The Promise of Preventive Cancer Vaccines
Abstract
:1. Cancer Prevention: A Primer
| Type of prevention | Institute of Medicine of the National Academies, USA [2] | IARC, World Health Organization [3] |
|---|---|---|
| Primary | Primary prevention refers to health promotion, which fosters wellness in general and thus reduces the likelihood of disease, disability, and premature death in a nonspecific manner, as well as specific protection against the inception of disease. | Primary prevention is prevention of disease by reducing exposure of individuals to risk factors or by increasing their resistance to them. |
| Secondary | Secondary prevention refers to the detection and management of presymptomatic disease, and the prevention of its progression to symptomatic disease. | Secondary prevention (applied during the preclinical phase) is the early detection and treatment of disease. Screening activities are an important component of secondary prevention. |
| Tertiary | Tertiary prevention refers to the treatment of symptomatic disease in an effort to prevent its progression to disability or premature death. The overlap with treatment is self-evident, and perhaps suggests that preventive medicine has grandiose territorial ambitions. Be that as it may, there is a legitimate focus on prevention even after disease develops, such as the prevention of early cancer from metastasizing […] | Tertiary prevention (appropriate in the clinical phase) is the use of treatment and rehabilitation programmes to improve the outcome of illness among affected individuals. |
| Cancer prevention | Aim | Target | Non-immunological examples | Immunological examples |
|---|---|---|---|---|
| Primary | Removal or avoidance of cancer risk factors | Healthy individuals | Healthy diet; Ban on carcinogens in the workplace; Quitting smoking; Tamoxifen in healthy women; Prophylactic mastectomy in hereditary breast cancer | Anti- HBV and HPV vaccines |
| Secondary | Early diagnosis and therapy | Pre-symptomatic cancer bearers | Pap test; Mammography; Colonoscopy | Anti- Her2 and MUC1 vaccines against preneoplastic or early neoplastic lesions |
| Tertiary | Prevention of relapse and metastasis | Survivors with occult neoplastic lesions | Prophylactic radiotherapy; Adjuvant chemotherapy | Adjuvant monoclonal antibodies; Adjuvant therapeutic vaccines; Intravesical instillations of Bacillus Calmette-Guerin |
1.1. Primary Cancer Prevention
1.2. Secondary Cancer Prevention
1.3. Tertiary Cancer Prevention
2. Toward Cancer Immunoprevention: Lessons Learned from Preclinical Testing
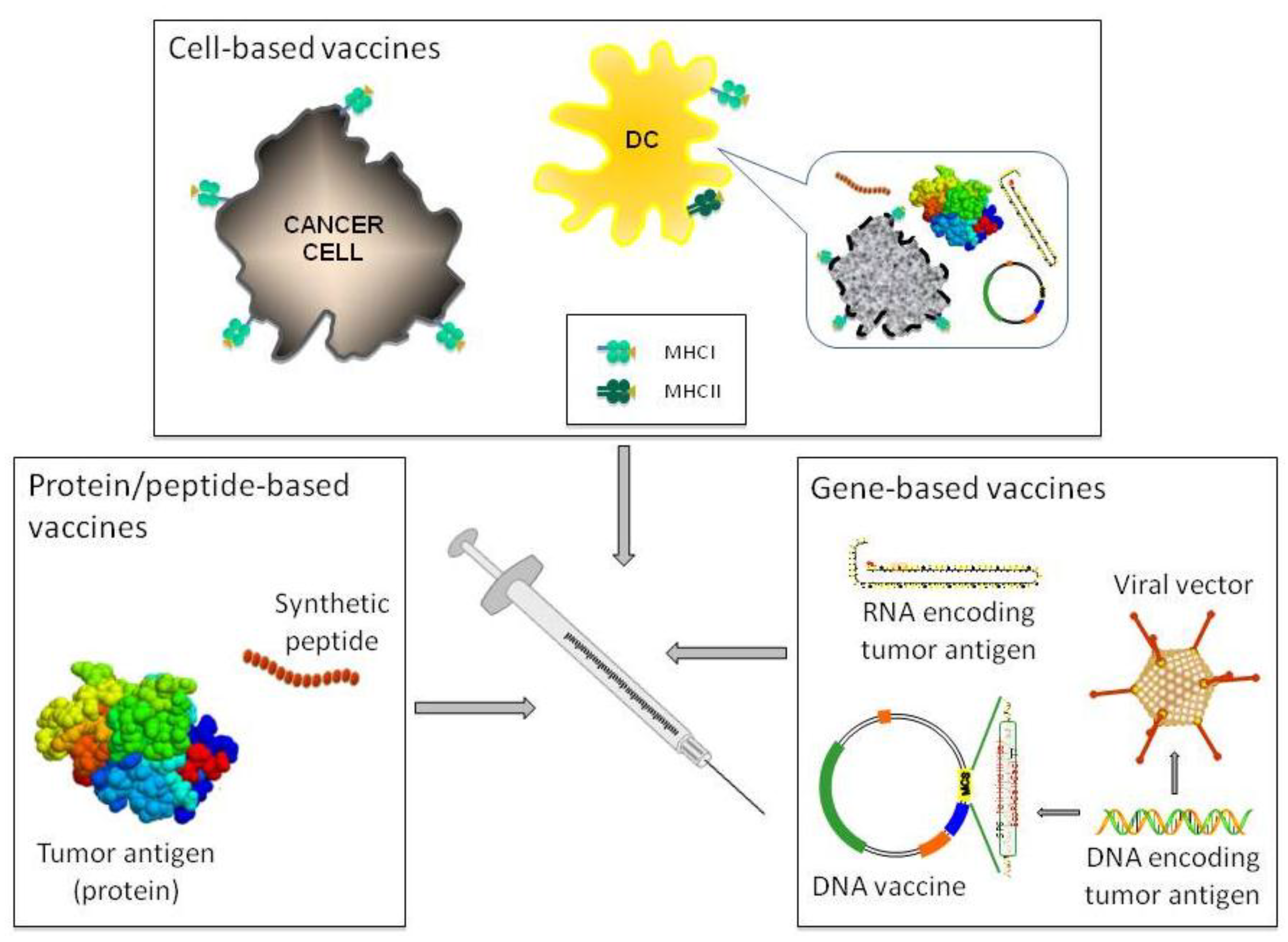
2.1. Genetically Modified Mouse Models
2.2. Companion Animals
3. Human Cancer Immunoprevention
3.1. Primary Immunoprevention: A Futuristic Option for Non-Infection Associated Tumors?
3.2. Secondary Immunoprevention: A Future Option Whose Efficacy Is Being Tested Now
3.3. Tertiary Immunoprevention: The Present Option
| ClinicalTrials.gov Identifier | Type of vaccine | Patients with: | Status |
|---|---|---|---|
| NCT00107211 | Autologous DC pulsed with HLA class II promiscuous-binding peptides from Her2 (DC1 vaccine) | Her2+ breast DCIS | Completed |
| NCT00773097 | MUC1 100-mer peptide with Poly-ICLC | Advanced colorectal adenoma | Completed |
| NCT02134925 | Advanced colon polyps | Recruiting | |
| NCT01431391 | Autologous DC pulsed with the fusion protein PA2024 (sipuleucel-T) | Castration refractory metastatic Prostate cancer | Completed |
| NCT00639639 | Autologous DC pulsed with CMV pp65-LAMP mRNA | Glioblastoma multiforme | Active, not recruiting |
| NCT00524277 | Her2-derived HLA class I peptide (GP2) with GM-CSF | Her2+ breast cancer | Active, not recruiting |
| NCT00841399 | Her2-derived HLA class I peptide (E75) with GM-CSF | Her2+ breast cancer | Completed |
| NCT00854789 | Completed | ||
| NCT01479244 | Active, not recruiting | ||
| NCT01510288 | GM-CSF-transfected allogeneic prostate cancer cells | Castration refractory metastatic Prostate cancer | Terminated |
| NCT01417000 | GM-CSF-transfected allogeneic pancreatic cancer cells and CRS-207 | Metastatic pancreatic Adenocarcinoma | Active, not recruiting |
| NCT02004262 | Recruiting | ||
| NCT02243371 | Recruiting | ||
| NCT00077532 | gp100-derived HLA class I peptide | Advanced melanoma | Completed |
4. Checkpoint Blockade as a Biological Adjuvant for Cancer Immunoprevention: Work in Progress

5. Target Antigens for Cancer Immunoprevention: Drivers or Passengers?
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fundamentals of Cancer Prevention, 3rd ed.; Alberts, D.; Hess, L.M. (Eds.) Springer-Verlag: Heildelberg, Germany, 2014.
- Katz, D.; Ali, A. Preventive Medicine, Integrative Medicine, and the Health of the Public. Commissioned paper for Institute of Medicine (IOM) of the National Academies. In Proceedings of the Summit on Integrative Medicine and the Health of the Public, Washington, DC, USA, 25–27 February 2009.
- Dos Santos Silva, I. Cancer Epidemiology: Principles and Method; IARC Publications: Lyon, France, 1999. [Google Scholar]
- Schottenfeld, D.; Fraumeni, J.F. Cancer Epidemiology and Prevention, 3rd ed.; Oxford University Press: Oxford, UK, 2006; pp. 872–897. [Google Scholar]
- Stubert, J.; Dieterich, M.; Gerber, B. Medical prevention of breast cancer. Breast Care (Basel) 2014, 9, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sinha, G. More evidence that aspirin lowers cancer risk. J. Natl. Cancer Inst. 2015, 107, 495. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.J.; Armstrong, T.D.; Jaffee, E.M. Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention. Clin. Cancer Res. 2015, 21, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lee, C.M.; Lin, S.M.; Chu, H.C.; Wu, T.C.; Yang, S.S.; Kuo, H.S.; et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009, 101, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Lazzeroni, M.; Bonanni, B. Cancer chemoprevention: Much has been done, but there is still much to do. State of the art and possible new approaches. Mol. Oncol. 2015, 9, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A. Surgical management of the breast: Breast conservation therapy and mastectomy. Surg. Clin. N. Am. 2013, 93, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, A.; Piccart, M. Adjuvant systemic therapy in breast cancer: Quo vadis? Ann. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Nicoletti, G.; Palladini, A.; Croci, S.; Murgo, A.; Antognoli, A.; Landuzzi, L.; Fabbi, M.; Ferrini, S.; Musiani, P.; et al. Antimetastatic activity of a preventive cancer vaccine. Cancer Res. 2007, 67, 11037–11044. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Nicoletti, G.; Landuzzi, L.; Cavallo, F.; Forni, G.; de Giovanni, C.; Nanni, P. Vaccines and other immunological approaches for cancer immunoprevention. Curr. Drug Targets 2011, 12, 1957–1973. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Malfertheiner, P.; Rappuoli, R. Development of vaccines against Helicobacter pylori. Expert Rev. Vaccines 2009, 8, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M. Currently approved prophylactic HPV vaccines. Expert Rev. Vaccines 2009, 8, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell. 2011, 144, 646–674. [Google Scholar]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Cicchelero, L.; de Rooster, H.; Sanders, N.N. Various ways to improve whole cancer cell vaccines. Expert Rev. Vaccines 2014, 13, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buque, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [PubMed]
- Braun, M.; Perret, R.; Scholz, G.; Romero, P. Peptide and protein-based cancer vaccines. In Cancer Immunotherapy; Curiel, T.J., Ed.; Springer: New York, NY, USA, 2013; pp. 111–146. [Google Scholar]
- Aurisicchio, L.; Ciliberto, G. Genetic cancer vaccines: Current status and perspectives. Expert Opin. Biol. Ther. 2012, 12, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Danishmalik, S.N.; Sin, J.I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vaccines Immunother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Morse, M.A.; Hobeika, A.; Lyerly, H.K. Novel recombinant alphaviral and adenoviral vectors for cancer immunotherapy. Semin. Oncol. 2012, 39, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; Offringa, R.; van der Burg, S.H.; Forni, G.; Melief, C.J. Vaccination for treatment and prevention of cancer in animal models. Adv. Immunol. 2006, 90, 175–213. [Google Scholar] [PubMed]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr. Opin. Immunol. 2004, 16, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Riccardo, F.; Macagno, M.; Bandini, S.; Cojoca, R.; Ercole, E.; Amici, A.; Cavallo, F. Chimeric DNA Vaccines against ErbB2+ Carcinomas: From Mice to Humans. Cancers (Basel) 2011, 3, 3225–3241. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Experimental mouse tumour models: What can be learnt about human cancer immunology? Nat. Rev. Immunol. 2012, 12, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Cavallo, F.; Nanni, P.; Forni, G. Vaccines for tumour prevention. Nat. Rev. Cancer 2006, 6, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; de Giovanni, C.; Pannellini, T.; Cavallo, F.; Forni, G.; Nanni, P. Cancer immunoprevention. Future Oncol. 2005, 1, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; de Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol. Immunother. 2011, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Mastini, C.; Amici, A.; Marchini, C.; Iezzi, M.; Lanzardo, S.; de Giovanni, C.; Montani, M.; Lollini, P.L.; Masucci, G.; et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010, 70, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Bolli, E.; Quaglino, E.; Arigoni, M.; Lollini, P.L.; Calogero, R.; Forni, G.; Cavallo, F. Oncoantigens for an immune prevention of cancer. Am. J. Cancer Res. 2011, 1, 255–264. [Google Scholar] [PubMed]
- Boggio, K.; Nicoletti, G.; di Carlo, E.; Cavallo, F.; Landuzzi, L.; Melani, C.; Giovarelli, M.; Rossi, I.; Nanni, P.; de Giovanni, C.; et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 1998, 188, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Mastini, C.; Forni, G.; Cavallo, F. ErbB2 transgenic mice: A tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr. Protoc. Immunol. 2008. [Google Scholar] [CrossRef]
- Husemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmuller, G.; et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008, 13, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Rolla, S.; Iezzi, M.; Spadaro, M.; Musiani, P.; de Giovanni, C.; Lollini, P.L.; Lanzardo, S.; Forni, G.; Sanges, R.; et al. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J. Clin. Investig. 2004, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, C.; Nicoletti, G.; Landuzzi, L.; Astolfi, A.; Croci, S.; Comes, A.; Ferrini, S.; Meazza, R.; Iezzi, M.; di Carlo, E.; et al. Immunoprevention of HER-2/neu transgenic mammary carcinoma through an interleukin 12-engineered allogeneic cell vaccine. Cancer Res. 2004, 64, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Landuzzi, L.; Nicoletti, G.; de Giovanni, C.; Rossi, I.; Croci, S.; Astolfi, A.; Iezzi, M.; di Carlo, E.; Musiani, P.; et al. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J. Immunol. 2004, 173, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Nicoletti, G.; de Giovanni, C.; Landuzzi, L.; di Carlo, E.; Cavallo, F.; Pupa, S.M.; Rossi, I.; Colombo, M.P.; Ricci, C.; et al. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J. Exp. Med. 2001, 194, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, C.; Nicoletti, G.; Quaglino, E.; Landuzzi, L.; Palladini, A.; Ianzano, M.L.; Dall’Ora, M.; Grosso, V.; Ranieri, D.; Laranga, R.; et al. Vaccines against human HER2 prevent mammary carcinoma in mice transgenic for human HER2. Breast Cancer Res. 2014. [Google Scholar] [CrossRef]
- Conti, L.; Lanzardo, S.; Iezzi, M.; Montone, M.; Bolli, E.; Brioschi, C.; Maiocchi, A.; Forni, G.; Cavallo, F. Optical imaging detection of microscopic mammary cancer in ErbB-2 transgenic mice through the DA364 probe binding alphav beta3 integrins. Contrast Media Mol. Imaging 2013, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Marchini, C.; Malinarich, S.; Quaglino, E.; Lanzardo, S.; Montani, M.; Iezzi, M.; Angeletti, M.; Ramadori, G.; Forni, G.; et al. Protective immunity against neu-positive carcinomas elicited by electroporation of plasmids encoding decreasing fragments of rat neu extracellular domain. Hum. Gene Ther 2008, 19, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, E.; Iezzi, M.; Mastini, C.; Amici, A.; Pericle, F.; di Carlo, E.; Pupa, S.M.; de Giovanni, C.; Spadaro, M.; Curcio, C.; et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004, 64, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Rovero, S.; Amici, A.; di Carlo, E.; Bei, R.; Nanni, P.; Quaglino, E.; Porcedda, P.; Boggio, K.; Smorlesi, A.; Lollini, P.L.; et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J. Immunol. 2000, 165, 5133–5142. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Ria, F.; Occhipinti, S.; di Sante, G.; Iezzi, M.; Spadaro, M.; Nicolo, C.; Ambrosino, E.; Merighi, I.F.; Musiani, P.; et al. Erbb2 DNA vaccine combined with regulatory T cell deletion enhances antibody response and reveals latent low-avidity T cells: Potential and limits of its therapeutic efficacy. J. Immunol. 2010, 184, 6124–6132. [Google Scholar] [CrossRef] [PubMed]
- Arigoni, M.; Barutello, G.; Lanzardo, S.; Longo, D.; Aime, S.; Curcio, C.; Iezzi, M.; Zheng, Y.; Barkefors, I.; Holmgren, L.; et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis 2012, 15, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Cancer vaccines: Between the idea and the reality. Nat. Rev. Immunol. 2003, 3, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Tumor immunology top 10 list. Immunol. Rev. 2008, 222, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Nicoletti, G.; Landuzzi, L.; de Giovanni, C.; Rossi, I.; di Carlo, E.; Musiani, P.; Muller, W.J.; Nanni, P. Down regulation of major histocompatibility complex class I expression in mammary carcinoma of HER-2/neu transgenic mice. Int J. Cancer 1998, 77, 937–941. [Google Scholar] [CrossRef]
- Aptsiauri, N.; Cabrera, T.; Mendez, R.; Garcia-Lora, A.; Ruiz-Cabello, F.; Garrido, F. Role of altered expression of HLA class I molecules in cancer progression. Adv. Exp. Med. Biol. 2007, 601, 123–131. [Google Scholar] [PubMed]
- Curcio, C.; Khan, A.S.; Amici, A.; Spadaro, M.; Quaglino, E.; Cavallo, F.; Forni, G.; Draghia-Akli, R. DNA immunization using constant-current electroporation affords long-term protection from autochthonous mammary carcinomas in cancer-prone transgenic mice. Cancer Gene Ther. 2008, 15, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.B.; Quaglino, E.; Radkevich-Brown, O.; Jones, R.F.; Piechocki, M.P.; Reyes, J.D.; Weise, A.; Amici, A.; Wei, W.Z. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer Res. 2010, 70, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Rolla, S.; Nicolo, C.; Malinarich, S.; Orsini, M.; Forni, G.; Cavallo, F.; Ria, F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J. Immunol. 2006, 177, 7626–7633. [Google Scholar] [CrossRef] [PubMed]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 32, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Barutello, G.; Curcio, C.; Spadaro, M.; Arigoni, M.; Trovato, R.; Bolli, E.; Zheng, Y.; Ria, F.; Quaglino, E.; Iezzi, M.; et al. Anti-tumor immunization of mothers delays tumor development in cancer prone offspring. Oncoimmunology 2015. [Google Scholar] [CrossRef]
- Riccardo, F.; Aurisicchio, L.; Impellizeri, J.A.; Cavallo, F. The importance of comparative oncology in translational medicine. Cancer Immunol. Immunother. 2015, 64, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Greggs, W.M., 3rd; Clouser, C.L.; Patterson, S.E.; Mansky, L.M. Broadening the use of antiretroviral therapy: The case for feline leukemia virus. Ther. Clin. Risk Manag. 2011, 7, 115–122. [Google Scholar] [PubMed]
- Bergman, P.J.; McKnight, J.; Novosad, A.; Charney, S.; Farrelly, J.; Craft, D.; Wulderk, M.; Jeffers, Y.; Sadelain, M.; Hohenhaus, A.E.; et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: A phase I trial. Clin. Cancer Res. 2003, 9, 1284–1290. [Google Scholar] [PubMed]
- Grosenbaugh, D.A.; Leard, A.T.; Bergman, P.J.; Klein, M.K.; Meleo, K.; Susaneck, S.; Hess, P.R.; Jankowski, M.K.; Jones, P.D.; Leibman, N.F.; et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am. J. Vet. Res. 2011, 72, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J.; Camps-Palau, M.A.; McKnight, J.A.; Leibman, N.F.; Craft, D.M.; Leung, C.; Liao, J.; Riviere, I.; Sadelain, M.; Hohenhaus, A.E.; et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 2006, 24, 4582–4585. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Iussich, S.; Maniscalco, L.; Lorda Mayayo, S.; la Rosa, G.; Arigoni, M.; de Maria, R.; Gattino, F.; Lanzardo, S.; Lardone, E.; et al. CSPG4-specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin. Cancer Res. 2014, 20, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Montano, D. Chemical and biological work-related risks across occupations in Europe: A review. J. Occup. Med. Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, A.R.; Onstad, M.A. Hereditary Breast Cancer: An update on risk assessment and genetic testing in 2015. Am. J. Obstet. Gynecol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.G.; Masciari, S.; Gelman, R.; Miron, A.; Miron, P.; Foley, K.; Richardson, A.L.; Krop, I.E.; Verselis, S.J.; Dillon, D.A.; et al. Prevalence of germline TP53 mutations in HER2+ breast cancer patients. Breast Cancer Res. Treat. 2013, 139, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Misguided cancer goal. Nature 2012. [CrossRef]
- Fracol, M.; Xu, S.; Mick, R.; Fitzpatrick, E.; Nisenbaum, H.; Roses, R.; Fisher, C.; Tchou, J.; Fox, K.; Zhang, P.; et al. Response to HER-2 pulsed DC1 vaccines is predicted by both HER-2 and estrogen receptor expression in DCIS. Ann. Surg. Oncol. 2013, 20, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. (Phila) 2013, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Vaccines for cancer prevention: A practical and feasible approach to the cancer epidemic. Cancer Immunol. Res. 2014, 2, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Signori, E.; Cavallo, F. The Fourteenth International Conference on Progress in Vaccination Against Cancer (PIVAC-14), September 24–26, 2014, Rome, Italy: Rethinking anti-tumor vaccines in a new era of cancer immunotherapy. Cancer Immunol. Immunother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Therapeutic cancer vaccine survives biotech bust. Nature 2015, 519, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.L.; Haynes, L.; Parker, C.; Iversen, P. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 2012, 104, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Leitman, S.F.; Dahut, W.; Schlom, J. Re: Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Small, E.J.; Whitmore, J.B.; Frohlich, M.W.; Schellhammer, P.F. Re: Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 2012. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G. Re: Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. 2012. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Kibel, A.S.; Adams, G.W.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vogelzang, N.J.; Corman, J.M.; Tyler, R.C.; McCoy, C.; et al. Antigen-specific immune responses through 24 months in the STAND trial: A randomized phase 2 study evaluating optimal sequencing of sipuleucel-T (sip-T) and androgen deprivation therapy (ADT) in biochemically-recurrent prostate cancer (BRPC). ASCO Meet. Abstr. 2015. Abstract Number: 171. [Google Scholar]
- Sabado, R.L.; Bhardwaj, N. Cancer immunotherapy: Dendritic-cell vaccines on the move. Nature 2015, 519, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Sue, R. New peptide vaccine for HER2-expressing breast tumors. J. Natl. Cancer Inst. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Clive, K.S.; Patil, R.; Benavides, L.C.; Gates, J.D.; Sears, A.K.; Stojadinovic, A.; Ponniah, S.; et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: From US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 2012, 118, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Silvestris, N.; Licchetta, A.; Santini, D.; Scartozzi, M.; Ria, R.; Pisconti, S.; Petrelli, F.; Vacca, A.; Lorusso, V. Metronomic chemotherapy from rationale to clinical studies: A dream or reality? Crit. Rev. Oncol. Hematol. 2015, 95, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Dronca, R.S.; Dong, H. Immunomodulatory antibody therapy of cancer: The closer the better. Clin. Cancer Res. 2014, 21, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Martin, F.; Apetoh, L.; Bouyer, F.; Ghiringhelli, F. Cancer chemotherapy: Not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol. Immunother. 2008, 57, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheng Sow, H.; Mattarollo, S.R. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. Oncoimmunology 2013, 2, e27058. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Waitz, R.; Fasso, M.; Allison, J.P. CTLA-4 blockade synergizes with cryoablation to mediate tumor rejection. Oncoimmunology 2012, 1, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Andersen, G.H.; Svane, I.M.; Andersen, M.H. The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4 T cells. Oncoimmunology 2013, 2, e23991. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Pauken, K.E.; Eagar, T.N.; Obu, T.; Wu, J.; Tang, Q.; Azuma, M.; Krummel, M.F.; Bluestone, J.A. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009, 10, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Joncker, N.T.; Raulet, D.H. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol. Rev. 2008, 224, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Hirschhorn-Cymerman, D.; Morales-Kastresana, A.; Sanmamed, M.F.; Wolchok, J.D. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin. Cancer Res. 2013, 19, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.J.; Vella, A.T. Betting on improved cancer immunotherapy by doubling down on CD134 and CD137 co-stimulation. Oncoimmunology 2013, 2, e22837. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Ishii, N.; Weinberg, A.D. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 2004, 4, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Shuford, W.W.; Newby, S.A.; Aruffo, A.; Ledbetter, J.A.; Hellstrom, K.E.; Mittler, R.S.; Chen, L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997, 3, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Song, D.G.; Powell, D.J., Jr. Finding a needle in a haystack: Activation-induced CD137 expression accurately identifies naturally occurring tumor-reactive T cells in cancer patients. Oncoimmunology 2013, 2, e27184. [Google Scholar] [CrossRef] [PubMed]
- Shevach, E.M.; Stephens, G.L. The GITR-GITRL interaction: Co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol 2006, 6, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, S.; Diop-Frimpong, B.; Chen, W.; Goel, S.; Naxerova, K.; Ancukiewicz, M.; Boucher, Y.; Jain, R.K.; Xu, L. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA 2012, 109, 16618–16623. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 2012, 366, 2517–2519. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Sondak, V.K.; Smalley, K.S.; Kudchadkar, R.; Grippon, S.; Kirkpatrick, P. Ipilimumab. Nat. Rev. Drug Discov. 2011, 10, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.M. Pembrolizumab: First global approval. Drugs 2014, 74, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Bagcchi, S. Pembrolizumab for treatment of refractory melanoma. Lancet Oncol. 2014, 15, e419. [Google Scholar] [CrossRef]
- PD-1 inhibitors raise survival in NSCLC. Cancer Discov 2014. [CrossRef]
- ESMO. Pembrolizumab Shows Promise in Several Solid Tumours. 2014. Avaliable online: http://www.esmo.org/Conferences/Past-Conferences/ESMO-2014-Congress/News-Articles/Pembrolizumab-Shows-Promise-in-Several-Solid-Tumours (accessed on 15 April 2015).
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Squibb, B.-M. CheckMate-017,A phase 3 study of Opdivo (nivolumab) compared to docetaxel in patients with second-line squamous cell non-small cell lung cancer, stopped early. 2015. Avaliable online: http://news.bms.com/press-release/checkmate-017-phase-3-study-opdivo-nivolumab-compared-docetaxel-patients-second-line-s (accessed on 15 April 2015).
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Sznol, M.; Kluger, H.M.; Callahan, M.K.; Postow, M.A.; Gordon, R.A.; Segal, N.H.; Rizvi, N.A.; Lesokhin, A.M.; Atkins, M.B.; Kirkwood, J.M.; et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). ASCO Meet. Abstr. 2014, 32. Abstract Number: LBA9003. [Google Scholar]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, C.; Dominguez, A.L.; Lollini, P.L.; Croft, M.; Mittler, R.S.; Borgstrom, P.; Lustgarten, J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int. J. Cancer 2005, 116, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schadendorf, D.; Messina, M.; Hodi, F.S.; O’Day, S. MDX010-20 investigators. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin. Cancer Res. 2013, 19, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Tang, L.H.; Klimstra, D.S.; Brennan, M.F.; Brody, J.R.; Rocha, F.G.; Jia, X.; Qin, L.X.; D’Angelica, M.I.; DeMatteo, R.P.; et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS ONE 2012, 7, e40157. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Disis, M.L. Designing vaccines to prevent breast cancer recurrence or invasive disease. Immunotherapy 2015, 7, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.P.; Stanton, S.E.; Disis, M.L. The Antigenic Repertoire of Premalignant and High-Risk Lesions. Cancer Prev. Res. (Phila) 2015, 8, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Robbins, P.F.; Yao, X.; Li, Y.F.; Turcotte, S.; Tran, E.; Wunderlich, J.R.; Mixon, A.; Farid, S.; Dudley, M.E.; et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Investig. 2014, 124, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.L.; Forni, G. Cancer immunoprevention: Tracking down persistent tumor antigens. Trends. Immunol. 2003, 24, 62–66. [Google Scholar] [CrossRef]
- Tuohy, V.K. Retired self-proteins as vaccine targets for primary immunoprevention of adult-onset cancers. Expert Rev. Vaccines 2014, 13, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.J.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lollini, P.-L.; Cavallo, F.; Nanni, P.; Quaglino, E. The Promise of Preventive Cancer Vaccines. Vaccines 2015, 3, 467-489. https://doi.org/10.3390/vaccines3020467
Lollini P-L, Cavallo F, Nanni P, Quaglino E. The Promise of Preventive Cancer Vaccines. Vaccines. 2015; 3(2):467-489. https://doi.org/10.3390/vaccines3020467
Chicago/Turabian StyleLollini, Pier-Luigi, Federica Cavallo, Patrizia Nanni, and Elena Quaglino. 2015. "The Promise of Preventive Cancer Vaccines" Vaccines 3, no. 2: 467-489. https://doi.org/10.3390/vaccines3020467