Friedel–Crafts Crosslinked Highly Sulfonated Polyether Ether Ketone (SPEEK) Membranes for a Vanadium/Air Redox Flow Battery
Abstract
:1. Introduction
2. Experimental Section
2.1. Membrane Preparation
2.2. Proton Conductivity
2.3. Ion Exchange Capacity (IEC)
2.4. Water Uptake
2.5. Permeability of Vanadium Ions

2.6. Fourier Transform Infra-Red Spectroscopy
2.7. Pulsed Field Gradient Nuclear Magnetic Resonance Spectroscopy (PFG NMR)
3. Results and Discussion
3.1. Crosslinking SPEEK

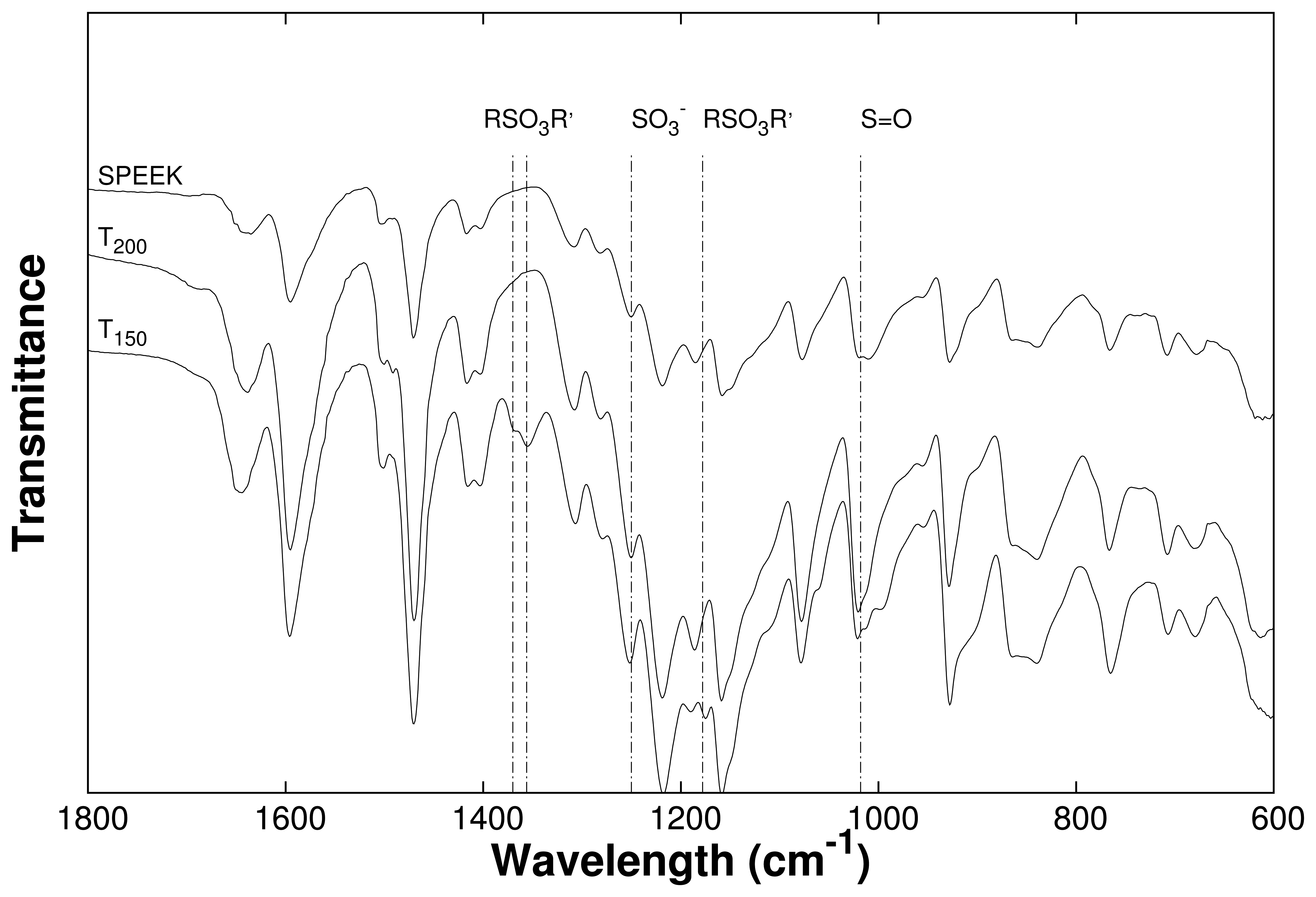
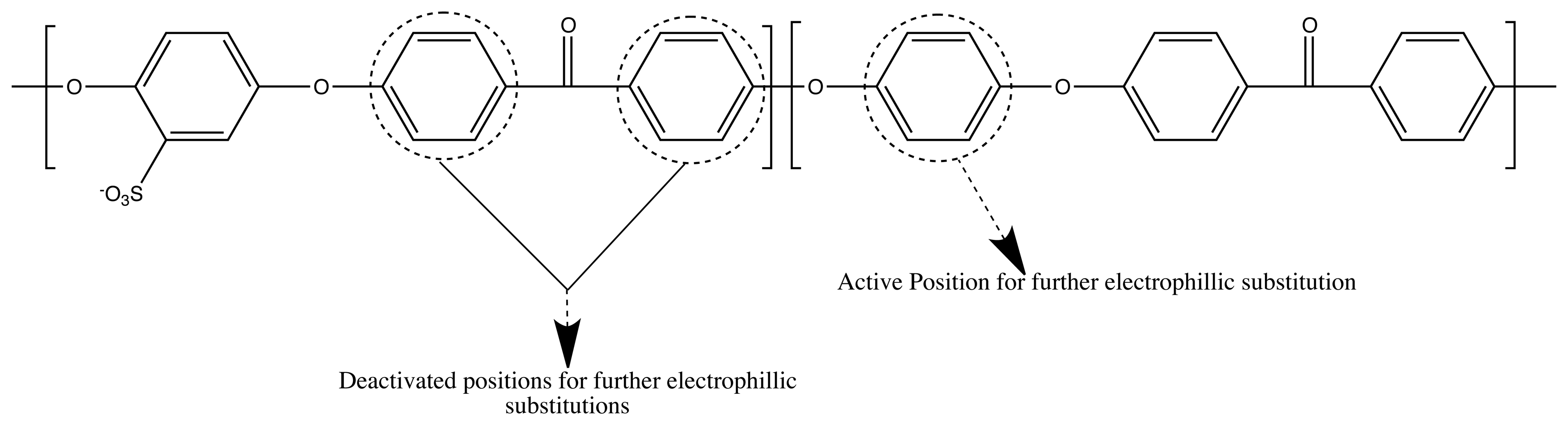
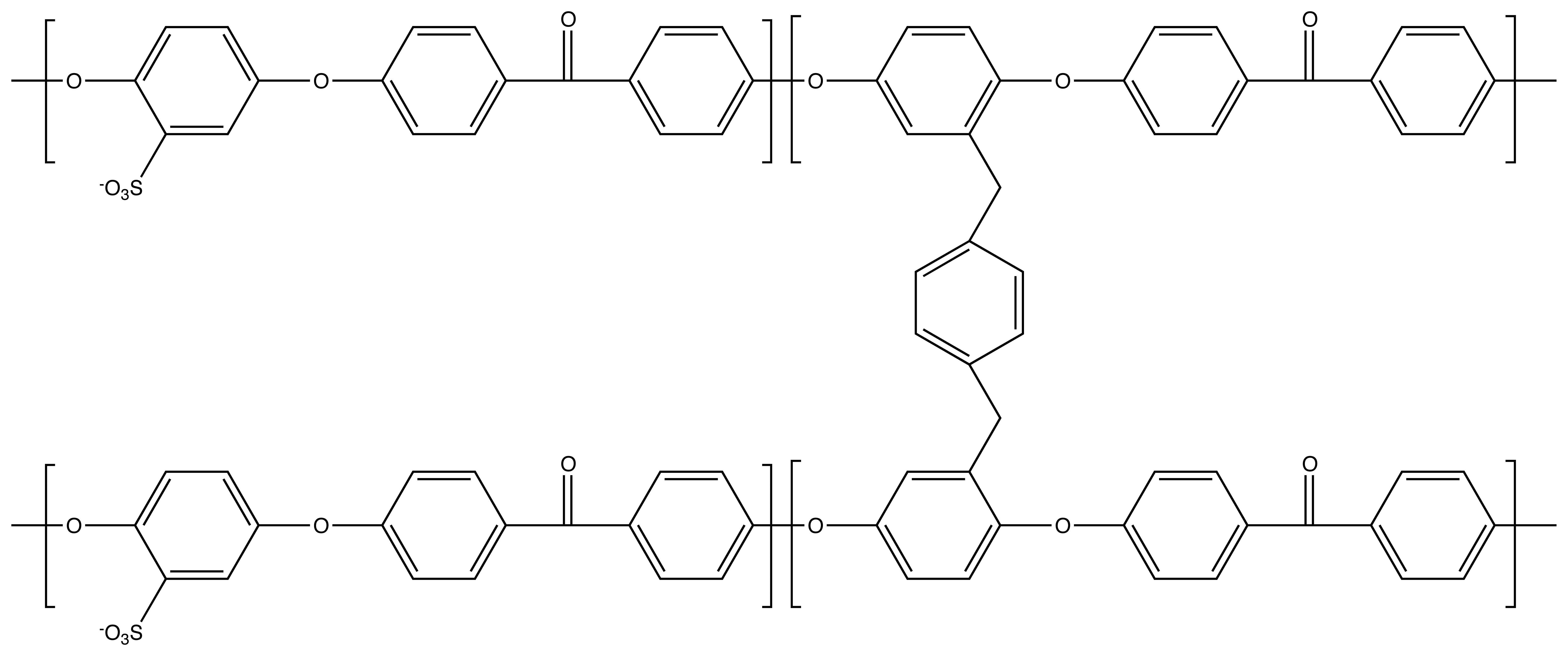
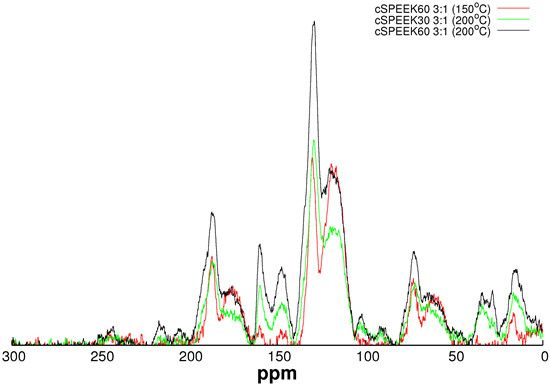
3.2. Channel Orientation by Water Diffusion Anisotropy
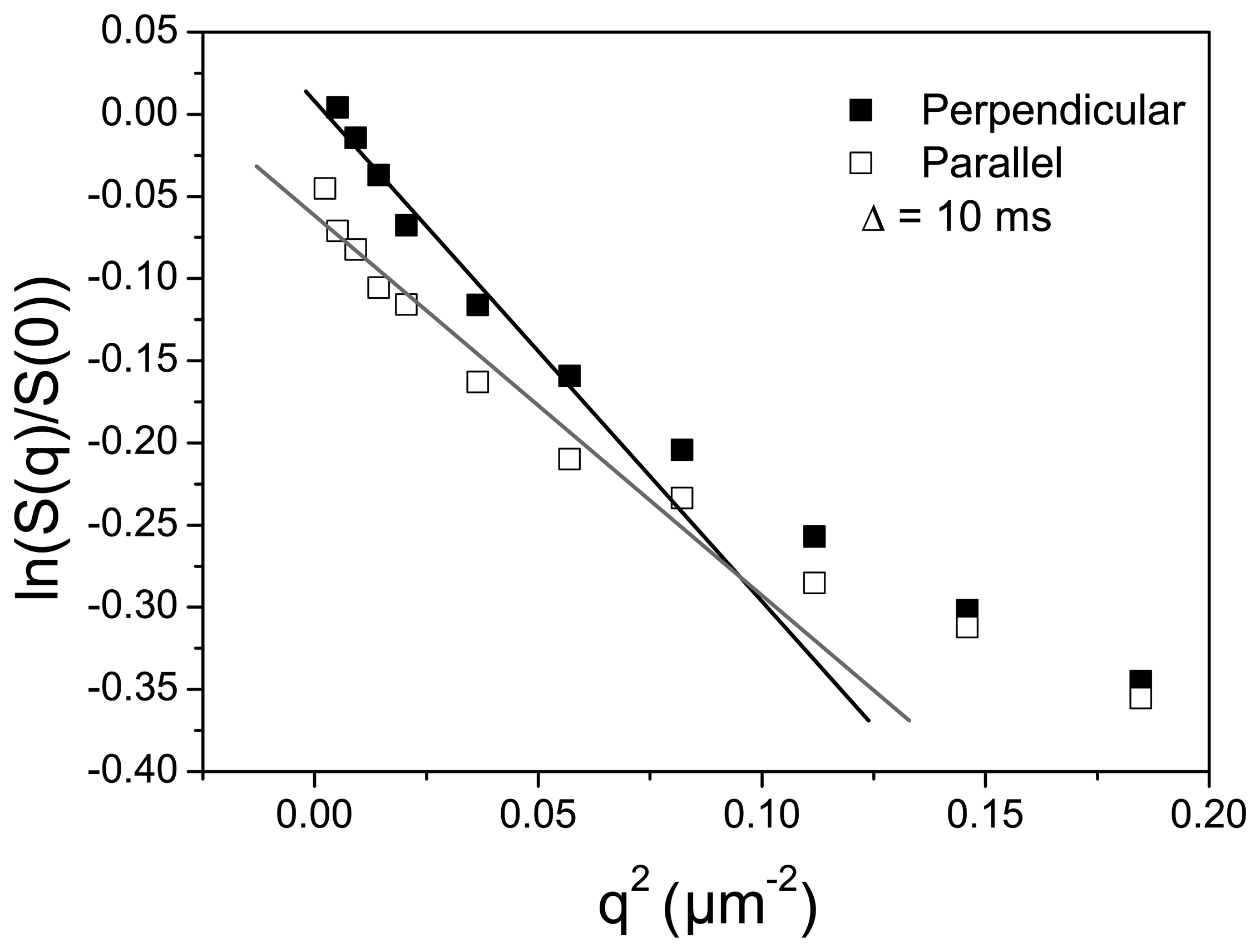
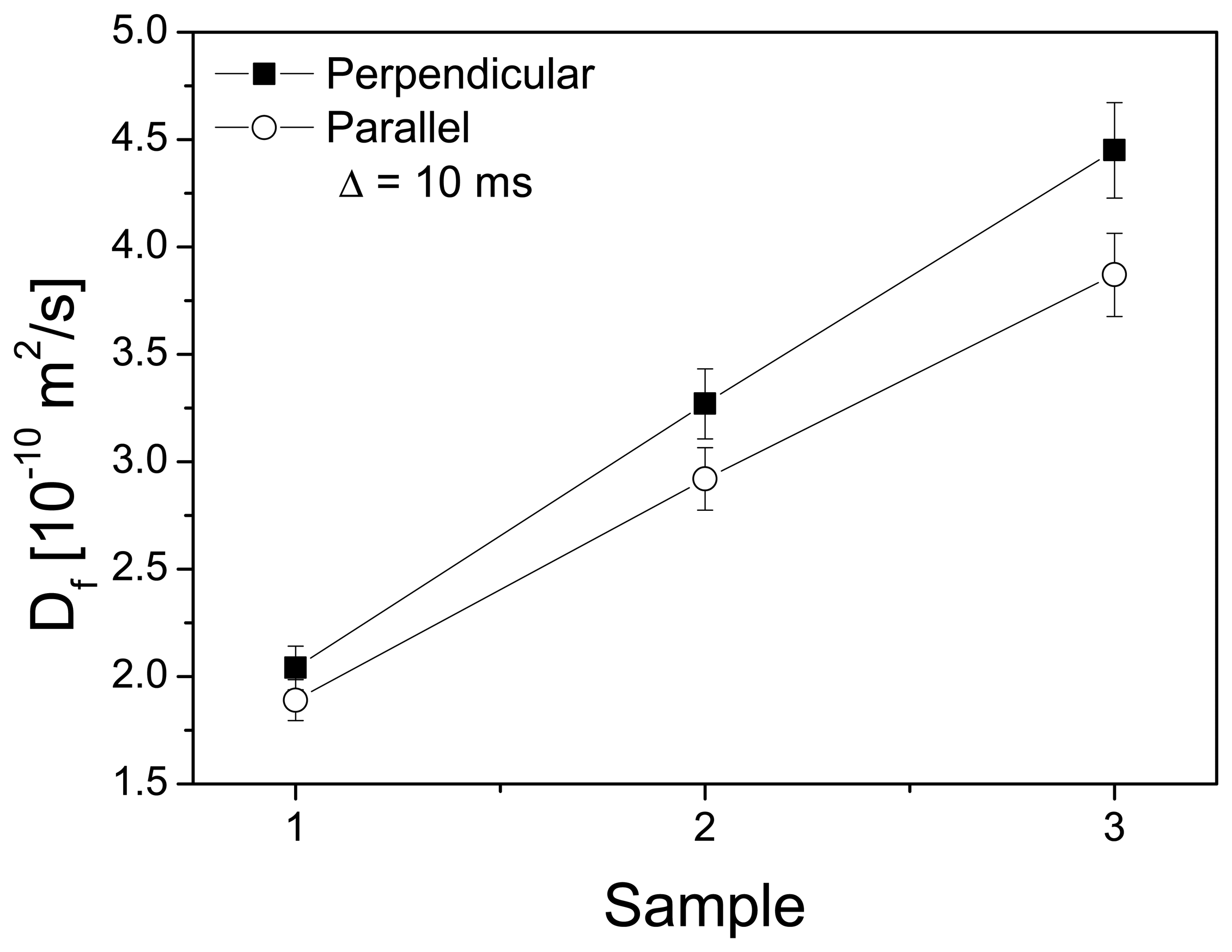
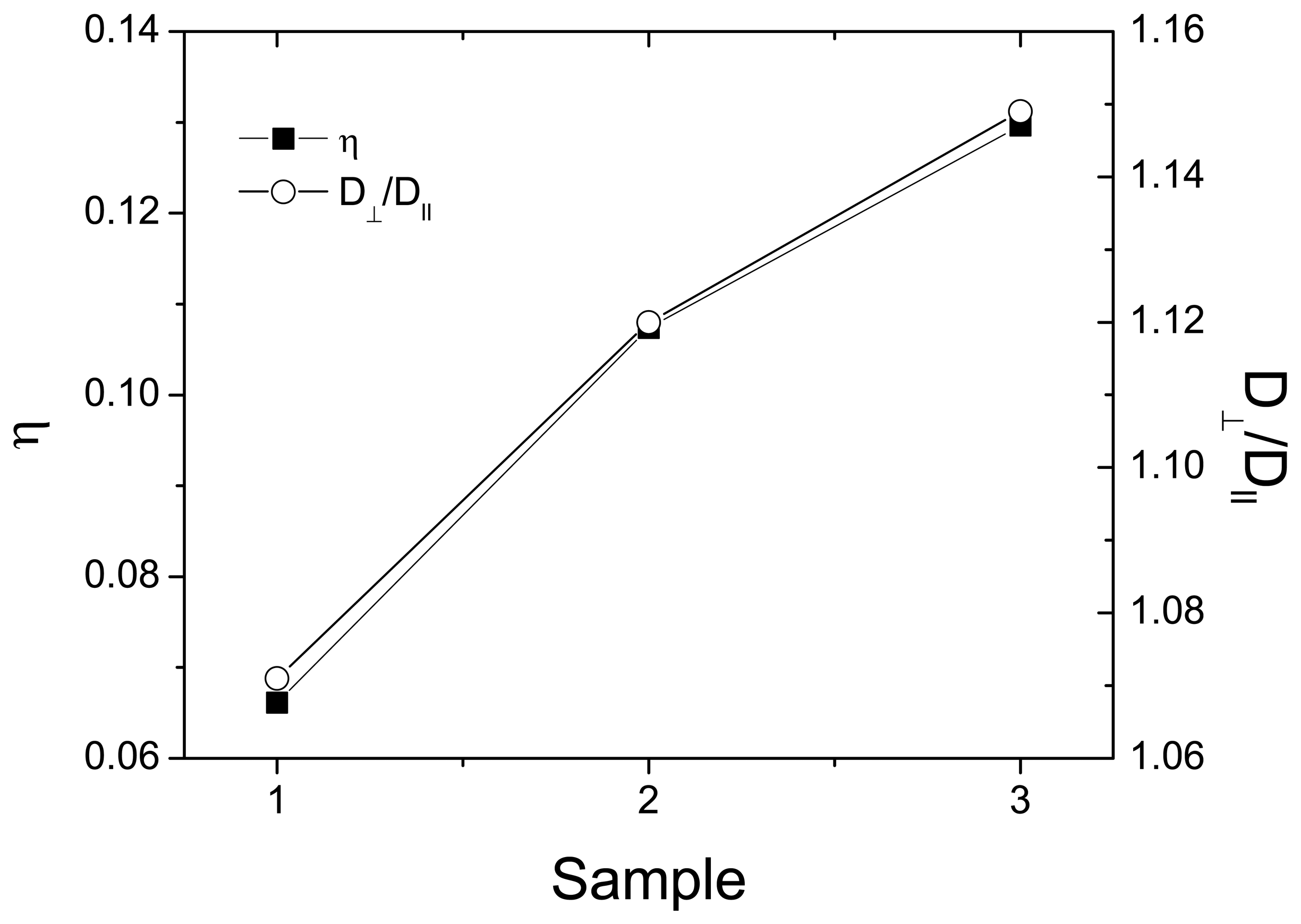
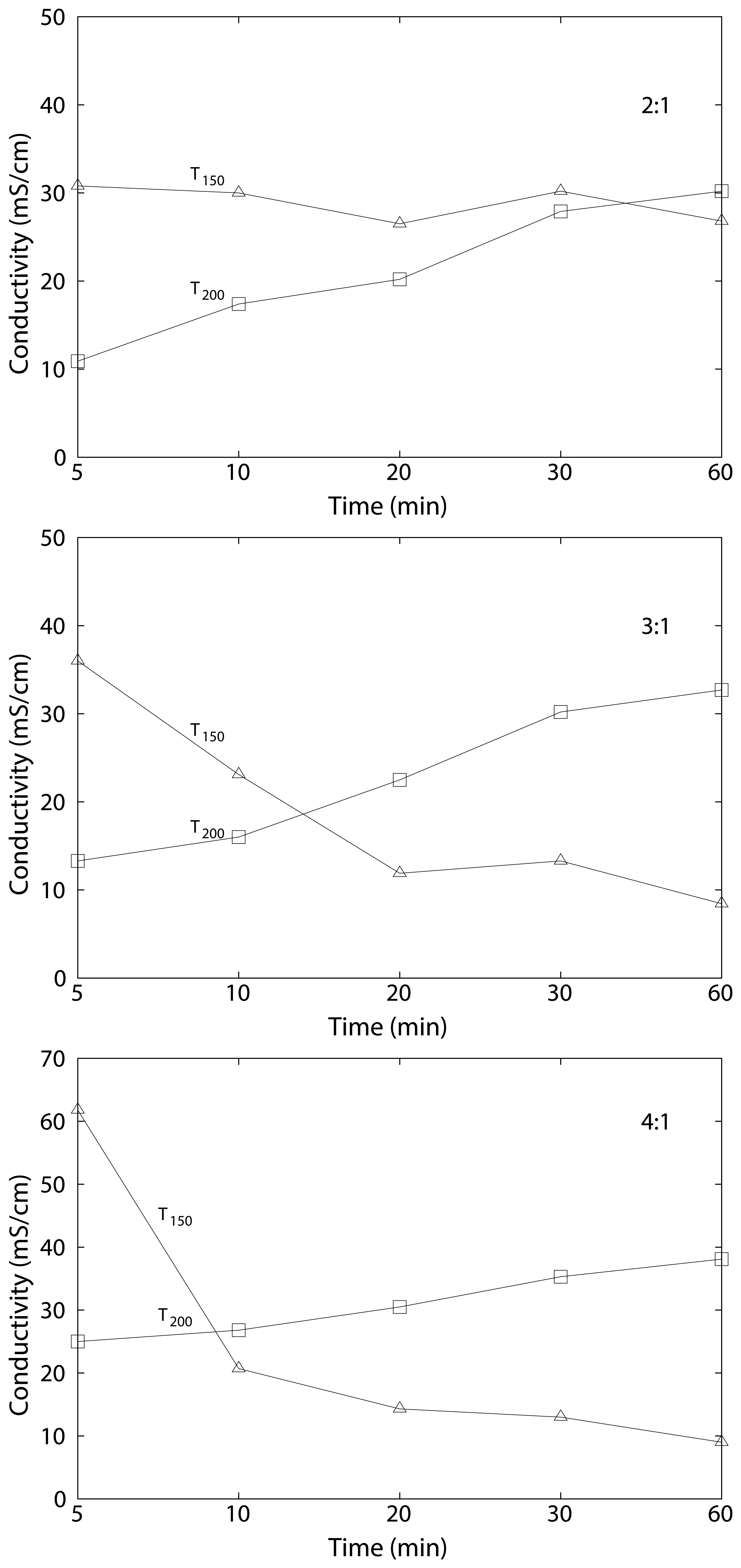
3.3. Swelling and Proton Conductivity
| 150 °C | 200 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| Swelling (%) | Proton Conductivity (mS/cm) | Swelling (%) | Proton Conductivity (mS/cm) | |||||
| 5 min | 60 min | 5 min | 60 min | 5 min | 60 min | 5 min | 60 min | |
| cSPEEK 2:1 | 24 ± 7 | 23 ± 3 | 30.8 | 26.8 | 27 ± 10 | 32 ± 2 | 10.9 | 30.2 |
| cSPEEK 3:1 | 42 ± 14 | 15 ± 1 | 36 | 8.4 | 35 ± 5 | 50 ± 8 | 13.3 | 36.3 |
| cSPEEK 4:1 | 56 ± 4 | 22 ± 3 | 61.8 | 9 | 45 ± 6 | 32 ± 1 | 25.9 | 38.1 |
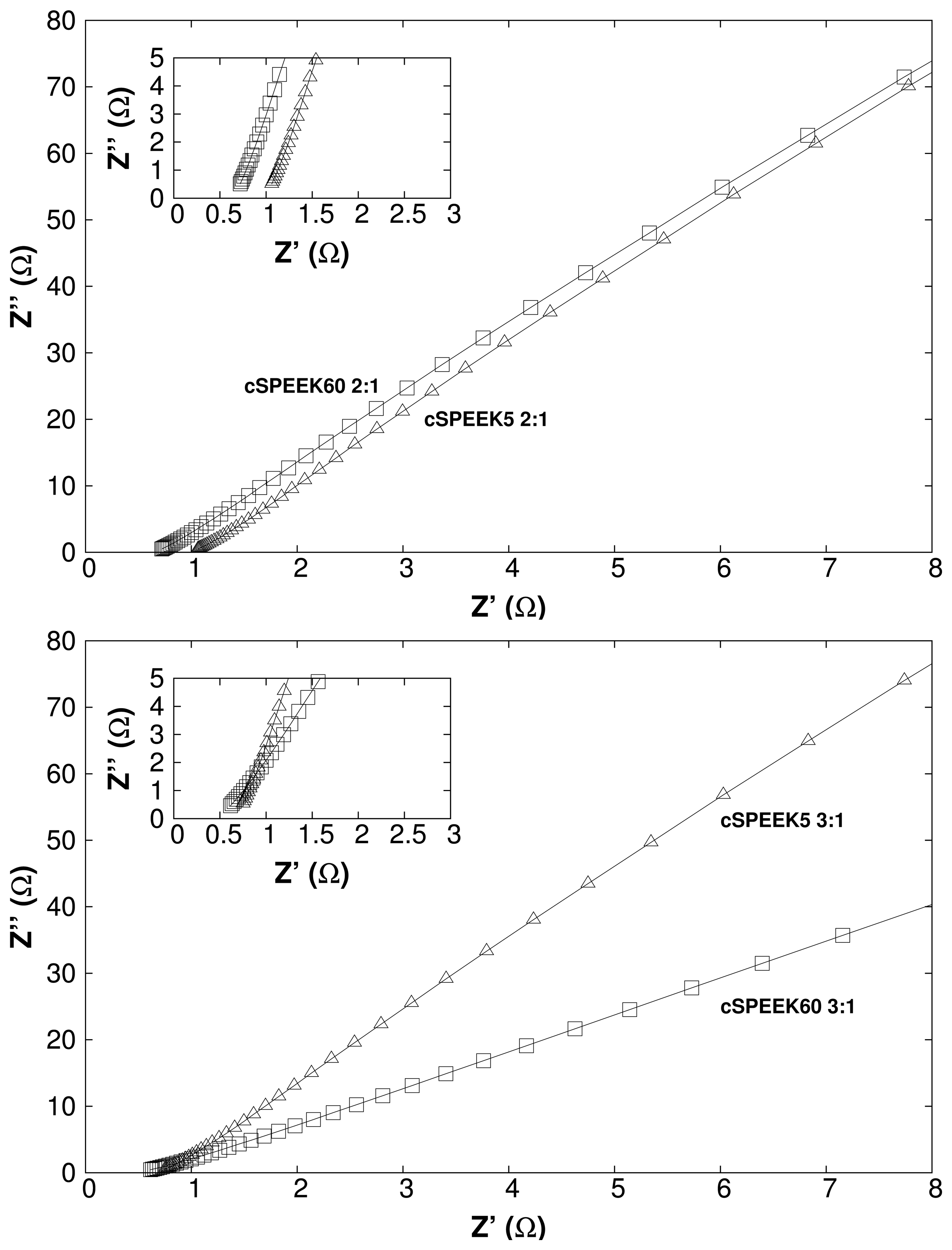


3.4. Vanadium Permeability
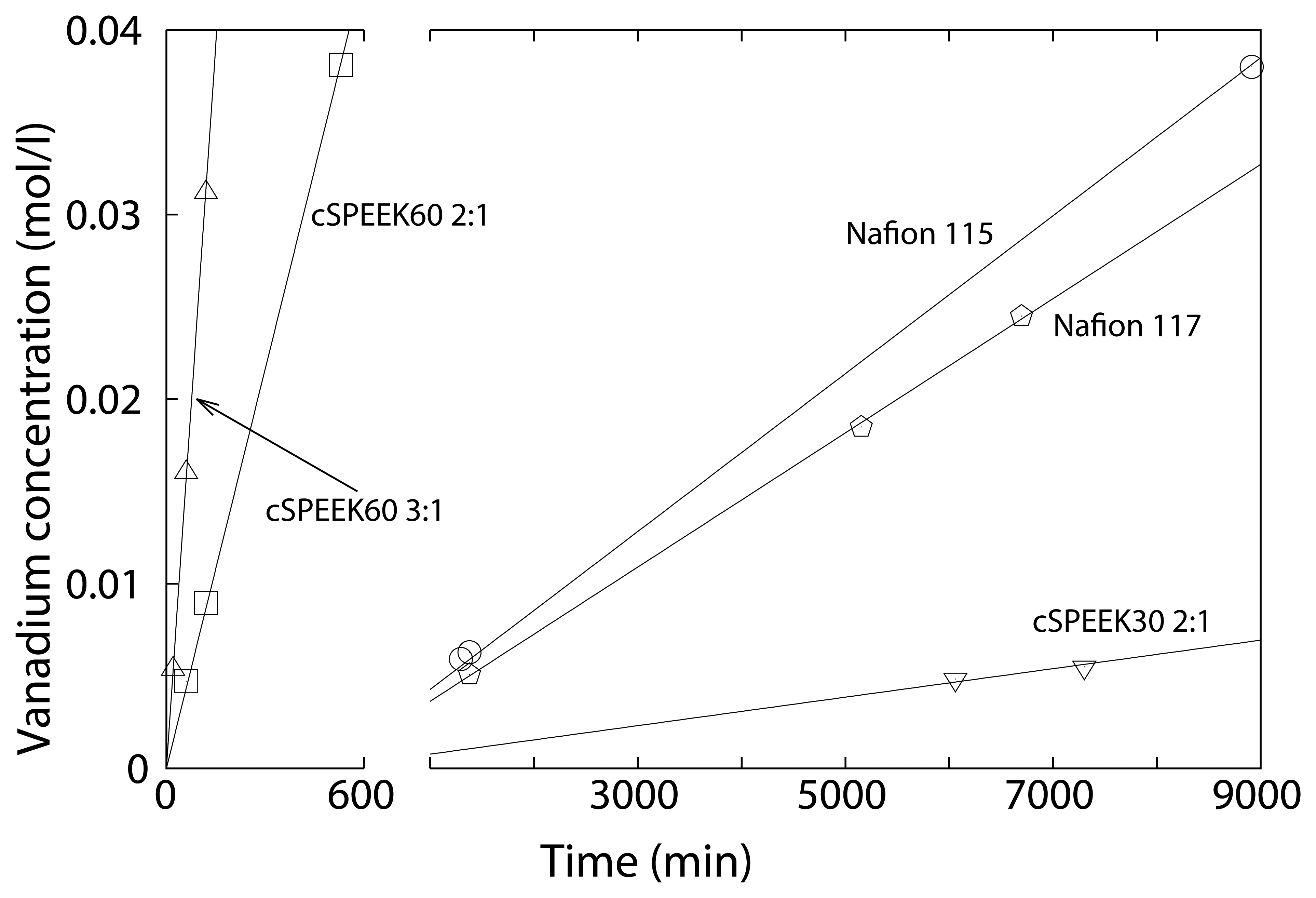
| Membranes | IEC | Permeability | |||
|---|---|---|---|---|---|
| Non-crosslinked | 150 °C | 200 °C | Non-crosslinked | 200 °C | |
| cSPEEK60 3:1 | 1.60 | 2.05 | 6.7 × 10−6 | ||
| cSPEEK60 2:1 | 1.43 | 1.97 | 4.8 × 10−6 | ||
| cSPEEK30 2:1 | 1.12 | 1.34 | 5.7 × 10−8 | ||
| Nafion 117 | 0.97 [36] | 1.9 × 10−6 | |||
| SPEEK | 2.24 | ∼ | |||
4. Conclusions
Appendix
References
- Hosseiny, S.S.; Saakes, M.; Wessling, M. A polyelectrolyte membrane based vanadium/air redox flow battery. Electrochem. Commun. 2011, 13, 751–754. [Google Scholar]
- Menictas, C.; Skyllas-Kazacos, M. Performance of vanadium-oxygen redox fuel cell. J. Appl. Electrochem. 2011, 41, 1223–1232. [Google Scholar]
- Haneko, H.; Akira, N.K.; Kanji, S.; Masato, N. Redox Battery. Eur. Patent 0517217, 9 December 1997. [Google Scholar]
- Haneko, H.; Akira, N.; Ken, N.; Kanji, S.; Masato, N. Redox Battery. U.S. Patent 5,318,865, 7 June 1994. [Google Scholar]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar]
- Hosseiny, S.S.; Wessling, M. Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Jia, C.; Liu, J.; Yan, C. A significantly improved membrane for vanadium redox flow battery. J. Power Sources 2010, 195, 4380–4383. [Google Scholar]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar]
- Luo, Q.; Zhang, H.; Chen, J.; You, D.; Sun, C.; Zhang, Y. Preparation and characterization of Nafion/SPEEK layered composite membrane and its application in vanadium redox flow battery. J. Membr. Sci. 2008, 325, 553–558. [Google Scholar]
- Daoust, D.; Devaux, J.; Godard, P. Part 3. General kinetic model of the sulfonation of PEEK fluoroarylketone chain-end repeat unit. Polym. Int. 2001, 50, 932–936. [Google Scholar]
- Shibuyal, N.; Porter, R.S. Kinetics of PEEK sulfonation in concentrated sulfuric acid. Macromolecules 1992, 25, 6495–6499. [Google Scholar]
- Zeng, J.; Jiang, C.; Wang, Y.; Chen, J.; Zhu, S.; Zhao, B.; Wang, R. Studies on polypyrrole modified nafion membrane for vanadium redox flow battery. Electrochem. Commun. 2008, 10, 372–375. [Google Scholar]
- Luu, D.X.; Kim, D. Strontium cross–linked sPEEK proton exchange membranes for fuel cell. Solid State Ionics 2011, 192, 627–631. [Google Scholar]
- Xuea, Y.; Fub, R.; Wub, C.; Leeb, J.Y. Acid–base hybrid polymer electrolyte membranes based on SPEEK. J. Membr. Sci. 2010, 350, 148–153. [Google Scholar]
- Kerres, J.; Ullrich, A.; Meier, F.; Häring, T. Synthesis and characterization of novel acid–base polymer blends for application in membrane fuel cells. Solid State Ionics 1999, 125, 243–249. [Google Scholar]
- Chen, J.; Maekawaa, Y.; Asanoa, M.; Yoshidaa, M. Double crosslinked polyetheretherketone-based polymer electrolyte membranes prepared by radiation and thermal crosslinking techniques. Polymer 2007, 48, 6002–6009. [Google Scholar]
- Vona, M.L.D.; Sgreccia, E.; Licoccia, S.; Alberti, G.; Torte, L.; Knauth, P. Analysis of temperature-promoted and solvent-assisted cross-linking in sulfonated Poly(ether ether ketone) (SPEEK) proton-conducting membranes. J. Phys. Chem. 2009, 113, 7505–7512. [Google Scholar]
- Yea, Y.S.; Yena, Y.C.; Chenga, C.C.; Chenb, W.Y.; Tsaib, L.T.; Changa, F.C. Sulfonated poly(ether ether ketone) membranes crosslinked with sulfonic acid containing benzoxazine monomer as proton exchange membranes. Polymer 2009, 50, 3196–3203. [Google Scholar]
- Deb, P.C.; Rajput, L.D.; Hande, V.R.; Sasane, S.; Kumar, A. Modification of sulfonated poly(ether ether ketone) with phenolic resin. Polym. Adv. Technol. 2007, 18, 419–426. [Google Scholar]
- Fenga, S.; Shanga, Y.; Wanga, Y.; Liua, G.; Xiea, X.; Donga, W.; Xua, J.; Mathurb, V.K. Synthesis and crosslinking of hydroxyl-functionalized sulfonated poly(ether ether ketone) copolymer as candidates for proton exchange membranes. J. Membr. Sci. 2010, 352, 14–21. [Google Scholar]
- Hande, V.R.; Rao, S.; Rath, S.K.; Thakur, A.; Patri, M. Crosslinking of sulphonated poly (ether ether ketone) using aromatic bis(hydroxymethyl) compound. J. Membr. Sci. 2008, 322, 67–73. [Google Scholar]
- Linkous, C.A.; Rhoden, S.L.; Linkous, W.G.; Pearman, B.P.; Mohajeri, N. Water uptake and conductivity of cross-linked SPEEK membranes. ECS Trans. 2008, 16, 705–710. [Google Scholar]
- Rhoden, S.L.N.H.; Linkous, C.A.; Mohajeri, N.; Díaz, D.J.; Brooker, P.; Slattery, D.K.; Fenton, J.M. Low equivalent weight Friedel-Crafts cross-linked sulfonated poly(ether ether ketone). J. Membr. Sci. 2011, 376, 290–301. [Google Scholar]
- Yao, H.; Zhu, J.; McKinney, M.A.; Wilkie, C.A. Cross-Linking of polystyrene by friedel-crafts chemistry: Multifunctional additives. J. Vinyl Addit. Technol. 2000, 6, 205–210. [Google Scholar]
- Compan, V.; Riande, E.; Fernandez-Carretero, F.; Berezina, N.; Sytcheva, A.R. Influence of polyaniline intercalations on the conductivity and permselectivity of perfluorinated cation-exchange membranes. J. Membr. Sci. 2008, 318, 255–263. [Google Scholar]
- Mohammadi, T.; Skyllas-Kazacos, M. Preparation of sulfonated composite membrane for vanadium redox flow battery applications. J. Membr. Sci. 1995, 107, 35–45. [Google Scholar]
- Luo, X.; Lu, Z.; Xi, J.; Wu, Z.; Zhu, W.; Chen, L.; Qiu, X. Influences of permeation of vanadium ions through PVDF-g-PSSA membranes on performances of vanadium redox flow batteries. J. Phys. Chem. B 2005, 109, 20310–20314. [Google Scholar]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar]
- Deb, P.C.; Rajput, L.D.; Hande, V.R.; Sasane, S.; Kumar, A. Modification of sulfonated poly(ether ether ketone) with phenolic resin. Polym. Adv. Technol. 2007, 18, 419–426. [Google Scholar]
- Stejkal, E.; Tanner, J. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar]
- Murray, K.A.; Holmes, A.B.; Moratti, S.C.; Rumbles, G. Conformational changes in regioregular polythiophenes due to crosslinking. J. Mater. Chem. 1999, 9, 2109–2116. [Google Scholar]
- Masamitsu, S.; Satoshi, M.; Haruyuki, O.; Masahiro, T. Photo-cross-linkable polymers with thermally degradable property. Chem. Mater. 2002, 14, 334–340. [Google Scholar]
- Okamura, H.; Takatori, Y.; Tsunooka, M.; Shirai, M. Synthesis of random and block copolymers of styrene and styrenesulfonic acid with low polydispersity using nitroxide-mediated living radical polymerization technique. Polymer 2002, 43, 3155–3162. [Google Scholar]
- Li, J.; Wilmsmeyer, K.G.; Madsen, L.A. Anisotropic diffusion and morphology in perfluorosulfonate ionomers investigated by NMR. Macromolecules 2008, 42, 255–262. [Google Scholar]
- Filipoi, C.; Demco, D.E.; Zhu, X.; Vinokur, R.; Conradi, O.; Fechete, R.; Möller, M. Channel orientation anisotropy in perfluorosulfonic acid/SiO2 composite proton exchange membranes: Water self-diffusion study using NMR. Chem. Phys. Lett. 2011, 513, 251–255. [Google Scholar]
- Xi, J.; Wu, Z.; Qiu, X.; Chen, L. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Merle, G.; Ioana, F.C.; Demco, D.E.; Saakes, M.; Hosseiny, S.S. Friedel–Crafts Crosslinked Highly Sulfonated Polyether Ether Ketone (SPEEK) Membranes for a Vanadium/Air Redox Flow Battery. Membranes 2014, 4, 1-19. https://doi.org/10.3390/membranes4010001
Merle G, Ioana FC, Demco DE, Saakes M, Hosseiny SS. Friedel–Crafts Crosslinked Highly Sulfonated Polyether Ether Ketone (SPEEK) Membranes for a Vanadium/Air Redox Flow Battery. Membranes. 2014; 4(1):1-19. https://doi.org/10.3390/membranes4010001
Chicago/Turabian StyleMerle, Géraldine, Filipoi Carmen Ioana, Dan Eugen Demco, Michel Saakes, and Seyed Schwan Hosseiny. 2014. "Friedel–Crafts Crosslinked Highly Sulfonated Polyether Ether Ketone (SPEEK) Membranes for a Vanadium/Air Redox Flow Battery" Membranes 4, no. 1: 1-19. https://doi.org/10.3390/membranes4010001