From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology
Abstract
:1. Introduction
2. Nanoparticles in Antigen Delivery
3. Nanoparticles in Adjuvanticity
4. Conclusions
- Facilitating the targeting to the cells of the mononuclear phagocyte system, which are the major APCs, because of the NP particulate nature;
- Possibility of precision targeting to the selected APCs by inserting specific molecules on the NP surface (ligands, receptors);
- Possibility of directing intracellular localization to specific compartments in order to facilitate antigen presentation in the context of a selected MHC type or both types (cross-presentation);
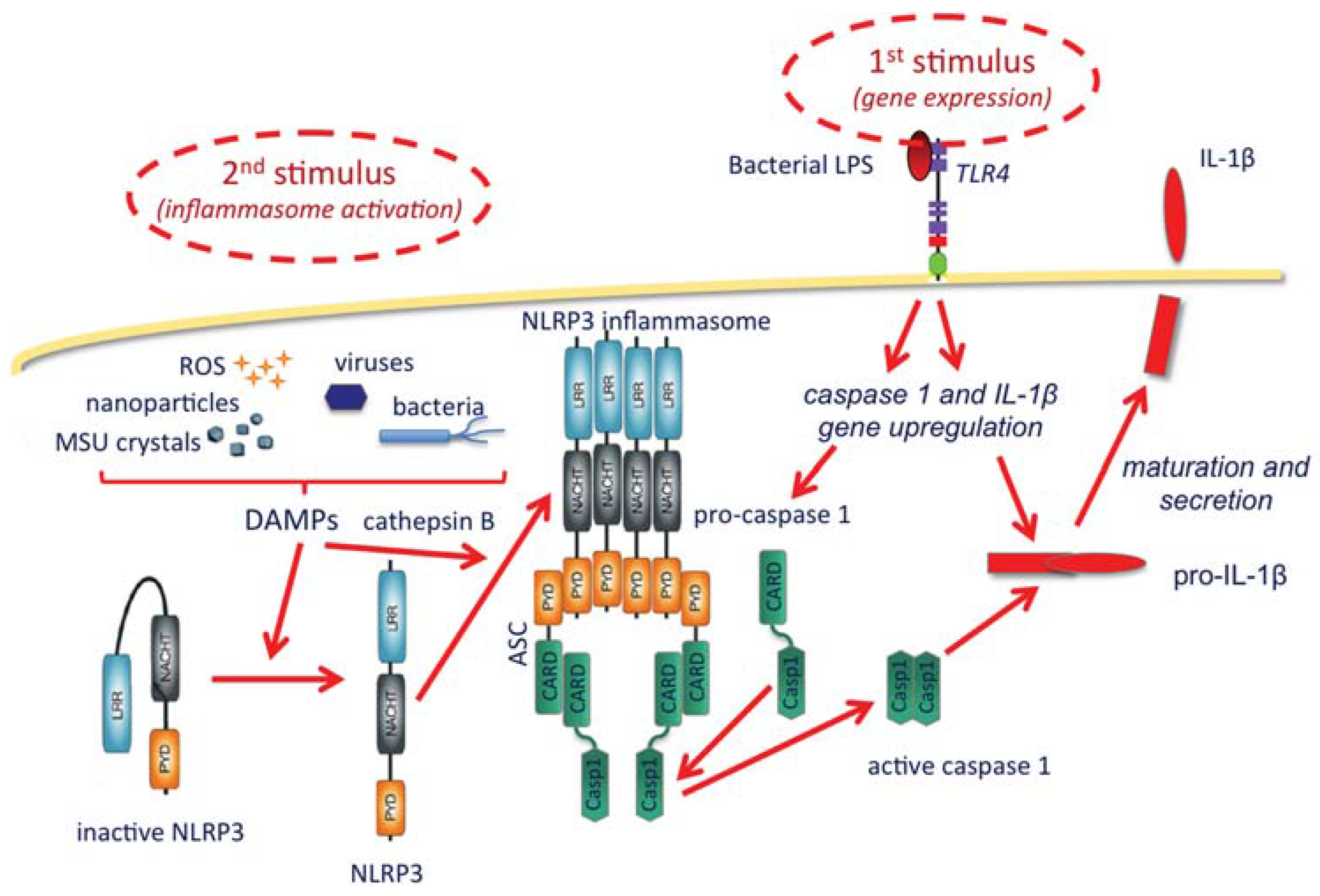
- Possibility of amplifying the establishment of adaptive protective immunity by inducing a localized and limited inflammatory reaction (controlled inflammasome activation and IL-1β production), an effect that could be increased by NP surface decoration with TLR ligands.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; van Nest, G. Development of vaccine adjuvants: A historical perspective. In Vaccine Adjuvants and Delivery Systems; Singh, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–31. [Google Scholar]
- Podda, A.; del Giudice, G. MF-59-adjuvanted vaccines: Increased immunogenicity with an optimal safety profile. Expert Rev. Vaccines 2003, 2, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, F.; Wiedermann, G.; Jonas, S.; Althaust, B.; Finkel, B.; Gluck, R.; Herzog, C. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine 1997, 15, 1209–1213. [Google Scholar] [CrossRef]
- Rappuoli, R.; Mandl, C.W.; Black, S.; de Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011, 11, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.L.; Sajkov, D.; Woodman, D.J.; Honda-Okubo, Y.; Cox, M.M.J.; Heinzel, S.; Petrovsky, N. Randomized clinical trial of immunogeni city and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Schlesinger, S.J.; Huang, Y.; Hurley, A.; Lombardo, A.; Chen, Z.; Than, S.; Adesanya, P.; Bunce, C.; Boaz, M.; et al. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B’ HIV-1 candidate vaccine. PLoS ONE 2010, 5, e8617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K. Janeway’s Immunobiology, 8th ed.; Garland Science: Abingdon, UK, 2011. [Google Scholar]
- Pobre, K.; Tashani, M.; Ridda, I.; Rashid, H.; Wong, M.; Booy, R. Carrier priming or suppression: Understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine 2014, 32, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. The second touch hypothesis: T cell activation, homing and polarization. Version 2. F1000Res. 2014. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Altin, J.; Parish, C.R. Liposomal vaccines—Targeting the delivery of antigen. Methods 2006, 40, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Marín, E.; Rodriguez-Del Rio, E.; Frande-Cabanes, E.; Tobes, R.; Pareja, E.; Lecea-Cuello, M.J.; Ruiz-Sáez, M.; Madrazo-Toca, F.; Hölscher, C.; Alvarez-Dominguez, C. Phagosomes induced by cytokines function as anti-Listeria vaccines: Novel role for functional compartmentalization of STAT-1 protein and cathepsin-D. J. Biol. Chem. 2012, 287, 14310–14324. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, S.; Li, W.; Dong, H.; Zhou, M.; Cao, M.; Hu, H.M.; Wang, L.X. Therapeutic antitumor efficacy of B cells loaded with tumor-derived autophagasomes vaccine (DRibbles). J. Immunother. 2014, 37, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Parihar, P.; Sharma, J. Assessment of phagosomes infected with Mycobacterium tuberculosis as a vaccine candidate against tuberculosis. Indian J. Exp. Biol. 2014, 52, 1090–1097. [Google Scholar] [PubMed]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Doroud, D.; Vatanara, A.; Zahedifard, F.; Gholami, E.; Vahabpour, R.; Najafabadi, A.R.; Rafati, S. Cationic solid lipid nanoparticles loaded by cystein proteinase genes as a novel anti-leishmaniasis DNA vaccine delivery system: Characterization and in vitro evaluations. J. Control. Release 2010, 148, e105–e106. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Ochyl, L.J.; Schwendeman, A.; Moon, J.J. Lipid-Based Nanoparticles for Vaccine Applications. In Biomedical Engineering: Frontier Research and Converging Technologies; Jo, H., Jun, H.W., Shin, J., Lee, S.-H., Eds.; Springer: Heildeberg, Germany; Berlin, Germany, 2015; Volume 9, pp. 177–197. [Google Scholar]
- Orr, N.; Arnon, R.; Rubin, G.; Cohen, D.; Bercovier, H.; Lowell, G.H. Enhancement of anti-Shigella lipopolysaccharide (LPS) response by addition of the cholera toxin B subunit to oral and intranasal proteosome-Shigella flexneri 2a LPS vaccines. Infect. Immun. 1994, 62, 5198–5200. [Google Scholar] [PubMed]
- Levi, R.; Aboud-Pirak, E.; Leclerc, C.; Lowell, G.H.; Arnon, R. Intranasal immunization of mice against influenza with synthetic peptides anchored to proteosomes. Vaccine 1995, 13, 1353–1359. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Cutting, S.M. Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine 2003, 21, 4215–4224. [Google Scholar] [CrossRef]
- Clark, J.R.; March, J.B. Bacteriophages and biotechnology: Vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006, 24, 212–218. [Google Scholar] [CrossRef] [PubMed]
- D’Apice, L.; Costa, V.; Sartorius, R.; Trovato, M.; Aprile, M.; de Berardinis, P. Stimulation of innate and adaptive immunity by using filamentous bacteriophage fd targeted to DEC-205. J. Immunol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Gill, P. Nanocarriers, nanovaccines, and nanobacteria as nanobiotechnological concerns in modern vaccines. Sci. Iran. 2013, 20, 1003–1013. [Google Scholar]
- Cellular and Molecular Immunology; Abbas, A.K.; Lichtman, A.H. (Eds.) Elsevier: Philadelphia, PA, USA, 2005; pp. 66–80.
- Felnerova, D.; Viret, J.F.; Glück, R.; Moser, C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr. Opin. Biotechnol. 2004, 15, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Firdous, J.; Islam, M.A.; Park, S.-M.; Cheon, H.-S.; Shim, B.-S.; Yoon, H.-S.; Song, M.; Chang, J.; Choi, Y.-J.; Park, Y.-M.; et al. Induction of long-term immunity against respiratory syncytial virus glycoprotein by an osmotic polymeric nanocarrier. Acta Biomater. 2014, 10, 4606–4617. [Google Scholar] [CrossRef] [PubMed]
- Gianvincenzo, P.D.; Calvo, J.; Perez, S.; Álvarez, A.; Bedoya, L.M.; Alcamí, J.; Penadés, S. Negatively charged glyconanoparticles modulate and stabilize the secondary structures of a gp120 V3 loop peptide: Toward fully synthetic HIV vaccine candidates. Bioconjug. Chem. 2015, 26, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Del Rio, E.; Marradi, M.; Calderon-Gonzalez, R.; Frande-Cabanes, E.; Penadés, S.; Petrovsky, N.; Alvarez-Dominguez, C. A gold glyco-nanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 2015, 33, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Willingham, S.B.; Ting, J.P.; Re, F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, M.J.; Mark Saltzman, W.; Mellman, I.; Ledizet, M.; Fikrig, E.; Flavell, R.A.; et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 2009, 27, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Staruch, M.J.; Wood, D.D. The adjuvanticity of interleukin 1 in vivo. J. Immunol. 1983, 130, 2191–2194. [Google Scholar] [PubMed]
- Durum, S.K.; Higuchi, C.; Ron, Y. Accessory cells and T cell activation. The relationship between two components of macrophage accessory cell function: I-A and IL-1. Immunobiology 1984, 168, 213–231. [Google Scholar] [CrossRef]
- Boraschi, D.; Nencioni, L.; Villa, L.; Censini, S.; Bossù, P.; Ghiara, P.; Presentini, R.; Perin, F.; Frasca, D.; Doria, G.; et al. In vivo stimulation and restoration of the immune response by the noninflammatory fragment 163-171 of human IL-1β. J. Exp. Med. 1988, 168, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Ratsimandresy, R.A.; Dorfleutner, A.; Stehlik, C. An update on PYRIN domain-containing pattern recognition receptors: From immunity to pathology. Front. Immunol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.-L.; Rejman, J.; Demeester, J.; Irvine, D.J.; Gander, B.; de Smedt, S.C. Particulare vaccines: On the quest for optimal delivery and immune response. Drug Discov. Today 2011, 16, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Costantino, L.; Italiani, P. Interaction of nanoparticles with immunocompetent cells: Nanosafety considerations. Nanomedicine (Lond.) 2012, 7, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P. Immunosenescence and vaccine failure in the elderly: Strategies for improving response. Immunol. Lett. 2014, 162, 346–353. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boraschi, D.; Italiani, P. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines 2015, 3, 930-939. https://doi.org/10.3390/vaccines3040930
Boraschi D, Italiani P. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines. 2015; 3(4):930-939. https://doi.org/10.3390/vaccines3040930
Chicago/Turabian StyleBoraschi, Diana, and Paola Italiani. 2015. "From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology" Vaccines 3, no. 4: 930-939. https://doi.org/10.3390/vaccines3040930




