From Immunologically Archaic to Neoteric Glycovaccines
Abstract
:1. Introduction
2. Pure Polysaccharide Vaccines
3. Protein Conjugate Vaccines
4. Multiple Causes May Prevent the Efficacy of Bacterial Vaccines
5. The Immune System Comes of Age and Senesces
6. Herd Immunity
7. Bacterial Genomics and Proteomics (Sero- and Resistance-Typing)
8. Cell Wall Polysaccharides and Serotype Replacement
9. Needs for Novel Bacterial Polysaccharide Vaccines
10. Future Directions for Glycovaccines
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grabenstein, J.D.; Klugman, K.P. A century of pneumococcal vaccination research in humans. Clin. Microbiol. Infect. 2012, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lesinski, G.B.; Westerink, M.A. Vaccines against polysaccharide antigens. Curr. Drug Targets Infect. Disord. 2001, 1, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A. Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr. Top. Med. Chem. 2008, 8, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D. Conjugate vaccines. Clin. Exp. Immunol. 2000, 119, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Zutshi, S.; Prashanth, K.S.; Saikia, K.K.; Kumar, P. Identification of potential vaccine candidates against Streptococcus pneumoniae by reverse vaccinology approach. Appl. Biochem. Biotechnol. 2014, 172, 3026–3041. [Google Scholar] [CrossRef] [PubMed]
- Malley, R.; Lipsitch, M.; Stack, A.; Saladino, R.; Fleisher, G.; Pelton, S.; Thompson, C.; Briles, D.; Anderson, P. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 2001, 69, 4870–4873. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat. Rev. Microbiol. 2007, 5, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.J.; Yates, L.E.; Terra, V.S.; Cuccui, J.; Wren, B.W. Recombinant expression of Streptococcus pneumoniae capsular polysaccharides in Escherichia coli. Open Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Den Reijer, P.M.; Lemmens-den Toom, N.; Kant, S.; Snijders, S.V.; Boelens, H.; Tavakol, M.; Verkaik, N.J.; van Belkum, A.; Verbrugh, H.A.; van Wamel, W.J.B. Characterization of the Humoral Immune Response during Staphylococcus aureus Bacteremia and Global Gene Expression by Staphylococcus aureus in Human Blood. PLoS ONE 2013, 8, e53391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.A.; Bundle, D.R. Designing a new antifungal glycoconjugate vaccine. Chem. Soc. Rev. 2013, 42, 4327–4344. [Google Scholar] [CrossRef] [PubMed]
- Nitz, M.; Ling, C.-C.; Otter, A.; Cutler, J.E.; Bundle, D.R. The unique solution structure and immunochemistry of the Candida albicans beta-1,2-mannopyranan cell wall antigens. J. Biol. Chem. 2002, 277, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Haji-Ghassemi, O.; Blackler, R.J.; Martin Young, N.; Evans, S.V. Antibody recognition of carbohydrate epitopes. Glycobiology 2015, 25, 920–952. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Bortnick, A.; Allman, D. What is and what should always have been: Long-lived plasma cells induced by T cell-independent antigens. J. Immunol. 2013, 190, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H. The logic of automated glycan assembly. Acc. Chem. Res. 2015, 48, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.C.; Martín-Lomas, M.; Penadés, S. Glyconanotechnology. Chem. Soc. Rev. 2013, 42, 4358–4376. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Nilo, A.; Castagner, B.; Boutureira, O.; Berti, F.; Bernardes, G.J.L. Synthetically defined glycoprotein vaccines: Current status and future directions. Chem. Sci. 2013, 4, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Villiers, C.; Freitas, H.; Couderc, R.; Villiers, M.-B.; Marche, P. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanopart. Res. 2010, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Pfrengle, F.; Rademacher, C.; Nycholat, C.M.; Gale, A.J.; von Drygalski, A.; Paulson, J.C. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Investig. 2013, 123, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Bird, L. B cell tolerance: STALling B cell responses. Nat. Rev. Immunol. 2013, 13, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Poe, J.C.; Tedder, T.F. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012, 33, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, L. CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance. Glycobiology 2014, 24, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Nitschke, L. The role of CD22 and Siglec-G in B-cell tolerance and autoimmune disease. Nat. Rev. Rheumatol. 2014, 10, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Hubben, G.A.A.; Bos, J.M.; Glynn, D.M.; van der Ende, A.; van Alphen, L.; Postma, M.J. Enhanced decision support for policy makers using a web interface to health-economic models—Illustrated with a cost-effectiveness analysis of nation-wide infant vaccination with the 7-valent pneumococcal conjugate vaccine in the Netherlands. Vaccine 2007, 25, 3669–3678. [Google Scholar] [CrossRef]
- Simon, R.; Levine, M.M. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum. Vaccines Immunother. 2012, 8, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.J.; O’Shaughnessy, C.M.; Siggins, M.K.; Bobat, S.; Kingsley, R.A.; Goulding, D.A.; Crump, J.A.; Reyburn, H.; Micoli, F.; Dougan, G.; et al. Differential Killing of Salmonella enterica Serovar Typhi by Antibodies Targeting Vi and Lipopolysaccharide O:9 Antigen. PLoS ONE 2016, 11, e0145945. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Johnson, H.L.; Nonyane, B.A.S.; Deloria-Knoll, M.; O’Brien, K.L.; AGEDD Adult Pneumococcal Burden Study Team; Andreo, F.; Beovic, B.; Blanco, S.; Boersma, W.G.; et al. Estimating the burden of pneumococcal pneumonia among adults: A systematic review and meta-analysis of diagnostic techniques. PLoS ONE 2013, 8, e60273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, C.; Anderson, R. Review: Current and new generation pneumococcal vaccines. J. Infect. 2014, 69, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Gamez, G.; Hammerschmidt, S. Combat pneumococcal infections: Adhesins as candidates for protein-based vaccine development. Curr. Drug Targets 2012, 13, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Terao, Y.; Mori-Yamaguchi, Y.; Domon, H.; Sakaue, Y.; Yagi, T.; Nishino, K.; Yamaguchi, A.; Nizet, V.; Kawabata, S. Streptococcus pneumoniae invades erythrocytes and utilizes them to evade human innate immunity. PLoS ONE 2013, 8, e77282. [Google Scholar] [CrossRef] [PubMed]
- Linterman, M.A.; Liston, A.; Vinuesa, C.G. T-follicular helper cell differentiation and the co-option of this pathway by non-helper cells. Immunol. Rev. 2012, 247, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Malito, E.; Bursulaya, B.; Chen, C.; Lo Surdo, P.; Picchianti, M.; Balducci, E.; Biancucci, M.; Brock, A.; Berti, F.; Bottomley, M.J.; et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM194. Proc. Natl. Acad. Sci. USA 2012, 109, 5229–5234. [Google Scholar] [CrossRef]
- Knuf, M.; Kowalzik, F.; Kieninger, D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine 2011, 29, 4881–4890. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.B.; Liao, H.-X.; Moody, M.A.; Kepler, T.B.; Alam, S.M.; Gao, F.; Wiehe, K.; Trama, A.M.; Jones, K.; Zhang, R.; et al. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science 2015. [Google Scholar] [CrossRef] [PubMed]
- Makwana, N.; Riordan, F.A.I. Bacterial meningitis: The impact of vaccination. CNS Drugs 2007, 21, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Glycoconjugate vaccines. Expert Opin. Biol. Ther. 2013, 13, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Jennings, H. Further approaches for optimizing polysaccharide-protein conjugate vaccines for prevention of invasive bacterial disease. J. Infect. Dis. 1992, 165, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.W.; Pichichero, M.E.; Insel, R.A.; Betts, R.; Eby, R.; Smith, D.H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: Structural and temporal requirements for priming in the human infant. J. Immunol. 1986, 137, 1181–1186. [Google Scholar] [PubMed]
- Arroabarren, E.; Anda, M.; Sanz, M.L. Anaphylaxis to pneumococcal vaccine; CRM(197): Novel cause of vaccine allergy. Pediatr. Allergy Immunol. 2016, 27, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Ponvert, C.; Scheinmann, P.; de Blic, J. Anaphylaxis to the 23-valent pneumococcal vaccine: A second ′explored case by means of immediate-reading skin tests with pneumococcal vaccines. Vaccine 2010, 28, 8256–8257. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, M.; Stallforth, P.; Kalinichenko, A.; Rathwell, D.C.K.; Gronewold, T.M.A.; Adibekian, A.; Mori, L.; Landmann, R.; Seeberger, P.H.; De Libero, G. A semisynthetic carbohydrate-lipid vaccine that protects against S. pneumoniae in mice. Nat. Chem. Biol. 2014, 10, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Tang, C.-W.; Daniels, N.J.; Compton, B.J.; Hayman, C.M.; Johnston, K.A.; Knight, D.A.; Gasser, O.; Poyntz, H.C.; Ferguson, P.M.; et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 2014, 10, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Compton, B.J.; Tang, C.; Authier-Hall, A.; Hayman, C.M.; Swinerd, G.W.; Kowalczyk, R.; Harris, P.; Brimble, M.A.; Larsen, D.S.; et al. NKT cell-dependent glycolipid–peptide vaccines with potent anti-tumour activity. Chem. Sci. 2015, 6, 5120–5127. [Google Scholar] [CrossRef]
- Compton, B.J.; Tang, C.-W.; Johnston, K.A.; Osmond, T.L.; Hayman, C.M.; Larsen, D.S.; Hermans, I.F.; Painter, G.F. Synthesis and Activity of 6′′-Deoxy-6′′-thio-α-GalCer and Peptide Conjugates. Org. Lett. 2015, 17, 5954–5957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-T.; Forestier, C.; Goff, R.D.; Li, C.; Teyton, L.; Bendelac, A.; Savage, P.B. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6′′-amino-6′′-deoxy-galactosylceramides. Org. Lett. 2002, 4, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- McFetridge, R.; Meulen, A.S.-T.; Folkerth, S.D.; Hoekstra, J.A.; Dallas, M.; Hoover, P.A.; Marchese, R.D.; Zacholski, D.M.; Watson, W.J.; Stek, J.E.; et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2015, 33, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.S. Streptococcus pneumoniae Serotype Distribution and Pneumococcal Conjugate Vaccine Serotype Coverage among Pediatric Patients in East and Southeast Asia, 2000–2014: A Pooled Data Analysis. Vaccines 2016. [Google Scholar] [CrossRef] [PubMed]
- Imöhl, M.; Möller, J.; Reinert, R.; Perniciaro, S.; van der Linden, M.; Aktas, O. Pneumococcal meningitis and vaccine effects in the era of conjugate vaccination: Results of 20 years of nationwide surveillance in Germany. BMC Infect. Dis. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tonder, A.J.; Bray, J.E.; Roalfe, L.; White, R.; Zancolli, M.; Quirk, S.J.; Haraldsson, G.; Jolley, K.A.; Maiden, M.C.J.; Bentley, S.D.; et al. Genomics Reveals the Worldwide Distribution of Multidrug-Resistant Serotype 6E Pneumococci. J. Clin. Microbiol. 2015, 53, 2271–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques-Normark, B.; Tuomanen, E.I. The pneumococcus: Epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Y.-J.; Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Leitner, W.W. Adjuvants in the Driver’s Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators. Vaccines 2014, 2, 252–296. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Cohen, J.M.; Jose, R.J.; de Vogel, C.; Baxendale, H.; Brown, J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015, 8, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Nevers, E.; Verhoeven, P.; Paul, S.; Grattard, F.; Pozzetto, B.; Berthelot, P.; Lucht, F. Staphylococcal vaccine development: Review of past failures and plea for a future evaluation of vaccine efficacy not only on staphylococcal infections but also on mucosal carriage. Expert Rev. Vaccines 2013, 12, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.; Chen, A.; Hirst, T.R.; Kara, E.E.; McColl, S.R.; Ogunniyi, A.D.; Paton, J.C.; Alsharifi, M. Intranasal vaccination with γ-irradiated Streptococcus pneumoniae whole-cell vaccine provides serotype-independent protection mediated by B-cells and innate IL-17 responses. Clin. Sci. 2016, 130, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gierahn, T.; Thompson, C.M.; Trzciński, K.; Ford, C.B.; Croucher, N.; Gouveia, P.; Flechtner, J.B.; Malley, R.; Lipsitch, M. Distinct Effects on Diversifying Selection by Two Mechanisms of Immunity against Streptococcus pneumoniae. PLoS Pathog. 2012, 8, e1002989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffitt, K.L.; Gierahn, T.M.; Lu, Y.; Gouveia, P.; Alderson, M.; Flechtner, J.B.; Higgins, D.E.; Malley, R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 2011, 9, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, K.; Skoberne, M.; Howard, A.; Gavrilescu, L.C.; Gierahn, T.; Munzer, S.; Dixit, B.; Giannasca, P.; Flechtner, J.B.; Malley, R. Toll-like receptor 2-dependent protection against pneumococcal carriage by immunization with lipidated pneumococcal proteins. Infect. Immun. 2014, 82, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, B.M.; Rharbaoui, F.; Groebe, L.; Guzman, C.A. Intranasal immunization promotes th17 immune responses. J. Immunol. 2009, 183, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, B.M.; Weissmann, S.F.; Guzman, C.A. NKT Cell Stimulation with α-Galactosylceramide Results in a Block of Th17 Differentiation after Intranasal Immunization in Mice. PLoS ONE 2012, 7, e30382. [Google Scholar] [CrossRef] [PubMed]
- Bittaye, M.; Cash, P. Streptococcus pneumoniae proteomics: Determinants of pathogenesis and vaccine development. Expert Rev. Proteom. 2015, 12, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens 2016. [Google Scholar] [CrossRef] [PubMed]
- Karauzum, H.; Datta, S.K. Adaptive Immunity Against Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 2016. [Google Scholar] [CrossRef]
- De Libero, G. Tissue distribution, antigen specificity and effector functions of gamma delta T cells in human diseases. Springer Semin. Immunopathol. 2000, 22, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ulm, H.; Rausch, M.; Li, X.; O’Riordan, K.; Lee, J.C.; Schneider, T.; Müller, C.E. Analysis of the Staphylococcus aureus capsule biosynthesis pathway in vitro: Characterization of the UDP-GlcNAc C6 dehydratases CapD and CapE and identification of enzyme inhibitors. Int. J. Med. Microbiol. 2014, 304, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, S.; Radcliff, F.J.; Grumann, D.; Read, H.; Johnson, S.; Monecke, S.; Ritchie, S.; Clow, F.; Goerke, C.; Bröker, B.M.; et al. Characterization of a Mouse-Adapted Staphylococcus aureus Strain. PLoS ONE 2013, 8, e71142. [Google Scholar] [CrossRef] [PubMed]
- Calix, J.J.; Porambo, R.J.; Brady, A.M.; Larson, T.R.; Yother, J.; Abeygunwardana, C.; Nahm, M.H. Biochemical, genetic, and serological characterization of two capsule subtypes among Streptococcus pneumoniae Serotype 20 strains: Discovery of a new pneumococcal serotype. J. Biol. Chem. 2012, 287, 27885–27894. [Google Scholar] [CrossRef] [PubMed]
- Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.M.; Wang, L.; Reeves, P.R. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect. Immun. 2001, 69, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Lattar, S.M.; Noto Llana, M.; Denoël, P.; Germain, S.; Buzzola, F.R.; Lee, J.C.; Sordelli, D.O. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect. Immun. 2014, 82, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Wang, L.; Kowarik, M.; Dowd, M.; Lipowsky, G.; Faridmoayer, A.; Shields, K.; Park, S.; Alaimo, C.; Kelley, K.A.; et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 2014, 209, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Liew, Y.K.; Awang Hamat, R.; van Belkum, A.; Chong, P.P.; Neela, V. Comparative Exoproteomics and Host Inflammatory Response in Staphylococcus aureus Skin and Soft Tissue Infections, Bacteremia, and Subclinical Colonization. Clin. Vaccine Immunol. 2015, 22, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, C.; Feng, Q.; Shi, Y.; Zuo, Q.; Yang, H.; Jing, H.; Wei, C.; Zhuang, Y.; Zou, Q.; et al. Protective efficacy of the chimeric Staphylococcus aureus vaccine candidate IC in sepsis and pneumonia models. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Zhang, J.-Y.; Wei, C.; Yang, L.-Y.; Zuo, Q.-F.; Zhuang, Y.; Feng, Y.-J.; Srinivas, S.; Zeng, H.; Zou, Q.-M. Immunisation With Immunodominant Linear B Cell Epitopes Vaccine of Manganese Transport Protein C Confers Protection against Staphylococcus aureus Infection. PLoS ONE 2016, 11, e0149638. [Google Scholar] [CrossRef] [PubMed]
- Scietti, L.; Sampieri, K.; Pinzuti, I.; Bartolini, E.; Benucci, B.; Liguori, A.; Haag, A.F.; Lo Surdo, P.; Pansegrau, W.; Nardi-Dei, V.; et al. Exploring host-pathogen interactions through genome wide protein microarray analysis. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Misawa, Y.; Kelley, K.A.; Wang, X.; Wang, L.; Park, W.B.; Birtel, J.; Saslowsky, D.; Lee, J.C. Staphylococcus aureus Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins. PLoS Pathog. 2015, 11, e1005061. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, A.; Stapels, D.A.C.; Weerwind, L.T.; Ko, Y.-P.; Ruyken, M.; Lee, J.C.; van Kessel, K.P.M.; Rooijakkers, S.H.M. The S. aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis. Microbiology 2016, 162, 1185–1194. [Google Scholar] [PubMed]
- Astley, R.A.; Coburn, P.S.; Parkunan, S.M.; Callegan, M.C. Modeling intraocular bacterial infections. Prog. Retin. Eye Res. 2016, 54, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Higginson, E.E.; Simon, R.; Tennant, S.M. Animal Models for Salmonellosis: Applications in Vaccine Research. Clin. Vaccine Immunol. 2016, 23, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Missiakas, D.; Schneewind, O. Mouse models for infectious diseases caused by Staphylococcus aureus. J. Immunol. Methods 2014, 410, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Saralahti, A.; Piippo, H.; Parikka, M.; Henriques-Normark, B.; Rämet, M.; Rounioja, S. Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 2014, 42, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Chiavolini, D.; Pozzi, G.; Ricci, S. Animal models of Streptococcus pneumoniae disease. Clin. Microbiol. Rev. 2008, 21, 666–685. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Zhang, J.; Kesselly, A.; Anosova, N.G.; Lam, H.; Kleanthous, H.; Yethon, J.A. Limitations of Murine Models for Assessment of Antibody-Mediated Therapies or Vaccine Candidates against Staphylococcus epidermidis Bloodstream Infection. Infect. Immun. 2016, 84, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Gritzfeld, J.F.; Wright, A.D.; Collins, A.M.; Pennington, S.H.; Wright, A.K.A.; Kadioglu, A.; Ferreira, D.M.; Gordon, S.B. Experimental human pneumococcal carriage. J. Vis. Exp. 2013, 72, e50115. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K.A.; Bangert, M.; Gritzfeld, J.F.; Ferreira, D.M.; Jambo, K.C.; Wright, A.D.; Collins, A.M.; Gordon, S.B. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog. 2013, 9, e1003274. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Wright, A.D.; Mitsi, E.; Gritzfeld, J.F.; Hancock, C.A.; Pennington, S.H.; Wang, D.; Morton, B.; Ferreira, D.M.; Gordon, S.B. First human challenge testing of a pneumococcal vaccine. Double-blind randomized controlled trial. Am. J. Respir. Crit. Care Med. 2015, 192, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.-A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Mandl, C.W.; Black, S.; Gregorio, E. De Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2012, 11, 865–872. [Google Scholar]
- Finco, O.; Rappuoli, R. Designing Vaccines for the Twenty-First Century Society. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pringle, H. In peril. Science 2015, 348, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Lawler, A. Making contact. Science 2015, 348, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Grubeck-Loebenstein, B. Vaccines for the elderly. Clin. Microbiol. Infect. 2012, 18 (Suppl. 5), 100–108. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Montecino-Rodriguez, E.; Dorshkind, K. Effects of aging on early B- and T-cell development. Immunol. Rev. 2005, 205, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Kaufmann, G.R.; Hodgkin, P.D.; Lewin, S.R.; Kelleher, A.D.; Davenport, M.P.; Zaunders, J.J. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol. Cell Biol. 2003, 81, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.A.; Lang, P.O.; Aspinall, R. Tracing thymic output in older individuals. Clin. Exp. Immunol. 2010, 161, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Swann, J.B. Thymus involution and regeneration: Two sides of the same coin? Nat. Rev. Immunol. 2013, 13, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Youm, Y.-H.; Vandanmagsar, B.; Rood, J.; Kumar, K.G.; Butler, A.A.; Dixit, V.D. Obesity accelerates thymic aging. Blood 2009, 114, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Haj, T.; Kessel, A.; Toubi, E. Age-related autoimmunity. BMC Med. 2013, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Brezar, V.; Godot, V.; Cheng, L.; Su, L.; Lévy, Y.; Seddiki, N. T-Regulatory Cells and Vaccination “Pay Attention and Do Not Neglect Them”: Lessons from HIV and Cancer Vaccine Trials. Vaccines 2016. [Google Scholar] [CrossRef] [PubMed]
- Ademokun, A.; Wu, Y.-C.; Martin, V.; Mitra, R.; Sack, U.; Baxendale, H.; Kipling, D.; Dunn-Walters, D.K. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell 2011, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, C.; Nishimoto, N.; Gartland, G.L.; Billips, L.G.; Burrows, P.D.; Kubagawa, H.; Cooper, M.D. B cells are generated throughout life in humans. J. Immunol. 1996, 156, 866–872. [Google Scholar] [PubMed]
- Ghia, P.; Melchers, F.; Rolink, A.G. Age-dependent changes in B lymphocyte development in man and mouse. Exp. Gerontol. 2000, 35, 159–165. [Google Scholar] [CrossRef]
- Wu, Y.-C.B.; Kipling, D.; Dunn-Walters, D.K. Age-Related Changes in Human Peripheral Blood IGH Repertoire Following Vaccination. Front. Immunol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.L.; Blomberg, B.B.; Frasca, D. B cells, E2A, and aging. Immunol. Rev. 2005, 205, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.L. Impaired B lymphopoiesis in old age: A role for inflammatory B cells? Immunol. Res. 2013, 57, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Goodell, M.A. Hematopoietic stem cell aging: Wrinkles in stem cell potential. Stem Cell Rev. 2007, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Q.; Morita, Y.; Jiang, H.; Gross, A.; Lechel, A.; Hildner, K.; Guachalla, L.M.; Gompf, A.; Hartmann, D.; et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 2012, 148, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Ito, S.; Isobe, K. Loss of GADD34 induces early age-dependent deviation to the myeloid lineage. Immunol. Cell Biol. 2014, 92, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Funatsu, Y.; Oishi, K.; Akeda, Y.; Hiraoka, R.; Takeshita, K.; Asami, T.; Yagi, K.; Kimizuka, Y.; Ishii, M.; et al. Comparison of the immunogenicity and safety of polysaccharide and protein-conjugated pneumococcal vaccines among the elderly aged 80 years or older in Japan: An open-labeled randomized study. Vaccine 2015, 33, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Duraisingham, S.S.; Rouphael, N.; Cavanagh, M.M.; Nakaya, H.I.; Goronzy, J.J.; Pulendran, B. Systems biology of vaccination in the elderly. Curr. Top. Microbiol. Immunol. 2013, 363, 117–142. [Google Scholar] [PubMed]
- Esposito, S.; Principi, N. Safety and tolerability of pneumococcal vaccines in children. Expert Opin. Drug Saf. 2016, 15, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Leclerc, C.; Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004, 4, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Solvason, N.; Kearney, J.F. The human fetal omentum: A site of B cell generation. J. Exp. Med. 1992, 175, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.; Smolders, M.A.; Gemen, E.F.; Antonius, T.A.; Leuvenink, J.; de Vries, E. Development of lymphocyte subpopulations in preterm infants. Scand. J. Immunol. 2011, 73, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Boxus, M.; Lockman, L.; Fochesato, M.; Lorin, C.; Thomas, F.; Giannini, S.L. Antibody avidity measurements in recipients of Cervarix vaccine following a two-dose schedule or a three-dose schedule. Vaccine 2014, 32, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.M.; El-Kady, E.M.; Eissa, S.A.; Wahby, A.F. Assessment of antibody level and avidity against Bordetella pertussis in a cohort of Egyptian individuals aged 1–18 years. J. Adv. Res. 2016, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Usinger, W.R.; Lucas, A.H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 1999, 67, 2366–2370. [Google Scholar] [PubMed]
- Van Westen, E.; Rodenburg, G.D.; van Gils, E.J.; Tcherniaeva, I.; Berbers, G.A.; Cowell, L.; Goldblatt, D.; Rots, N.Y.; van den Dobbelsteen, G.P.; Sanders, E.A. Levels and functionality of antibodies after pneumococcal conjugate vaccine in schedules with different timing of the booster dose. Vaccine 2013, 31, 5834–5842. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Vaz, A.R.; Miller, E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 1998, 177, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.J.E.; Ferrari, M.; Graham, A.L.; Grenfell, B.T. Understanding Herd Immunity. Trends Immunol. 2015, 36, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Vaccination and herd immunity to infectious diseases. Nature 1985, 318, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G. Herd immunity: Recent uses in vaccine assessment. Expert Rev. Vaccines 2008, 7, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Milwid, R.; Steriu, A.; Arino, J.; Heffernan, J.; Hyder, A.; Schanzer, D.; Gardner, E.; Haworth-Brockman, M.; Isfeld-Kiely, H.; Langley, J.M.; et al. Toward Standardizing a Lexicon of Infectious Disease Modeling Terms. Front. Public Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Lessler, J.; Cummings, D.A.T. Mechanistic Models of Infectious Disease and Their Impact on Public Health. Am. J. Epidemiol. 2016, 183, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Quadri-Sheriff, M.; Hendrix, K.S.; Downs, S.M.; Sturm, L.A.; Zimet, G.D.; Finnell, S.M.E. The role of herd immunity in parents’ decision to vaccinate children: A systematic review. Pediatrics 2012, 130, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Khandaker, G.; Booy, R. Vaccination and herd immunity: What more do we know? Curr. Opin. Infect. Dis. 2012, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Graeden, E.; Fielding, R.; Steinhouse, K.E.; Rubin, I.N. Modeling the Effect of Herd Immunity and Contagiousness in Mitigating a Smallpox Outbreak. Med. Decis. Mak. 2015, 35, 648–659. [Google Scholar] [CrossRef] [PubMed]
- A call to arms. Nat. Rev. Drug Discov. 2007, 6, 8–12.
- 2013 Runners-Up. Your microbes, your health. Science 2013. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Ramsay, M.E. Editorial Commentary: The Story of Sisyphus: Why We Need a Universal Pneumococcal Vaccine to Replace Current Conjugate Vaccines. Clin. Infect. Dis. 2015, 61, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.; Minamisava, R.; Policena, G.; Cristo, E.B.; Domingues, C.M.S.; de Cunto Brandileone, M.C.; Almeida, S.C.G.; Toscano, C.M.; Bierrenbach, A.L. Evaluating the impact of PCV-10 on invasive pneumococcal disease in Brazil: A time-series analysis. Hum. Vaccines Immunother. 2016, 12, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, C.; Bewick, T.; Sheppard, C.; Greenwood, S.; Mckeever, T.M.; Trotter, C.L.; Slack, M.; George, R.; Lim, W.S. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur. Respir. J. 2015, 45, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Grau, I.; Ardanuy, C.; Cubero, M.; Benitez, M.A.; Liñares, J.; Pallares, R. Declining mortality from adult pneumococcal infections linked to children’s vaccination. J. Infect. 2016, 72, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Jauneikaite, E.; Tocheva, A.S.; Jefferies, J.M.C.; Gladstone, R.A.; Faust, S.N.; Christodoulides, M.; Hibberd, M.L.; Clarke, S.C. Current methods for capsular typing of Streptococcus pneumoniae. J. Microbiol. Methods 2015, 113, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Chochua, S.; Satzke, C.; Dunne, E.M.; Mulholland, K.; Klugman, K.P.; Vidal, J.E. Single-plex quantitative assays for the detection and quantification of most pneumococcal serotypes. PLoS ONE 2015, 10, e0121064. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; van Belkum, A.; Sluijter, M.; Luijendijk, A.; de Groot, R.; Rümke, H.C.; Verbrugh, H.A.; Hermans, P.W.M. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004, 363, 1871–1872. [Google Scholar] [CrossRef]
- Croucher, N.J.; Finkelstein, J.A.; Pelton, S.I.; Mitchell, P.K.; Lee, G.M.; Parkhill, J.; Bentley, S.D.; Hanage, W.P.; Lipsitch, M. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat. Genet. 2013, 45, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chewapreecha, C.; Harris, S.R.; Croucher, N.J.; Turner, C.; Marttinen, P.; Cheng, L.; Pessia, A.; Aanensen, D.M.; Mather, A.E.; Page, A.J.; et al. Dense genomic sampling identifies highways of pneumococcal recombination. Nat. Genet. 2014, 46, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Straume, D.; Stamsås, G.A.; Håvarstein, L.S. Natural transformation and genome evolution in Streptococcus pneumoniae. Infect. Genet. Evol. 2015, 33, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Claverys, J.-P.; Prudhomme, M.; Martin, B. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 2006, 60, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Hanage, W.P. Interim results of an ecological experiment—Conjugate vaccination against the pneumococcus and serotype replacement. Hum. Vaccines Immunother. 2016, 12, 358–374. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Barquist, L.; Parkhill, J.; Bentley, S.D. A high-resolution view of genome-wide pneumococcal transformation. PLoS Pathog. 2012, 8, e1002745. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Harris, S.R.; Fraser, C.; Quail, M.A.; Burton, J.; van der Linden, M.; McGee, L.; von Gottberg, A.; Song, J.H.; Ko, K.S.; et al. Rapid pneumococcal evolution in response to clinical interventions. Science 2011, 331, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Olarte, L.; Barson, W.J.; Barson, R.M.; Lin, P.L.; Romero, J.R.; Tan, T.Q.; Givner, L.B.; Bradley, J.S.; Hoffman, J.A.; Hultén, K.G.; et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin. Infect. Dis. 2015, 61, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.R.; Penman, B.S.; Lourenço, J.; Buckee, C.O.; Maiden, M.C.J.; Gupta, S. Vaccination Drives Changes in Metabolic and Virulence Profiles of Streptococcus pneumoniae. PLoS Pathog. 2015, 11, e1005034. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, M.; Falkenhorst, G.; Perniciaro, S.; Imöhl, M. Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany. PLoS ONE 2015, 10, e0131494. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Agudelo, C.L.; Jordan-Villegas, A.; Garcia, C.; McCracken, G.H. The Effect of 13-Valent Pneumococcal Conjugate Vaccine on the Serotype Distribution and Antibiotic Resistance Profiles in Children With Invasive Pneumococcal Disease. J. Pediatr. Infect. Dis. Soc. 2016. [Google Scholar] [CrossRef]
- McDaniel, L.S.; Swiatlo, E. Should Pneumococcal Vaccines Eliminate Nasopharyngeal Colonization? mBio 2016. [Google Scholar] [CrossRef] [PubMed]
- Reiss-Mandel, A.; Regev-Yochay, G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: Myth or reality? Hum. Vaccines Immunother. 2016, 12, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.R.; Wright, L.; Clarke, S.R.; Moseby, H.; Tarkowski, A.; Vendrengh, M.; Foster, S.J. Identification of conserved antigens from staphylococcal and streptococcal pathogens. J. Med. Microbiol. 2012, 61, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.W.; Sever, J.L. Group B Streptococcus and pregnancy: A review. Am. J. Obstet. Gynecol. 2008, 198, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.L.; Avci, F.Y.; Kasper, D.L. A maternal vaccine against group B Streptococcus: Past, present, and future. Vaccine 2013, 31, D13–D19. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.A.; Padbury, J.F. Neonatal sepsis: An old problem with new insights. Virulence 2014, 5, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hong, S.-W.; Han, D.; Yi, J.; Jung, J.; Yang, B.-G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016. [Google Scholar] [CrossRef] [PubMed]
- Wesemann, D.R.; Portuguese, A.J.; Meyers, R.M.; Gallagher, M.P.; Cluff-Jones, K.; Magee, J.M.; Panchakshari, R.A.; Rodig, S.J.; Kepler, T.B.; Alt, F.W. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 2013, 501, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.S. Anti-endotoxin vaccines: Back to the future. Virulence 2014, 5, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Baker, S. A return to the pre-antimicrobial era? Science 2015, 347, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Viale, P.; Giannella, M.; Lewis, R.; Trecarichi, E.M.; Petrosillo, N.; Tumbarello, M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev. Anti. Infect. Ther. 2013, 11, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Guerry, P.; Poly, F.; Riddle, M.; Maue, A.C.; Chen, Y.-H.; Monteiro, M.A. Campylobacter polysaccharide capsules: Virulence and vaccines. Front. Cell. Infect. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Maue, A.C.; Poly, F.; Guerry, P. A capsule conjugate vaccine approach to prevent diarrheal disease caused by Campylobacter jejuni. Hum. Vaccines Immunother. 2014, 10, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Tubo, N.J.; Fife, B.T.; Pagan, A.J.; Kotov, D.I.; Goldberg, M.F.; Jenkins, M.K. Most microbe-specific naïve CD4+ T cells produce memory cells during infection. Science 2016, 351, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Lagousi, T.; Routsias, J.; Piperi, C.; Tsakris, A.; Chrousos, G.; Theodoridou, M.; Spoulou, V. Discovery of Immunodominant B Cell Epitopes within Surface Pneumococcal Virulence Proteins in Pediatric Patients with Invasive Pneumococcal Disease. J. Biol. Chem. 2015, 290, 27500–27510. [Google Scholar] [PubMed]
- 2013 Runners-Up. In vaccine design, looks do matter. Science 2013. [Google Scholar] [CrossRef]
- Jordan, R.E.; Fernandez, J.; Brezski, R.J.; Greenplate, A.R.; Knight, D.M.; Raju, T.S.; Lynch, A.S. A peptide immunization approach to counteract a Staphylococcus aureus protease defense against host immunity. Immunol. Lett. 2016, 172, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Amon, R.; Reuven, E.M.; Leviatan Ben-Arye, S.; Padler-Karavani, V. Glycans in immune recognition and response. Carbohydr. Res. 2014, 389, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Cartmell, J.; Bailey, J.J.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS ONE 2012, 7, e35106. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M.; Byrne, S.N.; Payne, R.J. Synthetic self-adjuvanting glycopeptide cancer vaccines. Front. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Paulson, J.C. Immunology: Glyco-engineering “super-self”. Nat. Chem. Biol. 2014, 10, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Rillahan, C.D.; Cheng, T.-Y.; Van Rhijn, I.; Macauley, M.S.; Moody, D.B.; Paulson, J.C. Targeted delivery of mycobacterial antigens to human dendritic cells via Siglec-7 induces robust T cell activation. J. Immunol. 2014, 193, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
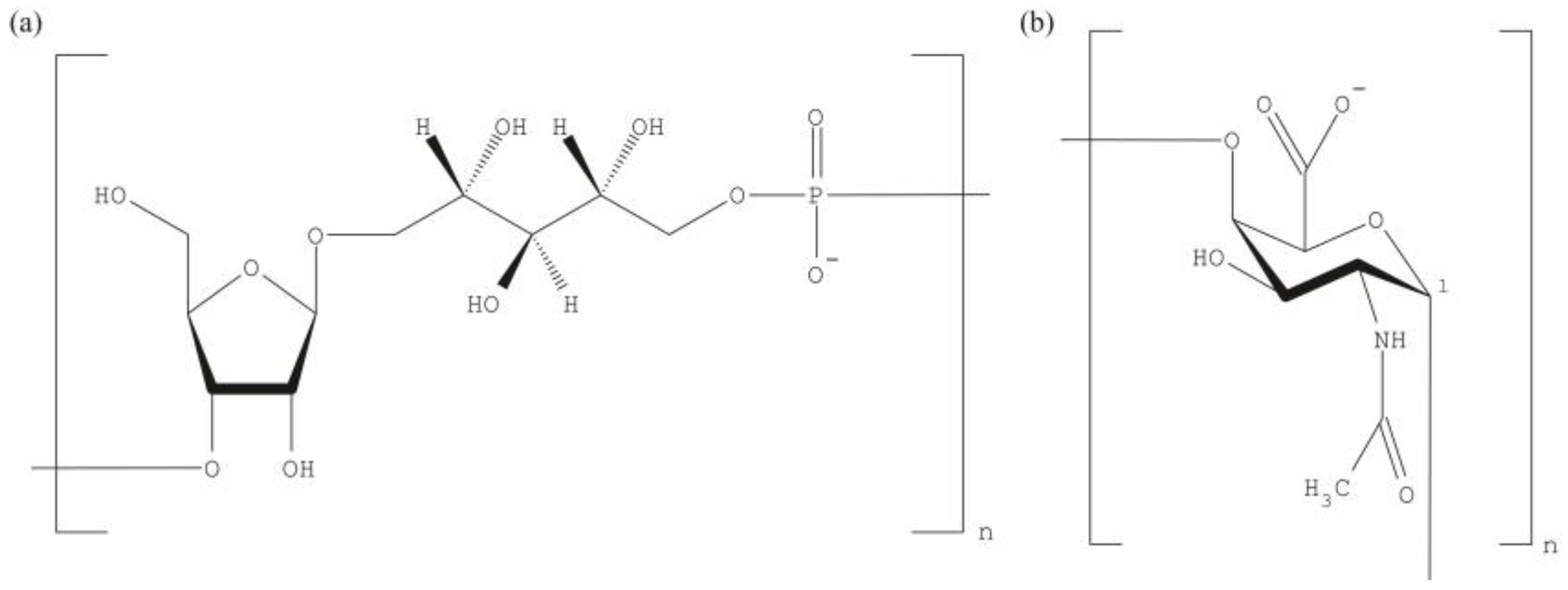
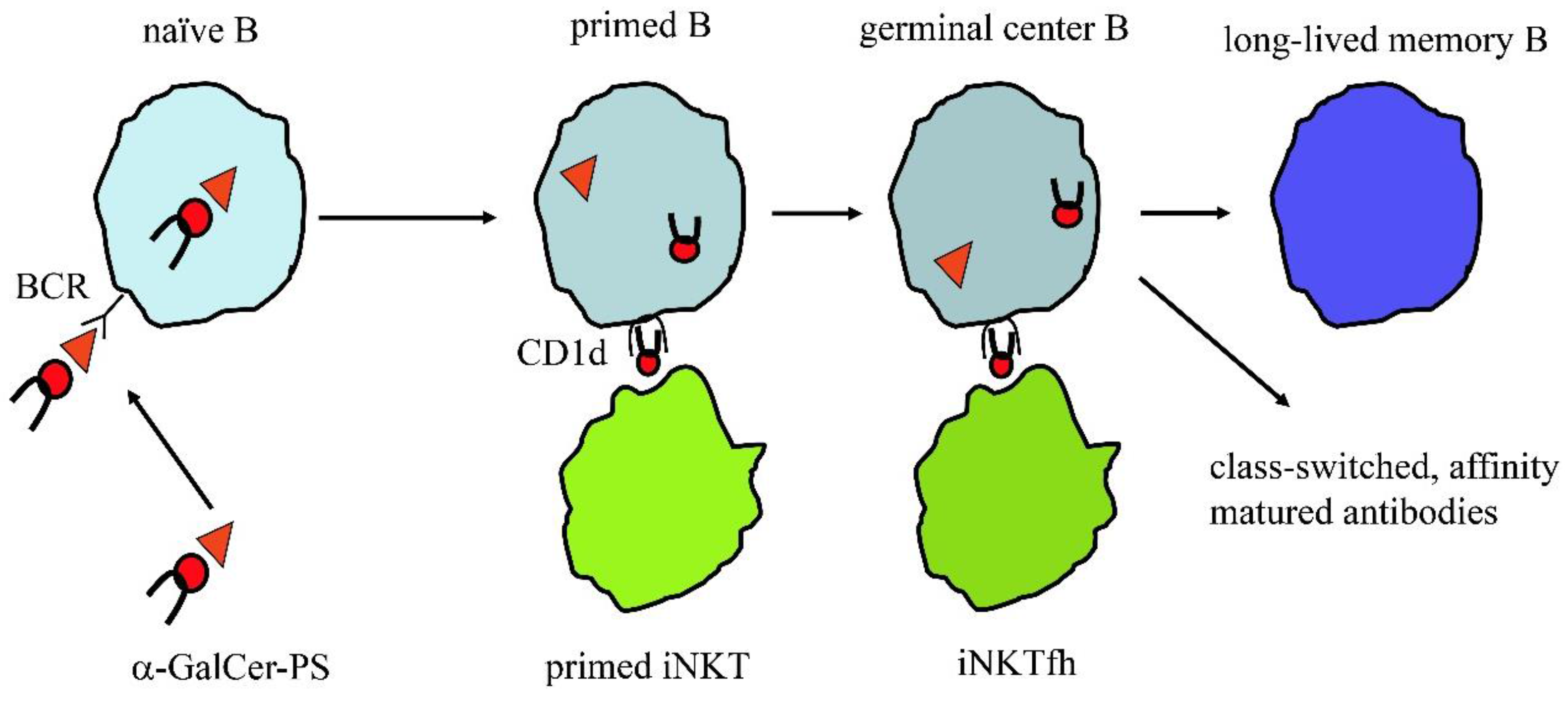
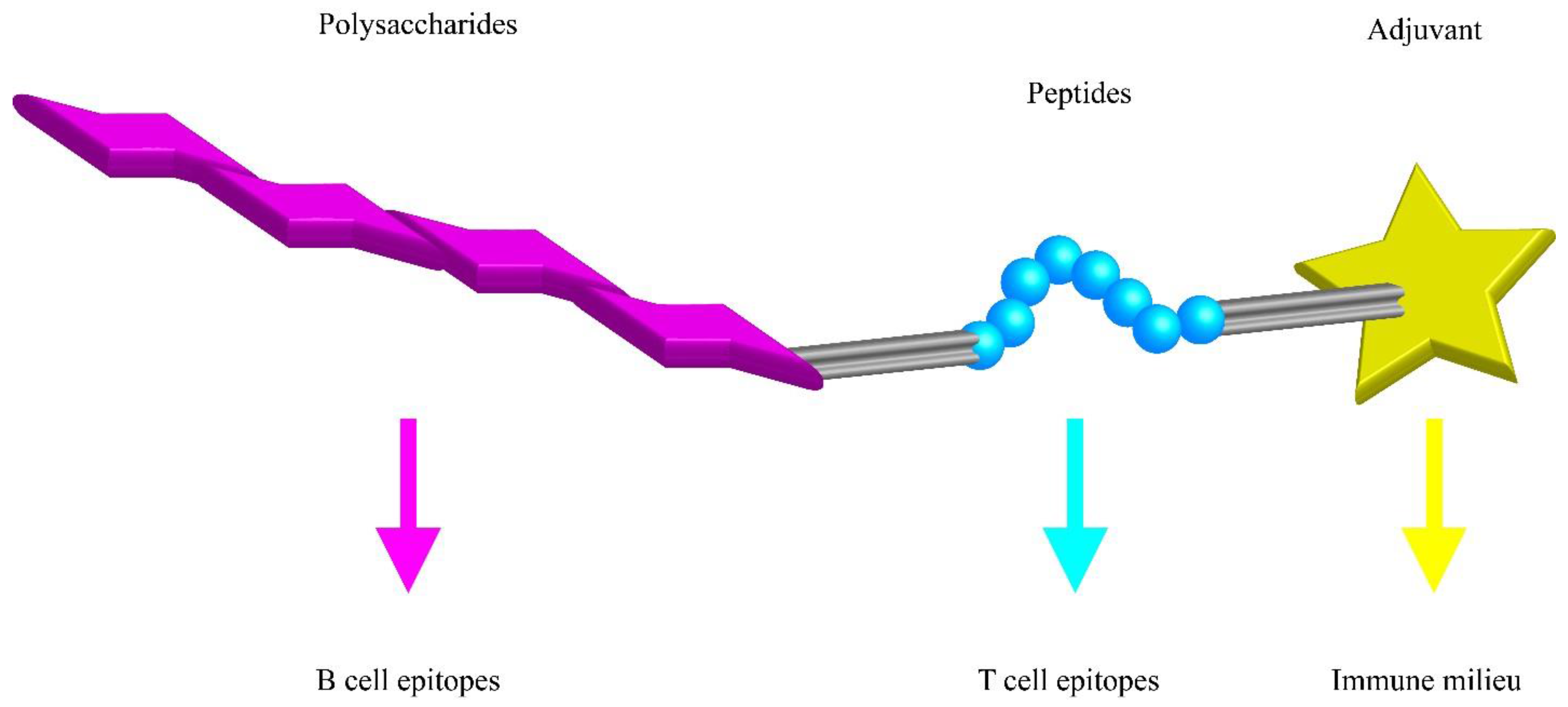
| Vaccine | Date | Conjugate | FDA | EMA | PS |
|---|---|---|---|---|---|
| ActHIB | 27 September 1996 | TT | Sanofi Pasteur, S.A. | Hib (PRP) | |
| Hexacima | 17 April 2013 | TT | Sanofi Pasteur S.A. | Hib (PRP) | |
| Hexyon | 17 April 2013 | TT | Sanofi Pasteur MSD SNC | Hib (PRP) | |
| Hiberix | 19 August 2009 | TT | GlaxoSmithKline Biologicals, S.A. | Hib (PRP) | |
| Infanrix Hexa | 23 October 2000 | TT | GlaxoSmithKline Biologicals S.A. | Hib (PRP) | |
| Menactra | 14 January 2005 | DT | Sanofi Pasteur, Inc. | Men (A, C, Y and W-135) | |
| MenHibrix | 14 June 2012 | TT | GlaxoSmithKline Biologicals | Men (C and Y), Hib (PRP) | |
| Menomune-A/C/Y/W-135 | 9 January 2009 | Sanofi Pasteur, Inc. | Men (A, C, Y and W-135) | ||
| Menveo | 15 March 2010 | CRM197 | GSK Vaccines S.r.l. | Men (A, C, Y and W-135) | |
| Menveo | 19 February 2010 | CRM197 | Novartis Vaccines and Diagnostics, Inc. | Men (A, C, Y and W-135) | |
| Nimenrix | 20 April 2012 | TT | Pfizer Limited | Men (A, C, Y and W-135) | |
| PedvaxHIB | 27 April 2011 | OMPC | Merck & Co, Inc. | Hib (PRP) | |
| Pentacel | 20 June 2008 | TT | Sanofi Pasteur Limited | Hib (PRP) | |
| Pneumovax 23 | 6 August 2008 | Merck & Co, Inc. | Pneumo (23-valent: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F) | ||
| Prevenar | 2 February 2001 | CRM197 | Pfizer Limited | Pneumo (7-valent: 4, 6B, 9V, 14, 18C, 19F, and 23F) | |
| Prevenar 13 | 9 December 2009 | CRM197 | Pfizer Limited | Pneumo (13-valent: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) | |
| Prevnar | 17 February 2000 | CRM197 | Wyeth Pharmaceuticals Inc. | Pneumo (7-valent: 4, 6B, 9V, 14, 18C, 19F, and 23F) | |
| Prevnar 13 | 24 February 2010 | CRM197 | Wyeth Pharmaceuticals Inc. | Pneumo (13-valent: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) | |
| Synflorix | 30 March 2009 | PD; TT; DT depending on serotype | GlaxoSmithKline Biologicals S.A. | Pneumo (10-valent: 1, 4, 5, 6B, 7F, 9V, 14 and 23F; 18C; 19F) | |
| TYPHIM Vi | 27 March 2014 | Sanofi Pasteur, S.A. | Typh (Vi) | ||
| Vaxelis | 15 February 2016 | OMPC | Sanofi Pasteur MSD SNC | Hib (PRP) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallari, M.; De Libero, G. From Immunologically Archaic to Neoteric Glycovaccines. Vaccines 2017, 5, 4. https://doi.org/10.3390/vaccines5010004
Cavallari M, De Libero G. From Immunologically Archaic to Neoteric Glycovaccines. Vaccines. 2017; 5(1):4. https://doi.org/10.3390/vaccines5010004
Chicago/Turabian StyleCavallari, Marco, and Gennaro De Libero. 2017. "From Immunologically Archaic to Neoteric Glycovaccines" Vaccines 5, no. 1: 4. https://doi.org/10.3390/vaccines5010004





