013, 104, 1–8. [Google Scholar]
- Xu, R.; Zhou, Q.J.; Li, F.T.; Zhang, B.R. Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem. Eng. J. 2013, 222, 321–329. [Google Scholar]
- Lloret, L.; Hollmann, F.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Immobilisation of laccase on eupergit supports and its application for the removal of endocrine disrupting chemicals in a packed-bed reactor. Biodegradation 2012, 23, 373–386. [Google Scholar]
- Cabana, H.; Alexandre, C.; Agathos, S.N.; Jones, J.P. Immobilization of laccase from the white rot fungus coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresour. Technol. 2009, 100, 3447–3458. [Google Scholar]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Preparation and characterization of cross-linked laccase aggregates and their application to the elimination of endocrine disrupting chemicals. J. Biotechnol. 2007, 132, 23–31. [Google Scholar]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Continuous operation of a fluidized bed reactor for the removal of estrogens by immobilized laccase on eupergit supports. J. Biotechnol. 2012, 162, 404–406. [Google Scholar]
- Sanchez Marcano, J.G.; Tsotsis, T.T. Catalytic Membranes and Membrane Reactors; Wiley-VCH: Weinheim, Germany, 2002; pp. 133–168. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; Ngo, H.H.; Guo, W.; Magram, S.F.; Nghiem, L.D. The effects of mediator and granular activated carbon addition on degradation of trace organic contaminants by an enzymatic membrane reactor. Bioresour. Technol. 2014, 167, 169–177. [Google Scholar]
- Paolucci-Jeanjean, D.; Belleville, M.P.; Rios, G.M.; Zakhia, N. The effect of enzyme concentration and space time on the performance of a continuous recycle membrane reactor for one-step starch hydrolysis. Biochem. Eng. J. 2000, 5, 17–22. [Google Scholar]
- Grzeskowiak-Przywecka, A.; Slominska, L. Saccharification of potato starch in an ultrafiltration reactor. J. Food Eng. 2007, 79, 539–545. [Google Scholar]
- Perea, A.; Ugalde, U. Continuous hydrolysis of whey proteins in a membrane recycle reactor. Enzyme Microb. Tech. 1996, 18, 29–34. [Google Scholar]
- Cabrera-Padilla, R.Y.; Pinto, G.A.; Giordano, R.L.C.; Giordano, R.C. A new conception of enzymatic membrane reactor for the production of whey hydrolysates with low contents of phenylalanine. Process. Biochem. 2009, 44, 269–276. [Google Scholar]
- Calabro, V.; Curcio, S.; de Paola, M.G.; Iorio, G. Optimization of membrane bioreactor performances during enzymatic oxidation of waste bio-polyphenols. Desalination 2009, 236, 30–38. [Google Scholar]
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor. J. Biotechnol. 2009, 143, 69–78. [Google Scholar]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of estrogens by laccase from myceliophthora thermophila in fed-batch and enzymatic membrane reactors. J. Hazard. Mater. 2012, 213–214, 175–183. [Google Scholar]
- Escalona, I.; de Grooth, J.; Font, J.; Nijmeijer, K. Removal of bpa by enzyme polymerization using nf membranes. J. Membrane Sci. 2014, 468, 192–201. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol a and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeter. Biodegr. 2014. [Google Scholar] [CrossRef]
- Mendoza, L.; Jonstrup, M.; Hatti-Kaul, R.; Mattiasson, B. Azo dye decolorization by a laccase/mediator system in a membrane reactor: Enzyme and mediator reusability. Enzyme Microb. Tech. 2011, 49, 478–484. [Google Scholar]
- Gasser, C.A.; Yu, L.; Svojitka, J.; Wintgens, T.; Ammann, E.M.; Shahgaldian, P.; Corvini, P.F.X.; Hommes, G. Advanced enzymatic elimination of phenolic contaminants in wastewater: A nano approach at field scale. Appl. Microbiol. Biot. 2014, 98, 3305–3316. [Google Scholar]
- Rios, G.M.; Belleville, M.P.; Paolucci, D.; Sanchez, J. Progress in enzymatic membrane reactors - a review. J. Membrane Sci. 2004, 242, 189–196. [Google Scholar]
- Belleville, M.-P.; Paolucci-Jeanjean, D.; Rios, G.M. Separation, extraction and concentration processes in the food, beverage and nutraceutical industries (membrane bioreactors and the production of food ingredients). Woodead Food Ser. 2010, 11, 314–337. [Google Scholar]
- Kanwar, L.; Goswami, P. Isolation of a pseudomonas lipase produced in pure hydrocarbon substrate and its application in the synthesis of isoamyl acetate using membrane-immobilised lipase. Enzyme Microb. Tech. 2002, 31, 727–735. [Google Scholar]
- Tan, T.W.; Wang, F.; ZHang, H. Preparation of pva/chitosan lipase membrane reactor and its application in synthesis of monoglyceride. J. Mol. Catal. B Enzym. 2002, 18, 325–331. [Google Scholar]
- Hilal, N.; Nigmatullin, R.; Alpatova, A. Immobilization of cross-linked lipase aggregates within microporous polymeric membranes. J. Membrane Sci. 2004, 238, 131–141. [Google Scholar]
- Torras, C.; Nabarlatz, D.; Vallot, G.; Montane, D.; Garcia-Valls, R. Composite polymeric membranes for process intensification: Enzymatic hydrolysis of oligodextrans. Chem. Eng. J. 2008, 144, 259–266. [Google Scholar]
- Sousa, H.A.; Rodrigues, C.; Klein, E.; Afonso, C.A.M.; Crespo, J.G. Immobilisation of pig liver esterase in hollow fibre membranes. Enzyme Microb. Tech. 2001, 29, 625–634. [Google Scholar]
- Xu, J.; Wang, Y.J.; Hu, Y.; Luo, G.S.; Dai, Y.Y. Immobilization of lipase by filtration into a specially designed microstructure in the ca/ptfe composite membrane. J. Mol. Catal. B Enzym. 2006, 42, 55–63. [Google Scholar]
- Wang, Y.J.; Jian, X.; Luo, G.S.; Dai, Y.Y. Immobilization of lipase by ultrafiltration and cross-linking onto the polysulfone membrane surface. Bioresour. Technol. 2008, 99, 2299–2303. [Google Scholar]
- Sakaki, K.; Giorno, L.; Drioli, E. Lipase-catalyzed optical resolution of racemic naproxen in biphasic enzyme membrane reactors. J. Membrane Sci. 2001, 184, 27–38. [Google Scholar]
- Trusek-Holownia, A.; Noworyta, A. An integrated process: Ester synthesis in an enzymatic membrane reactor and water sorption. J. Biotechnol. 2007, 130, 47–56. [Google Scholar]
- Shamel, M.M.; Ramachandran, K.B.; Hasan, M.; Al-Zuhair, S. Hydrolysis of palm and olive oils by immobilised lipase using hollow fibre reactor. Biochem. Eng. J. 2007, 34, 228–235. [Google Scholar]
- Paiva, A.L.; Balcao, V.M.; Malcata, F.X. Kinetics and mechanisms of reactions catalyzed by immobilized lipases. Enzyme Microb. Tech. 2000, 27, 187–204. [Google Scholar]
- Belleville, M.P.; Lozano, P.; Iborra, J.L.; Rios, G.M. Preparation of hybrid membranes for enzymatic reaction. Sep. Purif. Technol. 2001, 25, 229–233. [Google Scholar]
- Gumi, T.; Paolucci-Jeanjean, D.; Belleville, M.P.; Rios, G.A. Enzymatic membrane reactor involving a hybrid membrane in supercritical carbon dioxide. J. Membrane Sci. 2007, 297, 98–103. [Google Scholar]
- Lozano, P.; Perez-Marin, A.B.; de Diego, T.; Gomez, D.; Paolucci-Jeanjean, D.; Belleville, M.P.; Rios, G.M.; Iborra, J.L. Active membranes coated with immobilized candida antarctica lipase b: Preparation and application for continuous butyl butyrate synthesis in organic media. J. Membrane Sci. 2002, 201, 55–64. [Google Scholar]
- Durante, D.; Casadio, R.; Martelli, L.; Tasco, G.; Portaccio, M.; de Luca, P.; Bencivenga, U.; Rossi, S.; Di Martino, S.; Grano, V.; et al. Isothermal and non-isothermal bioreactors in the detoxification of waste waters polluted by aromatic compounds by means of immobilised laccase from rhus vernicifera. J. Mol. Catal. B Enzym. 2004, 27, 191–206. [Google Scholar]
- Lopez-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisan, J.M.; Fernandez-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membrane Sci. 2014, 452, 229–240. [Google Scholar]
- Akay, G.; Erhan, E.; Keskinler, B.; Algur, O.F. Removal of phenol from wastewater using membrane-immobilized enzymes—part II. Cross-flow filtration. J. Membrane Sci. 2002, 206, 61–68. [Google Scholar]
- Erhan, E.; Keskinler, B.; Akay, G.; Algur, O.F. Removal of phenol from water by membrane-immobilized enzymes—Part I. Dead-end filtration. J. Membrane Sci. 2002, 206, 361–373. [Google Scholar]
- Diano, N.; Grano, V.; Fraconte, L.; Caputo, P.; Ricupito, A.; Attanasio, A.; Bianco, M.; Bencivenga, U.; Rossi, S.; Manco, I.; et al. Non-isothermal bioreactors in enzymatic remediation of waters polluted by endocrine disruptors: Bpa as a model of pollutant. Appl. Catal. B Environ. 2007, 69, 252–261. [Google Scholar]
- Lozano, P.; de Diego, T.; Belleville, M.P.; Rios, G.M.; Iborra, J.L. A dynamic membrane reactor with immobilized alpha-chymotrypsin for continuous kyotorphin synthesis in organic media. Biotechnol. Lett. 2000, 22, 771–775. [Google Scholar]
- De Cazes, M.; Belleville, M.-P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; de Gunzburg, J.; Barceló, D.; Sanchez-Marcano, J. Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catal. Today 2014, 236. [Google Scholar] [CrossRef]
- Bohdziewicz, J. Biodegradation of phenol by enzymes from pseudomonas sp. Immobilized onto ultrafiltration membranes. Process. Biochem. 1998, 33, 811–818. [Google Scholar]
- Lante, A.; Crapisi, A.; Krastanov, A.; Spettoli, P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process. Biochem. 2000, 36, 51–58. [Google Scholar]
- Moeder, M.; Martin, C.; Koeller, G. Degradation of hydroxylated compounds using laccase and horseradish peroxidase immobilized on microporous polypropylene hollow fiber membranes. J. Membrane Sci. 2004, 245, 183–190. [Google Scholar]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-a with immobilized laccase on TiO2 sol–gel coated pvdf membrane. J. Membrane Sci. 2014, 469, 19–30. [Google Scholar]
- Burton, S.G.; Boshoff, A.; Edwards, W.; Rose, P.D. Biotransformation of phenols using immobilised polyphenol oxidase. J. Mol. Catal. B Enzym. 1998, 5, 411–416. [Google Scholar]
- Edwards, W.; Bownes, R.; Leukes, W.D.; Jacobs, E.P.; Sanderson, R.; Rose, P.D.; Burton, S.G. A capillary membrane bioreactor using immobilized polyphenol oxidase for the removal of phenols from industrial effluents. Enzyme Microb. Tech. 1999, 24, 209–217. [Google Scholar]
- Edwards, W.; Leukes, W.D.; Rose, P.D.; Burton, S.G. Immobilization of polyphenol oxidase on chitosan-coated polysulphone capillary membranes for improved phenolic effluent bioremediation. Enzyme Microb. Tech. 1999, 25, 769–773. [Google Scholar]
- Boshoff, A.; Edwards, W.; Leukes, W.D.; Rose, P.D.; Burton, S.G. Immobilisation of polyphenol oxidase on nylon and polyethersulphone membranes: Effect on product formation. Desalination 1998, 115, 307–312. [Google Scholar]
- Vasileva, N.; Godjevargova, T.; Ivanova, D.; Gabrovska, K. Application of immobilized horseradish peroxidase onto modified acrylonitrile copolymer membrane in removing of phenol from water. Int. J. Biol. Macromol. 2009, 44, 190–194. [Google Scholar]
- Jolivalt, C.; Brenon, S.; Caminade, E.; Mougin, C.; Pontie, M. Immobilization of laccase from trametes versicolor on a modified pvdf microfiltration membrane: Characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membrane Sci. 2000, 180, 103–113. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cazes, M.; Abejón, R.; Belleville, M.-P.; Sanchez-Marcano, J. Membrane Bioprocesses for Pharmaceutical Micropollutant Removal from Waters. Membranes 2014, 4, 692-729. https://doi.org/10.3390/membranes4040692
De Cazes M, Abejón R, Belleville M-P, Sanchez-Marcano J. Membrane Bioprocesses for Pharmaceutical Micropollutant Removal from Waters. Membranes. 2014; 4(4):692-729. https://doi.org/10.3390/membranes4040692
Chicago/Turabian StyleDe Cazes, Matthias, Ricardo Abejón, Marie-Pierre Belleville, and José Sanchez-Marcano. 2014. "Membrane Bioprocesses for Pharmaceutical Micropollutant Removal from Waters" Membranes 4, no. 4: 692-729. https://doi.org/10.3390/membranes4040692




