Immunotherapy with an HIV-DNA Vaccine in Children and Adults
Abstract
:1. Introduction
2. Experimental
2.1. Study Subjects and Trial Design
| Characteristics | Paediatric Study (N = 20) | Adult Study (N = 12) |
|---|---|---|
| Age ± SD, year | 11.6 ± 2.6 | 41.7 ± 6.8 |
| Sex, no. male/female | 7/13 | 12/0 |
| Caucasian race, no. (%) | 18 (90%) | 10 (83%) |
| Placebo/HIV-DNA vaccine, no. | 10/10 | 4/8 |
| CD4+ T-cells/mm3 at baseline, mean ± SD | 763.6 ± 231.1 | 685.9 ± 137.7 |
| CD4+ T-cells/mm3 at last visit, mean ± SD | 803.1 ± 237.5 | 545.4 ± 148.1 |
| HIV-RNA level at baseline copies/mL, mean | <50 | <50 |
| HIV-RNA level at last visit copies/mL, mean | <50 | <50 |
| Duration of vaccination: duration of study weeks | 36:96 | 28:60 |
| Antiretroviral therapy during study, weeks | 96 | 21 |
| Observation period, weeks | 156 | 300 |
2.2. Vaccine Formulation and Administration Technology
2.3. Safety Evaluation
2.4. Laboratory Assays
2.4.1. CD4+ T-Cell Counts, Plasma HIV-1 RNA Levels and Cell-Associated HIV-DNA Quantification
2.4.2. Cellular Immune Responses
2.4.3. Humoral Immune Responses
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Patients Enrolled
3.2. Safety
| Grade 1 | Grade 2 | Grade 3 | ||||
|---|---|---|---|---|---|---|
| Local | Systemic | Local | Systemic | Local | Systemic | |
| Vaccinated children (n = 10) | 12 | 16 | 2 | 6 | 0 | 0 |
| Vaccinated adults (n = 8) | 39 | 17 | 0 | 4 | 1 | 0 |
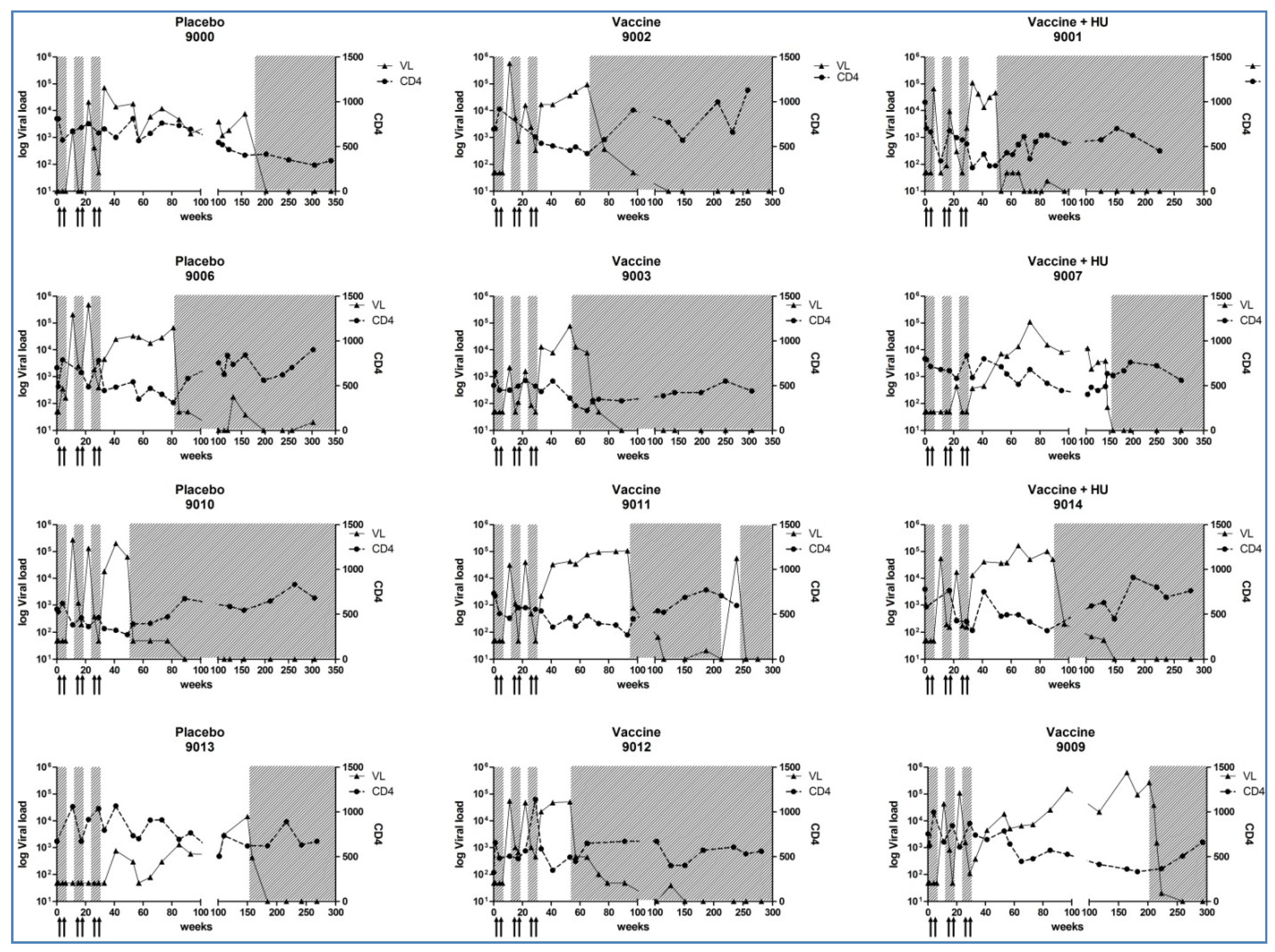
3.3. CD4+ T-Cell Counts, Plasma HIV-1 RNA Levels and Cell-Associated HIV-DNA Quantification
3.4. HIV-Specific Immune Responses
3.4.1. Cellular Immune Responses

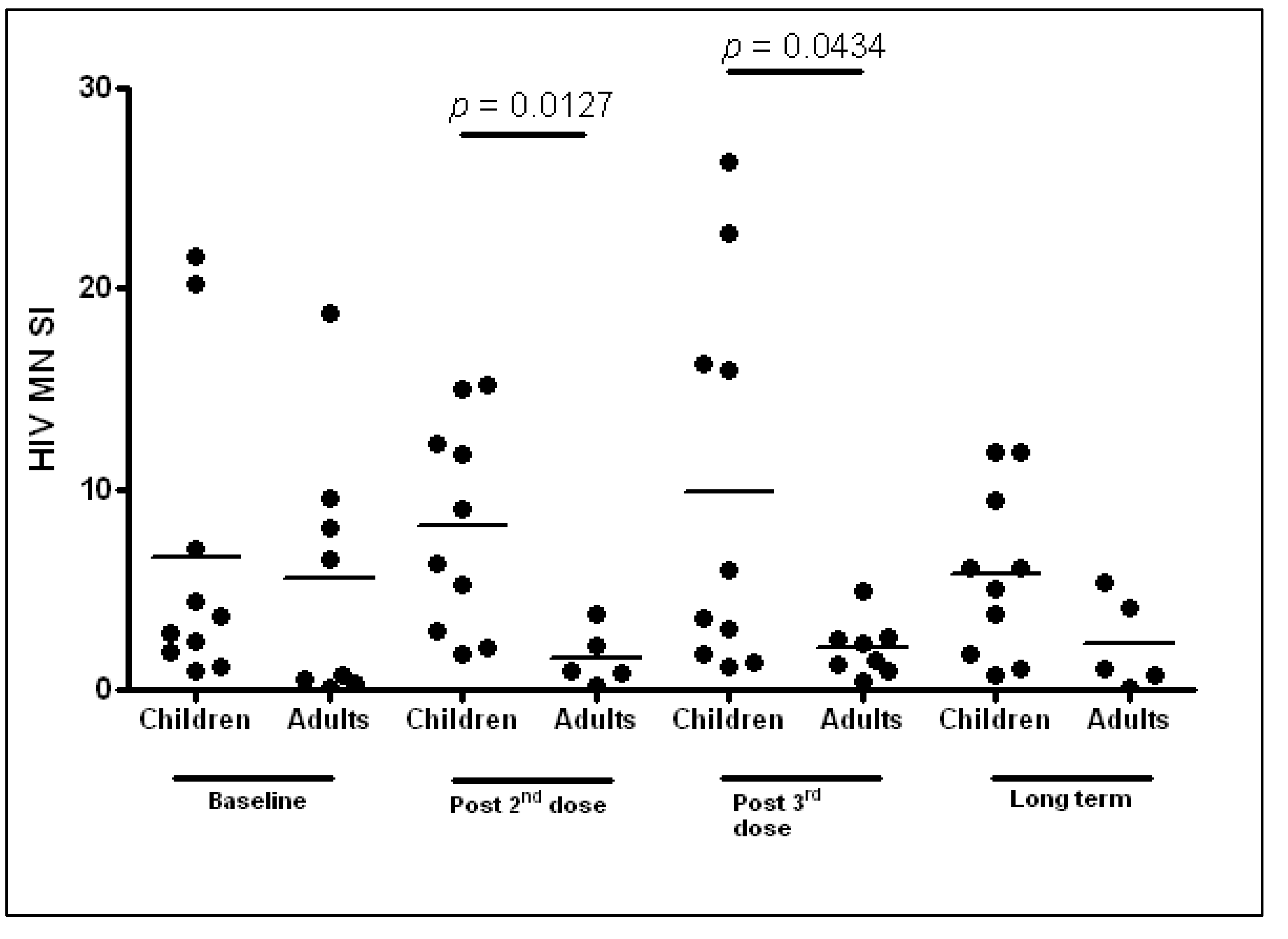

3.4.2. Humoral Responses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garcia, F.; León, A.; Gatell, J.M.; Plana, M.; Gallart, T. Therapeutic vaccines against HIV infection. Hum. Vaccines Immunother. 2012, 8, 569–581. [Google Scholar] [CrossRef]
- Patel, K.; Hernán, M.A.; Williams, P.L.; Seeger, J.D.; McIntosh, K.; Dyke, R.B.; Seage, G.R., III. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 Years and counting. Clin. Infect. Dis. 2008, 46, 1751–1760. [Google Scholar] [CrossRef]
- Piloya, T.; Bakeera-Kitaka, S.; Kekitiinwa, A.; Kamya, M.R. Lipodystrophy among HIV-infected children and adolescents on highly active antiretroviral therapy in Uganda: A cross sectional study. J. Int. AIDS Soc. 2012, 15. [Google Scholar] [CrossRef]
- Haberer, J.; Mellins, C. Pediatric adherence to HIV antiretroviral therapy. Curr. HIV/AIDS Rep. 2009, 6, 194–200. [Google Scholar] [CrossRef]
- Gakhar, H.; Kamali, A.; Holodniy, M. Health-related quality of life assessment after antiretroviral therapy: A review of the literature. Drugs 2013, 7, 651–672. [Google Scholar] [CrossRef]
- Feleke, Y.; Fekade, D.; Mezegebu, Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop. Med. J. 2012, 50, 221–230. [Google Scholar]
- Montagnier, L. 25 years after HIV discovery: Prospects for cure and vaccine. Virology 2010, 397, 248–254. [Google Scholar] [CrossRef]
- Gudmundsdotter, L.; Sjödin, A.; Boström, A.C.; Hejdeman, B.; Theve-Palm, R.; Alaeus, A.; Lidman, K.; Wahren, B. Therapeutic immunization for HIV. Springer Semin. Immunopathol. 2006, 28, 221–230. [Google Scholar] [CrossRef]
- Palma, P.; Romiti, M.L.; Li Pira, G.; Montesano, C.; Mora, N.; Aquilani, A.; Santilli, V.; Tchidjou, H.K.; Ivaldi, F.; Giovannelli, L.; et al. The PEDVAC trial: Preliminary data from the first therapeutic DNA vaccination in HIV-infected children. Vaccine 2011, 29, 6810–6816. [Google Scholar] [CrossRef]
- Gandhi, R.T.; O’Neill, D.; Bosch, R.J.; Chan, E.S.; Bucy, R.P.; Shopis, J.; Baglyos, L.; Adams, E.; Fox, L.; Purdue, L.; et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine 2009, 27, 6088–6094. [Google Scholar] [CrossRef]
- Palma, P.; Romiti, M.L.; Montesano, C.; Santilli, V.; Mora, N.; Aquilani, A.; Dispinseri, S.; Tchidjou, H.K.; Montano, M.; Eriksson, L.E.; et al. Therapeutic DNA vaccination of vertically HIV-infected children: Report of the first pediatric randomised trial (PEDVAC). PLoS One 2013, 8, e79957. [Google Scholar]
- Birx, D.L.; Loomis-Price, L.D.; Aronson, N.; Brundage, J.; Davis, C.; Deyton, L.; Garner, R.; Gordin, F.; Henry, D.; Holloway, W.; et al. Efficacy testing of recombinant human immunodeficiency virus (HIV) gp160 as a therapeutic vaccine in early-stage HIV-1-infected volunteers. J. Infect. Dis. 2000, 181, 881–889. [Google Scholar] [CrossRef]
- Redfield, R.R.; Birx, D.L.; Ketter, N.; Tramont, E.; Polonis, V.; Davis, C.; Brundage, J.F.; Smith, G.; Johnson, S.; Fowler, A.; et al. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N. Engl. J. Med. 1991, 324, 1677–1684. [Google Scholar] [CrossRef]
- Sandstrom, E.; Wahren, B.; Nordic VAC-04 Study Group. Therapeutic immunisation with recombinant gp160 in HIV-1 infection: A randomised double-blind placebo-controlled trial. Lancet 1999, 353, 1735–1742. [Google Scholar]
- Maino, V.C.; Suni, M.A.; Wormsley, S.B.; Carlo, D.J.; Wallace, M.R.; Moss, R.B. Enhancement of HIV type 1 antigen-specific CD4+ T cell memory in subjects with chronic HIV type 1 infection receiving an HIV type 1 immunogen. AIDS Res. Hum. Retrovir. 2000, 16, 539–547. [Google Scholar] [CrossRef]
- Moss, R.B.; Giermakowska, W.; Wallace, M.R.; Savary, J.; Jensen, F.; Carlo, D.J. T-helper-cell proliferative responses to whole-killed human immunodeficiency virus type 1 (HIV-1) and p24 antigens of different clades in HIV-1-infected subjects vaccinated with HIV-1 immunogen (Remune). Clin. Diagn. Lab. Immunol. 2000, 7, 724–727. [Google Scholar]
- Autran, B.; Murphy, R.L.; Costagliola, D.; Tubiana, R.; Clotet, B.; Gatell, J.; Staszewski, S.; Wincker, N.; Assoumou, L.; El-Habib, R.; et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452). AIDS 2008, 22, 1313–1322. [Google Scholar] [CrossRef]
- Kran, A.M.; Sørensen, B.; Nyhus, J.; Sommerfelt, M.A.; Baksaas, I.; Bruun, J.N.; Kvale, D. HLA- and dose-dependent immunogenicity of a peptide-based HIV-1 immunotherapy candidate (Vacc-4x). AIDS 2004, 18, 1875–1883. [Google Scholar] [CrossRef]
- Calarota, S.; Bratt, G.; Nordlund, S.; Hinkula, J.; Leandersson, A.C.; Sandström, E.; Wahren, B. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 1998, 351, 1320–1325. [Google Scholar]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Tebas, P.; Higgins, T.J.; Baine, Y.; Ciccarelli, R.B.; Ginsberg, R.S.; Weiner, D.B. Plasmid vaccination of stable HIV-positive subjects on antiviral treatment results in enhanced CD8 T-cell immunity and increased control of viral “blips”. Vaccine 2005, 23, 2066–2073. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Graham, B.S.; Chan, E.S.; Bosch, R.J.; Stocker, V.; Maenza, J.; Markowitz, M.; Little, S.; Sax, P.E.; Collier, A.C.; et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS One 2010, 5, e10555. [Google Scholar]
- Gudmundsdotter, L.; Wahren, B.; Haller, B.K.; Boberg, A.; Edback, U.; Bernasconi, D.; Butto, S.; Gaines, H.; Imami, N.; Gotch, F.; et al. Amplified antigen-specific immune responses in HIV-1 infected individuals in a double blind DNA immunization and therapy interruption trial. Vaccine 2011, 29, 5558–5566. [Google Scholar] [CrossRef]
- Lisziewicz, J.; Bakare, N.; Calarota, S.A.; Banhegyi, D.; Szlavik, J.; Ujhelyi, E.; Toke, E.R.; Molnar, L.; Lisziewicz, Z.; Autran, B.; et al. Single DermaVir immunization: Dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS One 2012, 7, e35416. [Google Scholar]
- Casazza, J.P.; Bowman, K.A.; Adzaku, S.; Smith, E.C.; Enama, M.E.; Bailer, R.T.; Price, D.A.; Gostick, E.; Gordon, I.J.; Ambrozak, D.R.; et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis. 2013, 207, 1829–1840. [Google Scholar] [CrossRef]
- Rodriguez, B.; Asmuth, D.M.; Matining, R.M.; Spritzler, J.; Jacobson, J.M.; Mailliard, R.B.; Li, X.D.; Martinez, A.I.; Tenorio, A.R.; Lori, F.; et al. Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: Results of the ACTG 5176 trial. J. Acquir. Immune Defic. Syndr. 2013, 64, 351–359. [Google Scholar] [CrossRef]
- Brave, A.; Ljungberg, K.; Boberg, A.; Rollman, E.; Isaguliants, M.; Lundgren, B.; Blomberg, P.; Hinkula, J.; Wahren, B.; et al. Multigene/Multisubtype HIV-1 Vaccine Induces Potent Cellular and Humoral Immune Responses by Needle-Free Intradermal Delivery. Mol. Ther. 2005, 12, 1197–1205. [Google Scholar] [CrossRef]
- Devito, C.; Zuber, B.; Schroder, U.; Benthin, R.; Okuda, K.; Broliden, K.; Wahren, B.; Hinkula, J. Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen in mice results in long-term HIV-1 neutralizing humoral mucosal and systemic immunity. J. Immunol. 2004, 173, 7078–7089. [Google Scholar] [CrossRef]
- Hinkula, J.; Devito, C.; Zuber, B.; Benthin, R.; Ferreira, D.; Wahren, B.; Schroder, U. A novel DNA adjuvant, N3, enhances mucosal and systemic immune responses induced by HIV-1 DNA and peptide immunizations. Vaccine 2006, 24, 4494–4497. [Google Scholar] [CrossRef]
- Brave, A.; Boberg, A.; Gudmundsdotter, L.; Rollman, E.; Hallermalm, K.; Ljungberg, K.; Blomberg, P.; Stout, R.; Paulie, S.; Sandstrom, E.; et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Mol. Ther. 2007, 15, 1724–1733. [Google Scholar] [CrossRef]
- Hallengard, D.; Haller, B.K.; Petersson, S.; Boberg, A.; Maltais, A.K.; Isaguliants, M.; Wahren, B.; Brave, A. Increased expression and immunogenicity of HIV-1 protease following inactivation of the enzymatic activity. Vaccine 2011, 29, 839–848. [Google Scholar] [CrossRef]
- Hallengard, D.; Applequist, S.E.; Nystrom, S.; Maltais, A.K.; Marovich, M.; Moss, B.; Earl, P.; Nihlmark, K.; Wahren, B.; Brave, A. Immunization with multiple vaccine modalities induce strong HIV-specific cellular and humoral immune responses. Viral Immunol. 2012, 25, 423–432. [Google Scholar]
- Lisziewicz, J.; Trocio, J.; Whitman, L.; Varga, G.; Xu, J.; Bakare, N.; Erbacher, P.; Fox, C.; Woodward, R.; Markham, P.; et al. DermaVir: A novel topical vaccine for HIV/AIDS. J. Investig. Dermatol. 2005, 124, 160–169. [Google Scholar] [CrossRef]
- Ljungberg, K.; Rollman, E.; Eriksson, L.; Hinkula, J.; Wahren, B. Enhanced immune responses after DNA vaccination with combined envelope genes from different HIV-1 subtypes. Virology 2002, 302, 44–57. [Google Scholar] [CrossRef]
- Brave, A.; Ljungberg, K.; Boberg, A.; Rollman, E.; Engstrom, G.; Hinkula, J.; Wahren, B. Reduced cellular immune responses following immunization with a multi-gene HIV-1 vaccine. Vaccine 2006, 24, 4524–4526. [Google Scholar] [CrossRef]
- Lisziewicz, J.; Toke, E.R. Nanomedicine applications towards the cure of HIV. Nanomedicine 2013, 9, 28–38. [Google Scholar]
- Toke, E.R.; Lorincz, O.; Somogyi, E.; Lisziewicz, J. Rational development of a stable liquid formulation for nanomedicine products. Int. J. Pharm. 2010, 392, 261–267. [Google Scholar] [CrossRef]
- Lisziewicz, J.; Trocio, J.; Xu, J.; Whitman, L.; Ryder, A.; Bakare, N.; Lewis, M.G.; Wagner, W.; Pistorio, A.; Arya, S.; et al. Control of viral rebound through therapeutic immunization with DermaVir. AIDS 2005, 19, 35–43. [Google Scholar] [CrossRef]
- Pensieroso, S.; Romiti, M.L.; Palma, P.; Castelli-Gattinara, G.; Bernardi, S.; Freda, E.; Rossi, P.; Cancrini, C. Switching from protease inhibitor-based-HAART to a protease inhibitor-sparing regimen is associated with improved specific HIV-immune responses in HIV-infected children. AIDS 2006, 20, 1893–1896. [Google Scholar] [CrossRef]
- Sandstrom, E.; Nilsson, C.; Hejdeman, B.; Brave, A.; Bratt, G.; Robb, M.; Cox, J.; Vancott, T.; Marovich, M.; Stout, R.; et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J. Infect. Dis. 2008, 198, 1482–1490. [Google Scholar] [CrossRef]
- Papagno, L.; Alter, G.; Assoumou, L.; Murphy, R.L.; Garcia, F.; Clotet, B.; Larsen, M.; Braibant, M.; Marcelin, A.G.; Costagliola, D.; et al. Comprehensive analysis of virus-specific T-cells provides clues for the failure of therapeutic immunization with ALVAC-HIV vaccine. AIDS 2011, 25, 27–36. [Google Scholar] [CrossRef]
- Nilsson, C.; Godoy-Ramirez, K.; Hejdeman, B.; Brave, A.; Gudmundsdotter, L.; Hallengard, D.; Currier, J.R.; Wieczorek, L.; Hasselrot, K.; Earl, P.L.; et al. Broad and potent cellular and humoral immune responses after a second late HIV-Modified vaccinia virus Ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS Res. Hum. Retrovir. 2013, 30, 299–311. [Google Scholar]
- Nowroozalizadeh, S.; Gudmundsdotter, L.; Hejdeman, B.; Andersson, L.; Esbjornsson, J.; Medstrand, P.; Sandstrom, E.; Gaines, H.; Wahren, B.; Jansson, M. Short-term HIV-1 treatment interruption is associated with dysregulated TLR-stimuli responsiveness. Hum. Vaccines Immunother. 2013, 9, 2103–2110. [Google Scholar]
- Rolland, M.; Tovanabutra, S.; deCamp, A.C.; Frahm, N.; Gilbert, P.B.; Sanders-Buell, E.; Heath, L.; Magaret, C.A.; Bose, M.; Bradfield, A.; et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat. Med. 2011, 17, 366–371. [Google Scholar] [CrossRef]
- Prendergast, A.; Goodliffe, H.; Clapson, M.; Cross, R.; Tudor-Williams, G.; Riddell, A.; Daniels, J.; Williams, A.; Goulder, P. Gag-specific CD4+ T-cell responses are associated with virological control of paediatric HIV-1 infection. AIDS 2011, 25, 1329–1331. [Google Scholar] [CrossRef]
- Riou, C.; Ganusov, V.V.; Campion, S.; Mlotshwa, M.; Liu, M.K.; Whale, V.E.; Goonetilleke, N.; Borrow, P.; Ferrari, G.; Betts, M.R.; et al. Distinct kinetics of Gag-specific CD4+ and CD8+ T cell responses during acute HIV-1 infection. J. Immunol. 2012, 188, 2198–2206. [Google Scholar] [CrossRef]
- Ferrando-Martinez, S.; Casazza, J.P.; Leal, M.; Machmach, K.; Munoz-Fernandez, M.A.; Viciana, P.; Koup, R.A.; Ruiz-Mateos, E. Differential Gag-specific polyfunctional T cell maturation patterns in HIV-1 elite controllers. J. Virol. 2012, 86, 3667–3674. [Google Scholar] [CrossRef]
- SenGupta, D.; Tandon, R.; Vieira, R.G.; Ndhlovu, L.C.; Lown-Hecht, R.; Ormsby, C.E.; Loh, L.; Jones, R.B.; Garrison, K.E.; Martin, J.N.; et al. Strong human endogenous retrovirus-specific T cell responses are associated with control of HIV-1 in chronic infection. J. Virol. 2011, 85, 6977–6985. [Google Scholar] [CrossRef]
- Jones, R.B.; Garrison, K.E.; Mujib, S.; Mihajlovic, V.; Aidarus, N.; Hunter, D.V.; Martin, E.; John, V.M.; Zhan, W.; Faruk, N.F.; et al. HERV-K-specific T cells eliminate diverse HIV-1/2 and SIV primary isolates. J. Clin. Investig. 2012, 122, 4473–4489. [Google Scholar]
- Tandon, R.; SenGupta, D.; Ndhlovu, L.C.; Vieira, R.G.; Jones, R.B.; York, V.A.; Vieira, V.A.; Sharp, E.R.; Wiznia, A.A.; Ostrowski, M.A.; et al. Identification of human endogenous retrovirus-specific T cell responses in vertically HIV-1-infected subjects. J. Virol. 2011, 85, 11526–11531. [Google Scholar] [CrossRef]
- Bakari, M.; Aboud, S.; Nilsson, C.; Francis, J.; Buma, D.; Moshiro, C.; Aris, E.A.; Lyamuya, E.F.; Janabi, M.; Godoy-Ramirez, K.; et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011, 29, 8417–8428. [Google Scholar]
- Weinberger, B.; Herndler-Brandstetter, D.; Schwanninger, A.; Weiskopf, D.; Grubeck-Loebenstein, B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008, 46, 1078–1084. [Google Scholar] [CrossRef]
- Deeks, S.G.; Verdin, E.; McCune, J.M. Immunosenescence and HIV. Curr. Opin. Immunol. 2012, 24, 501–506. [Google Scholar] [CrossRef]
- Palma, P.; Rinaldi, S.; Cotugno, N.; Santilli, V.; Pahwa, S.; Rossi, P.; Cagigi, A. Premature B-cell senescence as a consequence of chronic immune activation: Implications for vaccination of immune compromised individuals. Hum. Vaccines Immunother. 2014, 10. [Google Scholar] [PubMed]
- Cagigi, A.; Rinaldi, S.; di Martino, A.; Manno, E.C.; Zangari, P.; Aquilani, A.; Cotugno, N.; Nicolosi, L.; Villani, A.; Bernardi, S.; et al. Premature immune senescence during HIV-1 vertical infection relates with response to influenza vaccination. J. Allergy Clin. Immunol. 2014, 133, 592–594. [Google Scholar] [CrossRef]
- Hultstrom, A.L.; Hejdeman, B.; Leandersson, A.C.; Bratt, G.; Carbone, E.; Wahren, B. Human natural killer cells in asymptomatic human immunodeficiency virus-1 infection. Intervirology 2000, 43, 294–301. [Google Scholar] [CrossRef]
- Rydstrom, L.L.; Ygge, B.M.; Tingberg, B.; Naver, L.; Eriksson, L.E. Experiences of young adults growing up with innate or early acquired HIV infection—A qualitative study. J. Adv. Nurs. 2013, 69, 1357–1365. [Google Scholar] [CrossRef]
Appendix
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Palma, P.; Gudmundsdotter, L.; Finocchi, A.; Eriksson, L.E.; Mora, N.; Santilli, V.; Aquilani, A.; Manno, E.C.; Zangari, P.; Romiti, M.L.; et al. Immunotherapy with an HIV-DNA Vaccine in Children and Adults. Vaccines 2014, 2, 563-580. https://doi.org/10.3390/vaccines2030563
Palma P, Gudmundsdotter L, Finocchi A, Eriksson LE, Mora N, Santilli V, Aquilani A, Manno EC, Zangari P, Romiti ML, et al. Immunotherapy with an HIV-DNA Vaccine in Children and Adults. Vaccines. 2014; 2(3):563-580. https://doi.org/10.3390/vaccines2030563
Chicago/Turabian StylePalma, Paolo, Lindvi Gudmundsdotter, Andrea Finocchi, Lars E. Eriksson, Nadia Mora, Veronica Santilli, Angela Aquilani, Emma C. Manno, Paola Zangari, Maria Luisa Romiti, and et al. 2014. "Immunotherapy with an HIV-DNA Vaccine in Children and Adults" Vaccines 2, no. 3: 563-580. https://doi.org/10.3390/vaccines2030563





