Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future
Abstract
:1. Introduction
| 18–60 Years | > 60 Years |
|---|---|
| Seroconversion rate > 40% | Seroconversion rate > 30% |
| Mean geometric increase > 2.5 | Mean geometric increase > 2.0 |
| Seroprotection rate > 70% | Seroprotection rate > 60% |
2. Correlates of Protection
3. Haemagglutination Inhibition Assay
| Antibody cutoff level for clinical protection | |
|---|---|
| Protection Level | Antibody Cutoff Level |
| 50% | 1:110 |
| 70% | 1:215 |
| 80% | 1:330 |
| 90% | 1:629 |
4. Virus Neutralization Assay

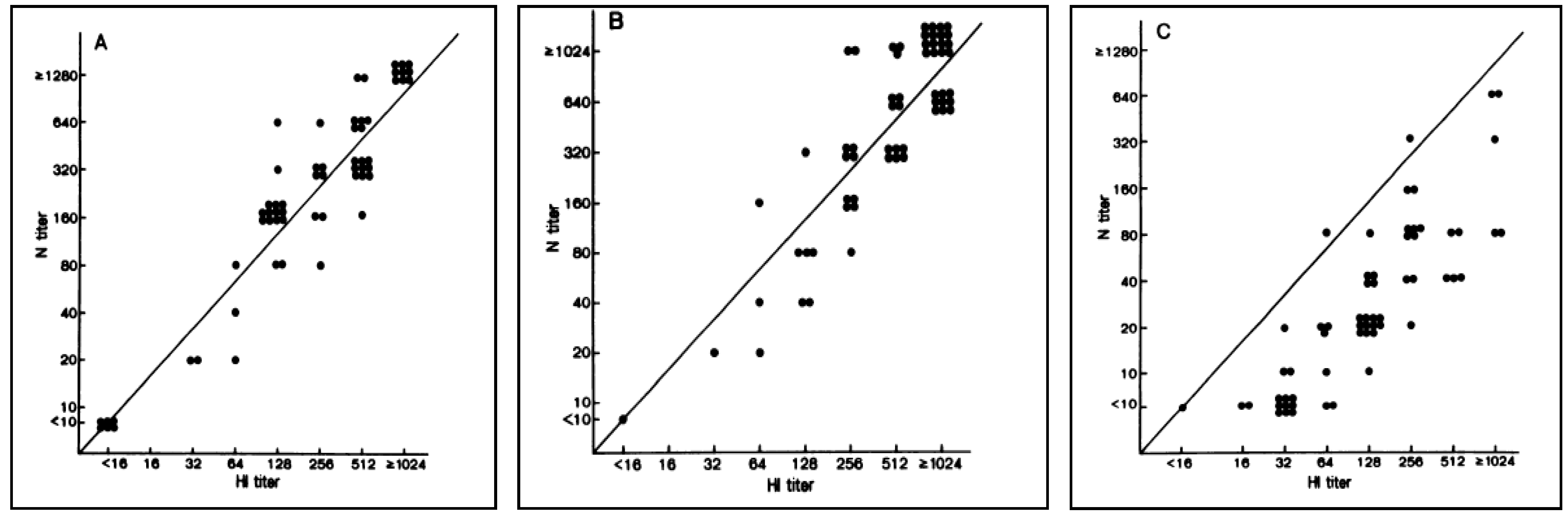
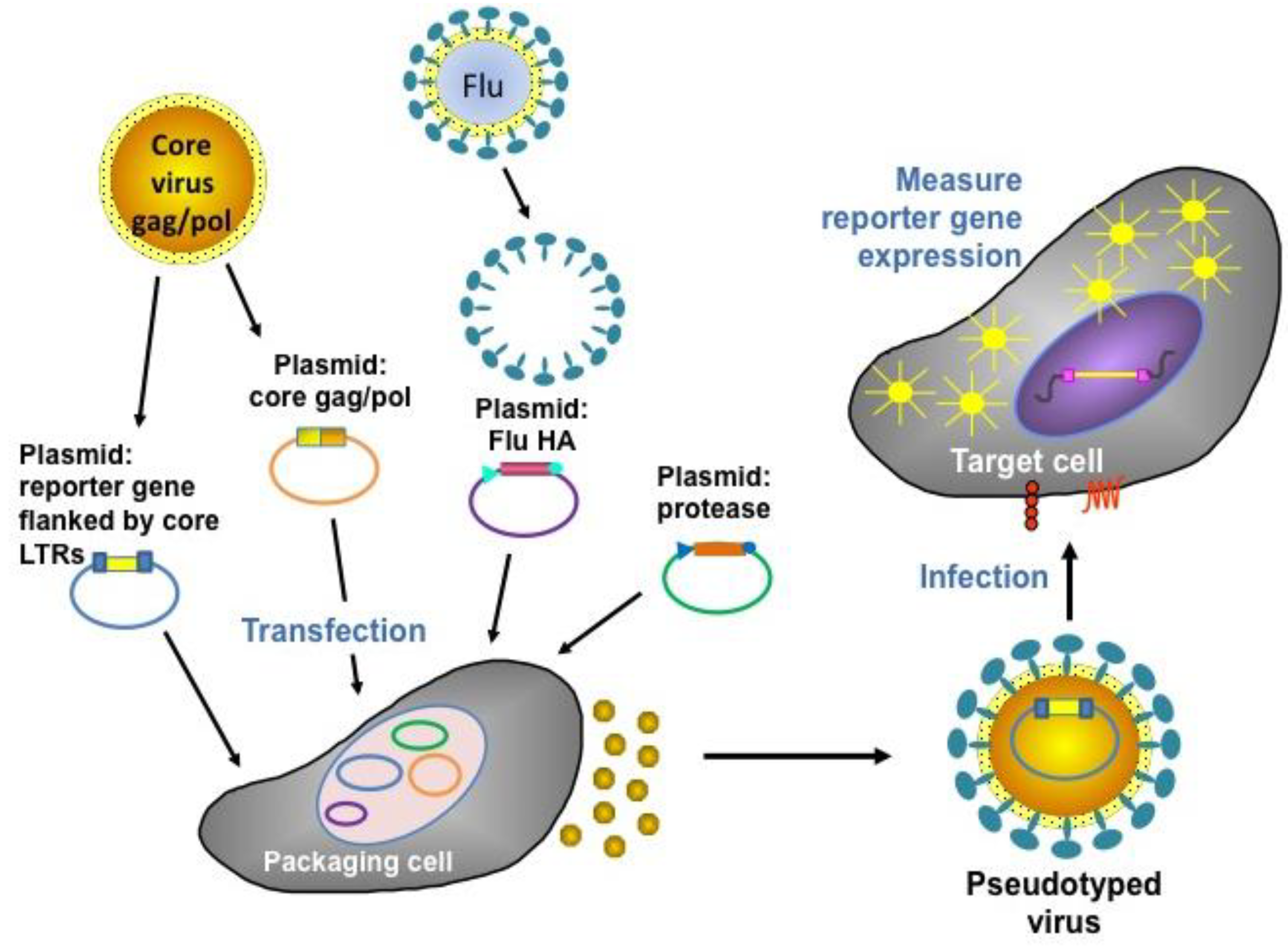
5. Pseudotype-Based Assays
6. Single Radial Haemolysis Assay

7. Neuraminidase Antibody Titration
8. Cell-Mediated Immunity
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Hilleman, M.R. Realities and enigmas of human viral influenza: Pathogenesis, epidemiology and control. Vaccine 2002, 20, 3068–3087. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, G.A.; Palache, A.M.; Forleo, E.; Fedson, D.S. Influenza vaccination in 2000: Recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine 2003, 21, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Prevention and control of Influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5208a1.htm (accessed on 04 November 2013).
- Monto, A.S. Vaccines and antiviral drugs in pandemic preparedness. Emerg. Infect. Dis. 2006, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S.; Opal, S.M. The controversy over H5N1 transmissibility research: An opportunity to define a practical response to a global threat. Hum. Vaccin. Immunother. 2013, 9, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, F.; Saluja, V.; ter Veer, W.; Amorij, J.P.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.; Huckriede, A. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010, 12, 215–222. [Google Scholar] [CrossRef]
- Onions, D.; Egan, W.; Jarrett, R.; Novicki, D.; Gregersen, J.P. Validation of the safety of MDCK cells as a substrate for the production of a cell-derived influenza vaccine. Biologicals 2010, 38, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Montomoli, E.; Capecchi, B.; Hoschler, K. Correlates of Protection against Influenza. In Influenza Vaccines for the Future; Schmidt, A., Weber, O., Kaufmann, S.H.E., Eds.; Springer: Basel, Switaland, 2011. [Google Scholar]
- Beyer, W.E.; Palache, A.M.; de Jong, J.C.; Osterhaus, A.D. Cold-adapted live influenza vaccine versus inactivated vaccine: Systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 2002, 20, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Brokstad, K.A.; Ogra, P. Influenza virus: Immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 2004, 59, 1–15. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010. [Google Scholar] [CrossRef]
- Howard, M.K.; Sabarth, N.; Savidis-Dacho, H.; Portsmouth, D.; Kistner, O.; Kreil, T.R.; Ehrlich, H.J.; Barrett, P.N. H5N1 whole-virus vaccine induces neutralizing antibodies in humans which are protective in a mouse passive transfer model. PLoS One 2011, 6, e23791. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.A.; Davis, A.E. Neutralization test in influenza: Use in individuals without hemagglutination inhibition antibody. J. Clin. Microbiol. 1979, 10, 382–384. [Google Scholar] [PubMed]
- Stephenson, I.; Heath, A.; Major, D.; Newman, R.W.; Hoschler, K.; Junzi, W.; Katz, J.M.; Weir, J.P.; Zambon, M.C.; Wood, J.M. Reproducibility of serologic assays for influenza virus A (H5N1). Emerg. Infect. Dis. 2009, 15, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Atmar, R.L.; Keitel, W.A.; Quarles, J.M.; Wells, J.; Arden, N.; Nino, D. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine 2012, 31, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Sultana, I.; Yang, K.; Getie-Kebtie, M.; Couzens, L.; Markoff, L.; Alterman, M.; Eichelberger, M.C. Stability of neuraminidase in inactivated influenza vaccines. Vaccine 2014, 32, 2225–2230. [Google Scholar] [CrossRef]
- Van Assen, S.; de Haan, A.; Holvast, A.; Horst, G.; Gorter, L.; Westra, J.; Kallenberg, C.G.; Telgt, D.S.; Palache, A.M.; Giezeman, K.M.; et al. Cell-mediated immune responses to inactivated trivalent influenza-vaccination are decreased in patients with common variable immunodeficiency. Clin. Immunol. 2011, 141, 161–168. [Google Scholar]
- Skowronski, D.M.; Hottes, T.S.; McElhaney, J.E.; Janjua, N.Z.; Sabaiduc, S.; Chan, T.; Gentleman, B.; Purych, D.; Gardy, J.; Patrick, D.M.; et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: Higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 2011, 203, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Note for Guidance on Harmonisation of Requirements for Influenza Vaccines; The European Agency for the Evaluation of Medicinal Products (EMEA): London, UK, 1996.
- Center for Disease Control and Prevention. Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5510a1.htm (accessed on 4 November 2013).
- McCullers, J.A.; Huber, V.C. Correlates of vaccine protection from influenza and its complications. Hum. Vaccin. Immunother. 2012, 8, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Haessler, S.D.; Brown, R.B. Complications of viral influenza. Am. J. Med. 2008, 121, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gilbert, P.B.; Corey, L.; McElrath, M.J.; Self, S.G. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 2007, 196, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Hannoun, C.; Megas, F.; Piercy, J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004, 103, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.P.; Cooney, M.K.; Hall, C.E.; Foy, H.M. Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am. J. Epidemiol. 1982, 116, 228–242. [Google Scholar] [PubMed]
- Ng, S.; Fang, V.J.; Ip, D.K.; Chan, K.H.; Leung, G.M.; Peiris, J.S.; Cowling, B.J. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J. Infect. Dis. 2013, 208, 1320–1324. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: Correlates of vaccine-induced immunity. Clin. Infect. Dis. 2008, 47, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Orenstein, W.A. Epidemiologic methods in immunization programs. Epidemiol. Rev. 1996, 18, 99–117. [Google Scholar] [CrossRef]
- Dunning, A.J. A model for immunological correlates of protection. Stat. Med. 2006, 25, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Flu Vaccine Effectiveness: Questions and Answers for Health Professionals. Available online: http://www.cdc.gov/flu/professionals/vaccination/effectivenessqa.htm (accessed on 10 November 2013).
- Halloran, M.E.; Longini, I.M., Jr.; Struchiner, C.J. Surrogates of Protection. In Design and Analysis of Vaccine Studies; Springer: New York, USA, 2009. [Google Scholar]
- Sadoff, J.C.; Wittes, J. Correlates, surrogates, and vaccines. J. Infect. Dis. 2007, 196, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Cross, G. Hemagglutination inhibition assays. Semin. Avian Exotic Pet Med. 2002, 11, 15–18. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Manual for the laboratory diagnosis and virological surveillance of influenza. 2011. Available online: http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf (accessed on 10 November 2013).
- Nauta, J.J.; Beyer, W.E.; Osterhaus, A.D. On the relationship between mean antibody level, seroprotection and clinical protection from influenza. Biologicals 2009, 37, 216–221. [Google Scholar] [CrossRef] [PubMed]
- De Jong, J.C.; Palache, A.M.; Beyer, W.E.; Rimmelzwaan, G.F.; Boon, A.C.; Osterhaus, A.D. Haemagglutination-inhibiting antibody to influenza virus. Dev. Biol. (Basel) 2003, 115, 63–73. [Google Scholar]
- Noah, D.L.; Hill, H.; Hines, D.; White, E.L.; Wolff, M.C. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin. Vaccine Immunol. 2009, 16, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; del Giudice, G.; della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.S. Efficacy and correlates of protection for cell culture-derived and egg-derived inactivated influenza vaccines in younger adults. Expert Rev. Vaccines 2011, 10, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Granstrom, M.; Voordouw, A.C. Registration of influenza vaccines for children in Europe. Vaccine 2011, 29, 7572–7575. [Google Scholar] [CrossRef] [PubMed]
- Cosma, A.; Buhler, S.; Nagaraj, R.; Staib, C.; Hammarin, A.L.; Wahren, B.; Goebel, F.D.; Erfle, V.; Sutter, G. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin. Diagn. Lab. Immunol. 2004, 11, 406–410. [Google Scholar] [PubMed]
- Van der Velden, M.V.; Aichinger, G.; Pollabauer, E.M.; Low-Baselli, A.; Fritsch, S.; Benamara, K.; Kistner, O.; Muller, M.; Zeitlinger, M.; Kollaritsch, H.; et al. Cell culture (Vero cell) derived whole-virus non-adjuvanted H5N1 influenza vaccine induces long-lasting cross-reactive memory immune response: Homologous or heterologous booster response following two dose or single dose priming. Vaccine 2012, 30, 6127–6135. [Google Scholar] [CrossRef] [PubMed]
- Adamson, W.E.; McGregor, E.C.; Kavanagh, K.; McMenamin, J.; McDonagh, S.; Molyneaux, P.J.; Templeton, K.E.; Carman, W.F. Population exposure to a novel influenza A virus over three waves of infection. J. Clin. Virol. 2011, 52, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.L.; Puck, J.; Hughes, B.J.; Cate, T.R. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J. Clin. Microbiol. 1980, 12, 426–432. [Google Scholar] [PubMed]
- Harmon, M.W.; Rota, P.A.; Walls, H.H.; Kendal, A.P. Antibody response in humans to influenza virus type B host-cell-derived variants after vaccination with standard (egg-derived) vaccine or natural infection. J. Clin. Microbiol. 1988, 26, 333–337. [Google Scholar] [PubMed]
- Ansaldi, F.; Bacilieri, S.; Amicizia, D.; Valle, L.; Banfi, F.; Durando, P.; Sticchi, L.; Gasparini, R.; Icardi, G.; Crovari, P. Antigenic characterisation of influenza B virus with a new microneutralisation assay: Comparison to haemagglutination and sequence analysis. J. Med. Virol. 2004, 74, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.; Abernathy, R.A.; Hu-Primmer, J.; Thompson, W.W.; Lu, X.; Lim, W.; Fukuda, K.; Cox, N.J.; Katz, J.M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 1999, 37, 937–943. [Google Scholar] [PubMed]
- Ehrlich, H.J.; Muller, M.; Oh, H.M.; Tambyah, P.A.; Joukhadar, C.; Montomoli, E.; Fisher, D.; Berezuk, G.; Fritsch, S.; Low-Baselli, A.; et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N. Engl. J. Med. 2008, 358, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Veguilla, V.; Hancock, K.; Schiffer, J.; Gargiullo, P.; Lu, X.; Aranio, D.; Branch, A.; Dong, L.; Holiday, C.; Liu, F.; et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J. Clin. Microbiol. 2011, 49, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Hoschler, K.; Hardelid, P.; Stanford, E.; Andrews, N.; Zambon, M. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross-sectional serological study. Lancet 2010, 375, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Allison, K.J.; van de Velde, L.A.; Li, Y.; Flynn, P.M.; McCullers, J.A. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine 2012, 30, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; Macdonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vaccine Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef]
- Stephenson, I.; Das, R.G.; Wood, J.M.; Katz, J.M. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: An international collaborative study. Vaccine 2007, 25, 4056–4063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Recommendations and laboratory procedures for detection of avian influenza A(H5N1) virus in specimens from suspected human cases. 2007. Available online: http://www.who.int/influenza/resources/documents/RecAIlabtestsAug07.pdf (accessed on 11 November 2013).
- Cox, R.J.; Pedersen, G.; Madhun, A.S.; Svindland, S.; Saevik, M.; Breakwell, L.; Hoschler, K.; Willemsen, M.; Campitelli, L.; Nostbakken, J.K.; et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M adjuvant in a phase I clinical trial. Vaccine 2011, 29, 8049–8059. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Medini, D.; Borgogni, E.; Zedda, L.; Bardelli, M.; Malzone, C.; Nuti, S.; Tavarini, S.; Sammicheli, C.; Hilbert, A.K.; et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. USA 2009, 106, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Allwinn, R.; Geiler, J.; Berger, A.; Cinatl, J.; Doerr, H.W. Determination of serum antibodies against swine-origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: How is the prevalence rate of protecting antibodies in humans? Med. Microbiol. Immunol. 2010, 199, 117–121. [Google Scholar] [CrossRef]
- Hancock, K.; Veguilla, V.; Lu, X.; Zhong, W.; Butler, E.N.; Sun, H.; Liu, F.; Dong, L.; DeVos, J.R.; Gargiullo, P.M.; et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 2009, 361, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, I.; Wood, J.M.; Nicholson, K.G.; Charlett, A.; Zambon, M.C. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004, 103, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kayali, G.; Setterquist, S.F.; Capuano, A.W.; Myers, K.P.; Gill, J.S.; Gray, G.C. Testing human sera for antibodies against avian influenza viruses: Horse RBC hemagglutination inhibition vs. microneutralization assays. J. Clin. Virol. 2008, 43, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Tanaka, K.; Baba, K.; Maeda, A.; Kunita, N.; Ueda, S. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J. Clin. Microbiol. 1990, 28, 1308–1313. [Google Scholar] [PubMed]
- Leroux-Roels, I.; Leroux-Roels, G. Current status and progress of prepandemic and pandemic influenza vaccine development. Expert Rev. Vaccines 2009, 8, 401–423. [Google Scholar] [CrossRef]
- Temperton, N.J.; Hoschler, K.; Major, D.; Nicolson, C.; Manvell, R.; Hien, V.M.; Do, Q.H.; de Jong, M.; Zambon, M.; Takeuchi, Y.; et al. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Other Respir. Viruses 2007, 1, 105–112. [Google Scholar] [CrossRef]
- Garcia, J.M.; Lai, J.C. Production of influenza pseudotyped lentiviral particles and their use in influenza research and diagnosis: An update. Expert Rev. Anti Infect. Ther. 2011, 9, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Molesti, E.; Milani, A.; Terregino, C.; Cattoli, G.; Temperton, N.J. Comparative serological assays for the study of H5 and H7 avian influenza viruses. Influenza Res. Treat. 2013. [Google Scholar] [CrossRef]
- Bottcher, E.; Matrosovich, T.; Beyerle, M.; Klenk, H.D.; Garten, W.; Matrosovich, M. Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium. J. Virol. 2006, 80, 9896–9898. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Molesti, E.; Bottcher-Friebertshauser, E.; Cattoli, G.; Corti, D.; Scott, S.D.; Temperton, N.J. The human Transmembrane Protease Serine 2 is necessary for the production of Group 2 influenza A virus pseudotypes. J. Mol. Genet. Med. 2012, 7, 309–314. [Google Scholar] [PubMed]
- Scott, S.; Molesti, E.; Temperton, N.; Ferrara, F.; Bottcher-Friebertshauser, E.; Daly, J. The use of equine influenza pseudotypes for serological screening. J. Mol. Genet. Med. 2012, 6, 304–308. [Google Scholar] [PubMed]
- Molesti, E.; Wright, E.; Terregino, C.; Rahman, R.; Cattoli, G.; Temperton, N. Multiplex evaluation of influenza neutralizing antibodies with potential applicability to in-field serological studies. J. Immunol. Res. 2014. [Google Scholar] [CrossRef]
- Alberini, I.; del Tordello, E.; Fasolo, A.; Temperton, N.J.; Galli, G.; Gentile, C.; Montomoli, E.; Hilbert, A.K.; Banzhoff, A.; del Giudice, G.; et al. Pseudoparticle neutralization is a reliable assay to measure immunity and cross-reactivity to H5N1 influenza viruses. Vaccine 2009, 27, 5998–6003. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Suguitan, A.L., Jr.; Pinna, D.; Silacci, C.; Fernandez-Rodriguez, B.M.; Vanzetta, F.; Santos, C.; Luke, C.J.; Torres-Velez, F.J.; Temperton, N.J.; et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Investig. 2010, 120, 1663–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.M.; Lagarde, N.; Ma, E.S.; de Jong, M.D.; Peiris, J.S. Optimization and evaluation of an influenza A (H5) pseudotyped lentiviral particle-based serological assay. J. Clin. Virol. 2010, 47, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, J.M.; Pepin, S.; Lagarde, N.; Ma, E.S.; Vogel, F.R.; Chan, K.H.; Chiu, S.S.; Peiris, J.S. Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin. PLoS One 2009, 4, e7918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultberg, A.; Temperton, N.J.; Rosseels, V.; Koenders, M.; Gonzalez-Pajuelo, M.; Schepens, B.; Ibanez, L.I.; Vanlandschoot, P.; Schillemans, J.; Saunders, M.; et al. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One 2011, 6, e17665. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ma, C.; Liu, Z.; He, W. Serologic cross-reactivity among humans and birds infected with highly pathogenic avian influenza A subtype H5N1 viruses in China. Immunol. Lett. 2011, 135, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Butler, E.N.; Veguilla, V.; Vassell, R.; Thomas, J.T.; Moos, M., Jr.; Ye, Z.; Hancock, K.; Weiss, C.D. Establishment of retroviral pseudotypes with influenza hemagglutinins from H1, H3, and H5 subtypes for sensitive and specific detection of neutralizing antibodies. J. Virol. Methods 2008, 153, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef]
- Kaminski, D.A.; Lee, F.E. Antibodies against conserved antigens provide opportunities for reform in influenza vaccine design. Front. Immunol. 2011, 2. [Google Scholar] [CrossRef]
- Hai, R.; Krammer, F.; Tan, G.S.; Pica, N.; Eggink, D.; Maamary, J.; Margine, I.; Albrecht, R.A.; Palese, P. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J. Virol. 2012, 86, 5774–5781. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, V.V.; Citron, M.; Ferrara, F.; Lu, X.; Callahan, C.; Heidecker, G.J.; Sarma, S.P.; Flynn, J.A.; Temperton, N.J.; Liang, X.; et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl. Acad. Sci. USA 2014, 111, E2514–E2523. [Google Scholar] [CrossRef] [PubMed]
- Molesti, E.; Ferrara, F.; Lapini, G.; Montomoli, E.; Temperton, N. Discordant correlation between serological assays observed when measuring heterosubtypic responses against avian influenza H5 and H7 viruses in unexposed individuals. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Schild, G.C.; Pereira, M.S.; Chakraverty, P. Single-radial-hemolysis: A new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull. World Health Organ. 1975, 52, 43–50. [Google Scholar]
- Russell, S.M.; McCahon, D.; Beare, A.S. A single radial haemolysis technique for the measurement of influenza antibody. J. Gen. Virol. 1975, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morley, P.S.; Hanson, L.K.; Bogdan, J.R.; Townsend, H.G.; Appleton, J.A.; Haines, D.M. The relationship between single radial hemolysis, hemagglutination inhibition, and virus neutralization assays used to detect antibodies specific for equine influenza viruses. Vet. Microbiol. 1995, 45, 81–92. [Google Scholar] [CrossRef]
- Chan, Y.C.; Tan, H.C.; Tan, S.H.; Balachandran, K. The use of the single radial haemolysis technique in the serological diagnosis of dengue and Japanese encephalitis virus infections. Bull. World Health Organ. 1985, 63, 1043–1053. [Google Scholar] [PubMed]
- Oxford, J.S.; Yetts, R.; Schild, G.C. Quantitation and analysis of the specificity of post-immunization antibodies to influenza B viruses using single radial haemolysis. J. Hyg. (Lond.) 1982, 88, 325–333. [Google Scholar] [CrossRef]
- Fulton, R.E.; DiNinno, V.L.; Frank, R.I.; Fildes, J.; Turner, I.J. Single radial hemolysis test for quantitation of complement-fixing antibodies to non-hemagglutinating viruses. J. Clin. Microbiol. 1984, 20, 248–254. [Google Scholar] [PubMed]
- Vaananen, P.; Hovi, T.; Helle, E.P.; Penttinen, K. Determination of mumps and influenza antibodies by haemolysis-in-gel. Arch. Virol. 1976, 52, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Boustred, J.; Seagroatt, V.; Schild, G.C. The use of single-radial-haemolysis for rubella antibody studies. J. Hyg. (Lond.) 1977, 79, 355–364. [Google Scholar] [CrossRef]
- Russell, S.M.; Benjamin, S.R.; Briggs, M.; Jenkins, M.; Mortimer, P.P.; Payne, S.B. Evaluation of the single radial haemolysis (SRH) technique for rubella antibody measurement. J. Clin. Pathol. 1978, 31, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Probert, M.; Russell, S.M. Measurement of parainfluenza-3 virus antibody by the single radial hemolysis technique. J. Clin. Microbiol. 1975, 2, 157–161. [Google Scholar] [PubMed]
- Hierholzer, J.C.; Tannock, G.A. Quantitation of antibody to non-hemagglutinating viruses by single radial hemolysis: Serological test for human coronaviruses. J. Clin. Microbiol. 1977, 5, 613–620. [Google Scholar] [PubMed]
- Wood, J.M.; Melzack, D.; Newman, R.W.; Major, D.L.; Zambon, M.; Nicholson, K.G.; Podda, A. A single radial haemolysis assay for antibody to H5 haemagglutinin. Int. Congr. Ser. 2001, 1219, 761–766. [Google Scholar] [CrossRef]
- Al-Khayatt, R.; Jennings, R.; Potter, C.W. Interpretation of responses and protective levels of antibody against attenuated influenza A viruses using single radial haemolysis. J. Hyg. (Lond.) 1984, 93, 301–312. [Google Scholar] [CrossRef]
- Callow, K.A.; Beare, A.S. Measurement of antibody to influenza virus neuraminidase by single radial hemolysis in agarose gels. Infect. Immun. 1976, 13, 1–8. [Google Scholar] [PubMed]
- Joss, A.W.; McPherson, J.K.; Williams, H. Single radial haemolysis: A survey of antibody titres in the highland region of Scotland to recent strains of influenza A. J. Hyg. (Lond.) 1978, 80, 1–11. [Google Scholar] [CrossRef]
- Mancini, G.; Donatelli, I.; Arangio-Ruiz, G.; Rozera, C.; Macchia, T. Comparison of haemagglutination-inhibition and single radial haemolysis techniques for detecting antibodies to influenza A and B viruses. J. Hyg. (Lond.) 1983, 91, 157–162. [Google Scholar] [CrossRef]
- Wood, J.M.; Gaines-Das, R.E.; Taylor, J.; Chakraverty, P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994, 12, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Farrohi, K.; Farrohi, F.K.; Noble, G.R.; Kaye, H.S.; Kendal, A.P. Evaluation of the single radial hemolysis test for measuring hemagglutinin- and neuraminidase-specific antibodies to H3N2 influenza strains and antibodies to influenza B. J. Clin. Microbiol. 1977, 5, 353–360. [Google Scholar] [PubMed]
- Nicholson, K.G.; Colegate, A.E.; Podda, A.; Stephenson, I.; Wood, J.; Ypma, E.; Zambon, M.C. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: A randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001, 357, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, S.X.; Liu, Y.X.; Zhang, P.H.; Zuo, S.Q.; Lin, Z.; Dang, R.L.; Ma, Y.H.; Zhang, C.; Zhang, L.; et al. Increased sensitivity for detecting avian influenza-specific antibodies by a modified hemagglutination inhibition assay using horse erythrocytes. J. Virol. Methods 2008, 153, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, I.; Nicholson, K.G.; Colegate, A.; Podda, A.; Wood, J.; Ypma, E.; Zambon, M. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 2003, 21, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Abdel-Messih, I.A.; Raupachova, J.; Hobzova, L.; Fragapane, E. A phase III, randomized, open-label study to assess the tolerability and immunogenicity of an H5N1 influenza vaccine administered to healthy adults with a 1-, 2-, 3-, or 6-week interval between first and second doses. Clin. Ther. 2010, 32, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Forsten, A.; Herbinger, K.H.; Cioppa, G.D.; Beygo, J.; Borkowski, A.; Groth, N.; Bennati, M.; von Sonnenburg, F. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine 2012, 30, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Tambyah, P.A.; Wilder-Smith, A.; Pavlova, B.G.; Barrett, P.N.; Oh, H.M.; Hui, D.S.; Yuen, K.Y.; Fritsch, S.; Aichinger, G.; Loew-Baselli, A.; et al. Safety and immunogenicity of two different doses of a Vero cell-derived, whole virus clade 2 H5N1 (A/Indonesia/05/2005) influenza vaccine. Vaccine 2012, 30, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Karvonen, A.; Tilman, S.; Borkowski, A.; Montomoli, E.; Banzhoff, A.; Clemens, R. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 2010, 126, e762–e770. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Richmond, P.C.; McVernon, J.; Skeljo, M.V.; Hartel, G.F.; Bennet, J.; Basser, R.L. Safety and immunogenicity of an inactivated thimerosal-free influenza vaccine in infants and children. Influenza Other Respir. Viruses 2009, 3, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D.; Laver, W.G.; Schulman, J.L.; Webster, R.G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J. Virol. 1968, 2, 281–288. [Google Scholar] [PubMed]
- Sylte, M.J.; Suarez, D.L. Influenza neuraminidase as a vaccine antigen. Curr. Top. Microbiol. Immunol. 2009, 333, 227–241. [Google Scholar] [PubMed]
- Kilbourne, E.D.; Couch, R.B.; Kasel, J.A.; Keitel, W.A.; Cate, T.R.; Quarles, J.H.; Grajower, B.; Pokorny, B.A.; Johansson, B.E. Purified influenza A virus N2 neuraminidase vaccine is immunogenic and non-toxic in humans. Vaccine 1995, 13, 1799–1803. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L.; Khakpour, M.; Kilbourne, E.D. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J. Virol. 1968, 2, 778–786. [Google Scholar] [PubMed]
- Naikhin, A.N.; Tsaritsina, I.M.; Oleinikova, E.V.; Syrodoeva, L.G.; Korchanova, N.L.; Denisov, G.M.; Shvartsman, Ya.S. The importance of antineuraminidase antibodies in resistance to influenza A and immunologic memory for their synthesis. J. Hyg. (Lond.) 1983, 91, 131–138. [Google Scholar] [CrossRef]
- Webster, R.G.; Laver, W.G.; Kilbourne, E.D. Reactions of antibodies with surface antigens of influenza virus. J. Gen. Virol. 1968, 3, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Moran, T.M.; Bona, C.A.; Popple, S.W.; Kilbourne, E.D. Immunologic response to influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. II. Sequential infection of mice simulates human experience. J. Immunol. 1987, 139, 2010–2014. [Google Scholar] [PubMed]
- Chen, Z.; Kim, L.; Subbarao, K.; Jin, H. The 2009 pandemic H1N1 virus induces anti-neuraminidase (NA) antibodies that cross-react with the NA of H5N1 viruses in ferrets. Vaccine 2012, 30, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Sylte, M.J.; Hubby, B.; Suarez, D.L. Influenza neuraminidase antibodies provide partial protection for chickens against high pathogenic avian influenza infection. Vaccine 2007, 25, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Hassantoufighi, A.; Zhang, H.; Sandbulte, M.; Gao, J.; Manischewitz, J.; King, L.; Golding, H.; Straight, T.M.; Eichelberger, M.C. A practical influenza neutralization assay to simultaneously quantify hemagglutinin and neuraminidase-inhibiting antibody responses. Vaccine 2010, 28, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lambre, C.R.; Terzidis, H.; Greffard, A.; Webster, R.G. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J. Immunol. Methods 1990, 135, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Report from Scientific Workshop on Serology Assays and Correlates of Protection for Influenza Vaccines; European Medicines Agency: London, UK, 2010.
- Fritz, R.; Sabarth, N.; Kiermayr, S.; Hohenadl, C.; Howard, M.K.; Ilk, R.; Kistner, O.; Ehrlich, H.J.; Barrett, P.N.; Kreil, T.R. A vero cell-derived whole-virus H5N1 vaccine effectively induces neuraminidase-inhibiting antibodies. J. Infect. Dis. 2012, 205, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, V.; Orekov, T.; Alabanza, C.; Porika, U.; Jiang, H.; Connolly, K.; Pincus, S. Influenza virus-like particles as a new tool for vaccine immunogenicity testing: Validation of a neuraminidase neutralizing antibody assay. J. Virol. Methods 2011, 173, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kendal, A.P.; Bozeman, F.M.; Ennis, F.A. Further studies of the neuraminidase content of inactivated influenza vaccines and the neuraminidase antibody responses after vaccination of immunologically primed and unprimed populations. Infect. Immun. 1980, 29, 966–971. [Google Scholar] [PubMed]
- Monto, A.S.; Kendal, A.P. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973, 1, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, J.D.; Schwartzman, L.M.; Gao, J.; Kash, J.C.; Morens, D.M.; Couzens, L.; Wan, H.; Eichelberger, M.C.; Taubenberger, J.K. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 2012, 432, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sandbulte, M.R.; Westgeest, K.B.; Gao, J.; Xu, X.; Klimov, A.I.; Russell, C.A.; Burke, D.F.; Smith, D.J.; Fouchier, R.A.; Eichelberger, M.C. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc. Natl. Acad. Sci. USA 2011, 108, 20748–20753. [Google Scholar] [CrossRef] [PubMed]
- Rockman, S.; Brown, L.E.; Barr, I.G.; Gilbertson, B.; Lowther, S.; Kachurin, A.; Kachurina, O.; Klippel, J.; Bodle, J.; Pearse, M.; et al. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J. Virol. 2013, 87, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Kasel, J.A.; Gerin, J.L.; Schulman, J.L.; Kilbourne, E.D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 1974, 129, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Kilbourne, E.D.; Johansson, B.E. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin. Diagn. Lab. Immunol. 1996, 3, 511–516. [Google Scholar] [PubMed]
- Sandbulte, M.R.; Jimenez, G.S.; Boon, A.C.; Smith, L.R.; Treanor, J.J.; Webby, R.J. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007, 4, e59. [Google Scholar] [CrossRef] [PubMed]
- Brett, I.C.; Johansson, B.E. Immunization against influenza A virus: Comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology 2005, 339, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Matthews, J.T.; Kilbourne, E.D. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine 1998, 16, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; Bodewes, R.; de Vries, R.P.; Kreijtz, J.H.; Bartelink, W.; van Amerongen, G.; Rimmelzwaan, G.F.; de Haan, C.A.; Osterhaus, A.D.; Rottier, P.J. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 2010, 84, 10366–10374. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D.; Johansson, B.E.; Grajower, B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl. Acad. Sci. USA 1990, 87, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Bucher, D.J.; Kilbourne, E.D. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J. Virol. 1989, 63, 1239–1246. [Google Scholar] [PubMed]
- Gamblin, S.J.; Skehel, J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L. The role of antineuraminidase antibody in immunity to influenza virus infection. Bull. World Health Organ. 1969, 41, 647–650. [Google Scholar] [PubMed]
- Rappuoli, R. Bridging the knowledge gaps in vaccine design. Nat. Biotechnol. 2007, 25, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Galli, G.; Castellino, F.; Golding, H.; Khurana, S.; del Giudice, G.; Rappuoli, R. Influenza vaccine immunology. Immunol. Rev. 2011, 239, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, S.; Weber, G.F.; Panoutsakopoulou, V.; Sanchirico, M.E.; Jansson, M.; Zawaideh, S.; Rittling, S.R.; Denhardt, D.T.; Glimcher, M.J.; Cantor, H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 2000, 287, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, W.J.; Hackett, J.C. The Influenza Viruses. In The Viruses; Springer: New York, NY, USA, 1989. [Google Scholar]
- Rimmelzwaan, G.F.; Nieuwkoop, N.; Brandenburg, A.; Sutter, G.; Beyer, W.E.; Maher, D.; Bates, J.; Osterhaus, A.D. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine 2000, 19, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Stambas, J.; Guillonneau, C.; Kedzierska, K.; Mintern, J.D.; Doherty, P.C.; la Gruta, N.L. Killer T cells in influenza. Pharmacol. Ther. 2008, 120, 186–196. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Krauss, S.L.; Riberdy, J.M.; Webster, R.G.; Woodland, D.L. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 2000, 81, 2689–2696. [Google Scholar]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Keating, R.; Hulse-Post, D.J.; Doherty, P.C. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006, 12, 48–54. [Google Scholar] [CrossRef]
- Zaiss, D.M.; Boog, C.J.; van Eden, W.; Sijts, A.J. Considerations in the design of vaccines that induce CD8 T cell mediated immunity. Vaccine 2010, 28, 7716–7722. [Google Scholar] [CrossRef] [PubMed]
- Yewdell, J.W.; Bennink, J.R.; Smith, G.L.; Moss, B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Murasko, D.M.; Bernstein, E.D.; Gardner, E.M.; Gross, P.; Munk, G.; Dran, S.; Abrutyn, E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol. 2002, 37, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Decman, V.; Ali, M.A.; Abt, M.C.; Wolf, A.I.; Monticelli, L.A.; Mozdzanowska, K.; Angelosanto, J.M.; Artis, D.; Erikson, J.; et al. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 2013, 9, e1003207. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Zhang, A.J.; Hung, I.F.; Xu, T.; Ip, W.C.; Wong, R.T.; Ng, J.C.; Chan, J.F.; Chan, K.H.; Yuen, K.Y. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin. Vaccine Immunol. 2012, 19, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Jegaskanda, S.; Job, E.R.; Kramski, M.; Laurie, K.; Isitman, G.; de Rose, R.; Winnall, W.R.; Stratov, I.; Brooks, A.G.; Reading, P.C.; et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J. Immunol. 2013, 190, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, K.; Brydak, L. The epidemiology and history of influenza. Biomed. Pharmacother. 2000, 54, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Briganti, E.; Manfredi, R.; Chiodo, F. Influenza. Recent Prog. Med. 2000, 91, 657–666. [Google Scholar]
- Hay, A.J.; Gregory, V.; Douglas, A.R.; Lin, Y.P. The evolution of human influenza viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1861–1870. [Google Scholar] [CrossRef]
- Han, T.; Marasco, W.A. Structural basis of influenza virus neutralization. Ann. NY Acad. Sci. 2011, 1217, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Haber, M. Estimation of the direct and indirect effects of vaccination. Stat. Med. 1999, 18, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fang, H.H.; Chen, J.T.; Zhou, J.C.; Feng, Z.J.; Li, C.G.; Qiu, Y.Z.; Liu, Y.; Lu, M.; Liu, L.Y.; et al. Immunogenicity, safety, and cross-reactivity of an inactivated, adjuvanted, prototype pandemic influenza (H5N1) vaccine: A phase II, double-blind, randomized trial. Clin. Infect. Dis. 2009, 48, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.N.; Berezuk, G.; Fritsch, S.; Aichinger, G.; Hart, M.K.; El-Amin, W.; Kistner, O.; Ehrlich, H.J. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2011, 377, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Influenza Vaccines Prepared from Viruses with the Potential to Cause a Pandemic and Intended for Use Outside of the Core Dossier Context; Committee for Human Medical Products: London, UK, 2007.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines 2014, 2, 707-734. https://doi.org/10.3390/vaccines2040707
Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines. 2014; 2(4):707-734. https://doi.org/10.3390/vaccines2040707
Chicago/Turabian StyleTrombetta, Claudia Maria, Daniele Perini, Stuart Mather, Nigel Temperton, and Emanuele Montomoli. 2014. "Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future" Vaccines 2, no. 4: 707-734. https://doi.org/10.3390/vaccines2040707






