A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data
Abstract
:1. Introduction
1.1. Antioxidants
- (a)
- Endogenous antioxidants—the ones which are produced by the body. Examples are: superoxide dismutase, catalase, glutathione peroxidase, and glutathione.
- (b)
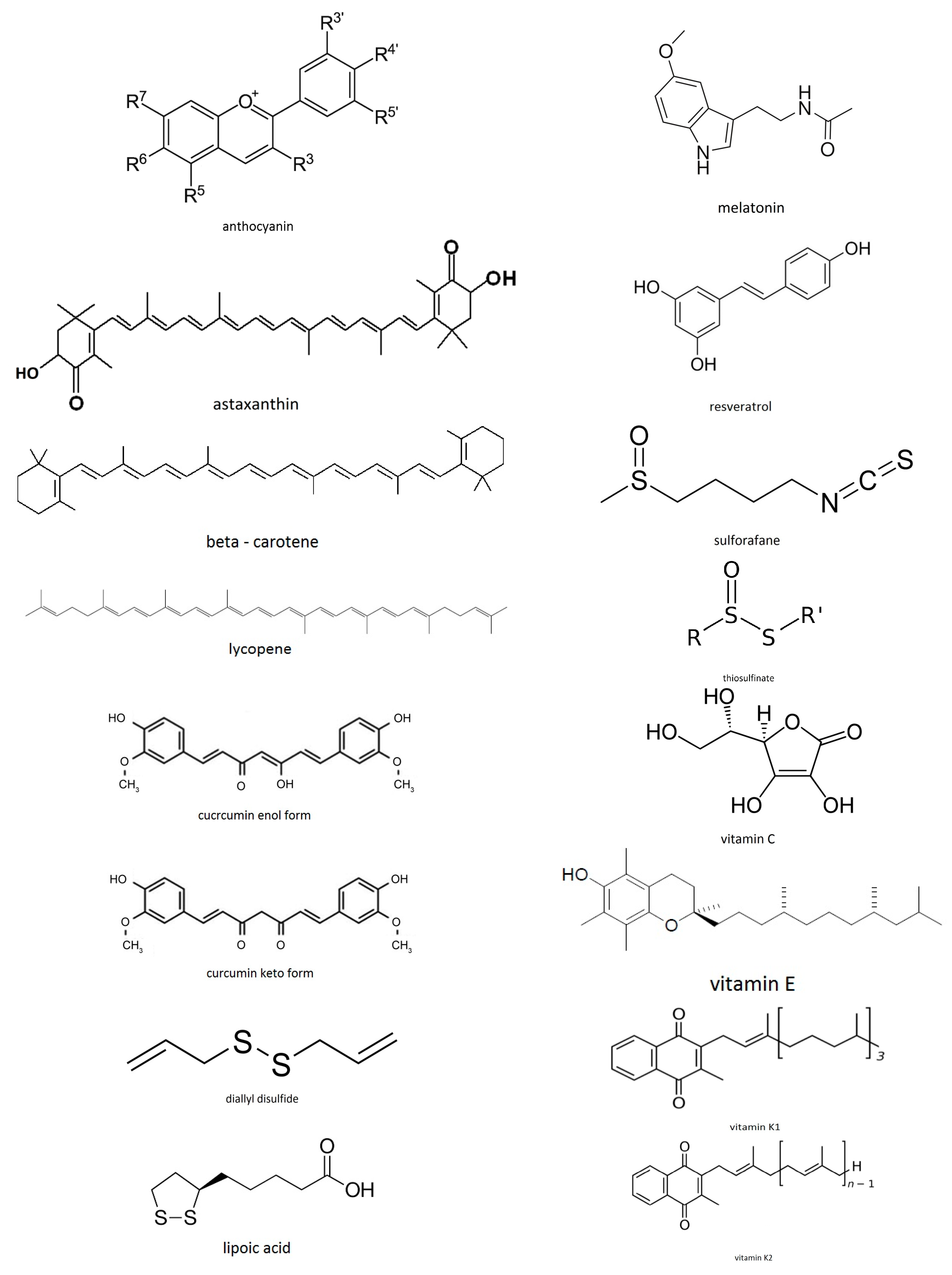
- Exogenous antioxidants—the ones which come from the diet. Examples are: vitamin C, carotenoids, polyphenols, sulforafanes, curcumin, anthocyanins, etc.
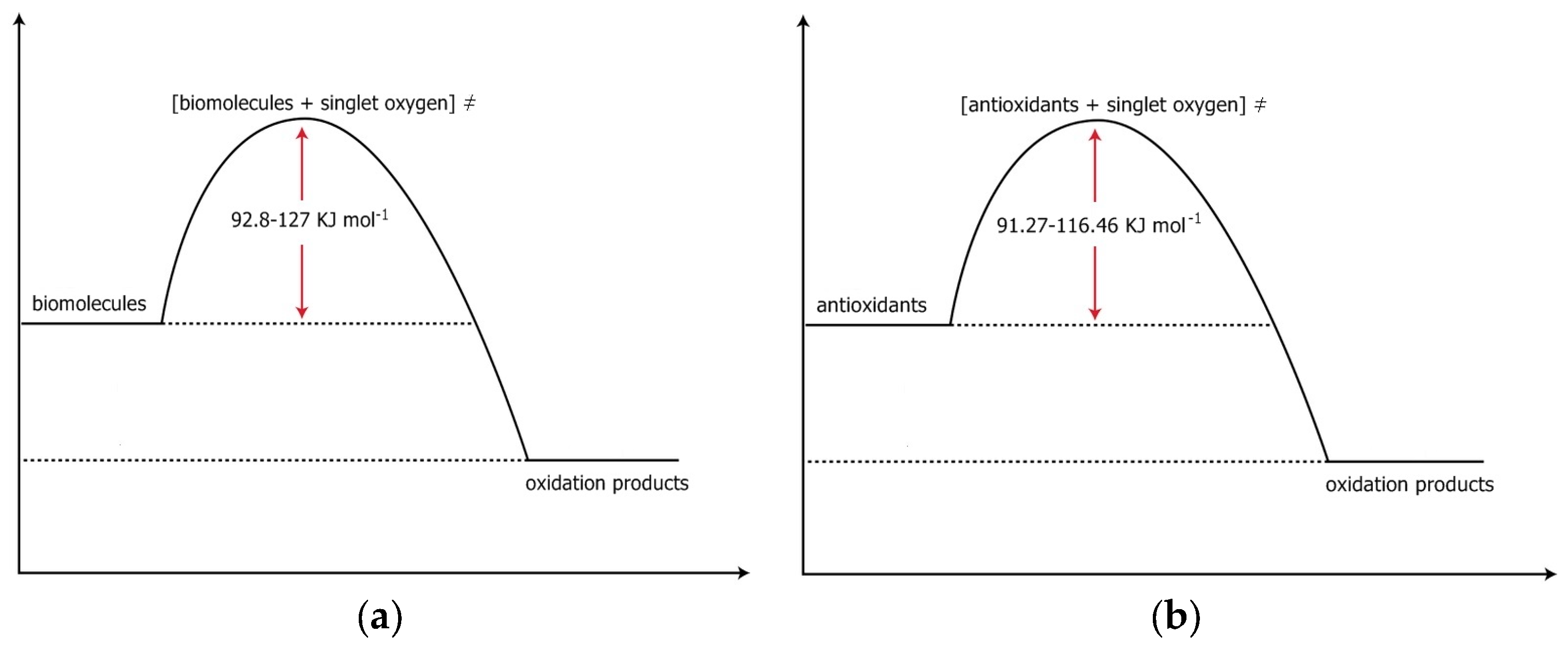
1.2. The Electronic Structure and the Energy above Ground State of Two Excited States of Oxygen. The First Excited State, the Singlet Oxygen State
| π* ↑ ↑ π* | π* ↑↓ π* | π* ↑ ↓ π* |
| Ground state | First excited state | Second excited state |
| 2S + 1 = 3 | 2S + 1 = 1 | 2S + 1 = 1 |
| 3Σg− | 1Δg, 92 kJmol−1 above ground state | 1Σg+, 155 kJmol−1 above ground state |
1.3. Diffusion—Controlled Reactions, Activation Energy of Diffusion
1.4. Food Antioxidants
1.5. Oxidative Stress, Singlet Oxygen and Antioxidants—The Goal of Our Research (Meta-Analysis and Review of Literature Data)
2. Method of Calculations
3. Results
Application of the Arrhenius and the Eyring-Polanyi Equations to Cases Related to Thermal and with Oxygen Treatment of Various Food Antioxidants
4. Discussion
4.1. Analytically the Various Cases that were Studied Are:
4.1.1. Destructive Processes due to Normal Body Temperature
4.1.2. Anthocyanins (Also Anthocyans)
4.1.3. Carotenoids or Tetraterpenoids
4.1.4. Polyphenols also Polyhydroxyphenols
4.1.5. Fumaric Acid or Trans-Butenedioic Acid
4.1.6. Oxygen-Free Reaction of Diallyl Disulfide
- (a)
- The kinetics of quercetin (a plant polyphenol from the flavonoid group) oxidation in the absence of air [105]. The authors state that: “The results, … show clearly that the absence, …. or at least substantial reduction, of oxygen content markedly slows down quercetin oxidation.” This means that quercetin oxidation becomes slower, that is, the ΔG≠ value becomes higher.
- (b)
- “Circulating air increased the rate of lycopene degradation” [106]. This suggests that the absence of oxygen decreased the degradation, which means that increased the ΔG≠ value.
- (c)
- “Oxygen-free conditions reduce lycopene losses significantly” [107]. This means that without the presence of oxygen lycopene is degraded more slowly meaning that the ΔG≠ value is higher.
4.2. Mechanism of the Studied Antioxidants’ Reactions with Oxygen
4.3. Questions That Need to Be Answered
4.4. Future Strategies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petrou, A.L.; Terzidaki, A. A meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases. Biochem. J. 2017, 474, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.R.; Keller, J.N. Oxidative stress and cerebral endothelial cells: Regulation of the blood-brain-barrier and antioxidant based interventions. Biochim. Biophys. Acta 2012, 1822, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defense mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.A.; Rodgers, M.A.J. Singlet Molecular Oxygen. Chem. Soc. Rev. 1981, 10, 205–231. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Tournaire, C.; Croux, S.; Maurette, M.-T.; Beck, I.; Hocquaux, M.; Braun, A.M.; Oliveros, E. Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1Δg) quenching. J. Photochem. Photobiol. B Biol. 1993, 19, 205–215. [Google Scholar] [CrossRef]
- Morales, J.; Gunther, G.; Zanacco, A.L.; Iemp, E. Singlet Oxygen reactions with flavonoids. A theoretical–experimental study. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Ranal, F.; Birtic, S.; Cuine, S.; Triantaphylides, C.; Ravanat, J.-L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Niedre, M.J.; Yu, C.S.; Patterson, M.S.; Wilson, B.C. Singlet oxygen luminescence as an in vivo photodynamic therapy dose metric: Validation in normal mouse skin with topical amino-levulinic acid. Br. J. Cancer 2005, 92, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bellnier, D.A.; Wood, L.M.; Potter, W.R.; Weishaupt, K.R.; Oseroff, A.R. Design and construction of a light-delivery system for photodynamic therapy. Med. Phys. 1999, 26, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Boyle, D.G.; Weishaupt, K.R.; Henderson, B.A.; Wityk, K.E. Photoradiation Therapy—Clinical and Drug Advances. Adv. Exp. Med. Biol. 1983, 160, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tyrell, R.M.; Keyse, S.M. The interaction of UVA radiation with cultured cells. J. Photochem. Photobiol. 1990, 4, 349–361. [Google Scholar] [CrossRef]
- Wyld, L.; Tomlinson, M.; Reed, M.W.R.; Brown, N.J. Aminolaevulinic acid-induced photodynamic therapy: Cellular responses to glucose starvation. Br. J. Cancer 2002, 86, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.L. The Free Energy of Activation as the critical factor in geochemical processes. Chem. Geol. 2012, 308, 50–59. [Google Scholar] [CrossRef]
- Petrou, A.L.; Terzidaki, A. Calcium carbonate and calcium sulfate precipitation, crystallization and dissolution: Evidence for the activated steps and the mechanisms from the enthalpy and entropy of activation values. Chem. Geol. 2014, 381, 144–153. [Google Scholar] [CrossRef]
- Katakis, D.; Gordon, G. Mechanisms of Inorganic Reactions; Wiley-Interscience: New York, NY, USA, 1987. [Google Scholar]
- Petrou, A.L.; Economou-Eliopoulos, M. Platinum-group mineral formation: Evidence of an interchange process from the entropy of activation values. Geochim. Cosmochim. Acta 2009, 73, 5635–5645. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms; Mc Graw-Hill: New York, NY, USA, 1981. [Google Scholar]
- Johnson, H.A.; Pavelec, M. Thermal injury due to normal body temperature. Am. J. Pathol. 1972, 66, 557–564. [Google Scholar] [PubMed]
- Davies, K.M. Plant Pigments and Their Manipulation; Wiley-Blackwell: Boca Raton, FL, USA, 2004; p. 6. ISBN 1-4051-1737-0. [Google Scholar]
- Archetti, M.; Döring, T.F.; Hagen, S.B.; Hughes, N.M.; Leather, S.R.; Lee, D.W.; Lev-Yadun, S.; Manetas, Y.; Ougham, H.J.; Schaberg, P.G.; et al. Unravelling the evolution of autumn colours: An interdisciplinary approach. Trends Ecol. Evol. 2011, 24, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, X.; Gao, J.; Guo, Y.; Huang, Z.; Du, Y. The Tomato Hoffman’s Anthocyaninless Gene Encodes a bHLH Transcription Factor Involved in Anthocyanin Biosynthesis That Is Developmentally Regulated and Induced by Low Temperatures. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive Oxygen Species in Plant Cell Death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2015, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Francis, F.J. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Cisse, M.; Vaillant, F.; Acosta, O.; Dhuique-Mayer, C.; Dornier, M. Thermal degradation kinetics of anthocyanins from blood orange, blackberry, and roselle using the Arrhenius, Eyring, and Ball Models. J. Agric. Food Chem. 2009, 57, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.; Crozier, A.; Duthie, G.G. Potential health benefits of berries. Curr. Nutr. Food Sci. 2005, 1, 71–86. [Google Scholar] [CrossRef]
- Stintsing, F.C.; Reinhold, C. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Anthocyanin pigments-Bioactivity and coloring properties. J. Food Sci. 2004, 69, 419–425. [Google Scholar] [CrossRef]
- Susan, D.; Arnum, V. Vitamin A in Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley: New York, NY, USA, 1998; pp. 99–107. [Google Scholar] [CrossRef]
- Foods Highest in Lycopene, Nutrition Data, USDA Nutrient Database, Version SR-21. Available online: nutritiondata.com (accessed on 19 August 2014).
- α-Carotene, β-carotene, β-Cryptoxanthin, Lycopene, Lutein, and Zeaxanthin. Available online: http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/carotenoids (accessed on 29 May 2017).
- Urrea, D.; Eim, V.S.; Gonzalez-Centeno, M.R.; Minjares-Fuentes, R.; Castell-Palou, A.; Juarez, M.D.; Rossello, C. Effects of air drying temperature on antioxidant activity and carotenoids content of carrots (Daucus carota). In Proceedings of the European Drying Conference-EuroDrying, Palma, Balearic Island, Spain, 26–28 October 2011. [Google Scholar]
- Abdul-Hammed, M.; Bolarinwa, I.F.; Adebayo, L.O.; Akindele, S.L. Kinetics of the degradation of carotenoid antioxidants in tomato paste. Adv. J. Food Sci. Technol. 2016, 11, 734–741. [Google Scholar] [CrossRef]
- Stefanovich, A.F.; Karel, M. Kinetics of beta-carotene degradation at temperatures typical of air drying of foods. J. Food Process. Preserv. 1982, 6, 227–242. [Google Scholar] [CrossRef]
- Zaibunnisa, A.H.; Norashikin, S.; Mamot, S.; Osman, H. Stability of curcumin in turmeric oleoresin-β-cyclodextrin inclusion complex during storage. Malays. J. Anal. Sci. 2009, 13, 165–169. [Google Scholar]
- Papadopoulou, A.; Frazier, R.A. Characterization of protein-polyphenol interactions. Trends Food Sci. Technol. 2004, 15, 186–190. [Google Scholar] [CrossRef]
- Flavonoids. Available online: http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids (accessed on 26 February 2016).
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An overview. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and Safety of Polyphenol Consumption. Am. J. Clin. Nutr. 2005, 81, 3265–3295. [Google Scholar] [CrossRef]
- Solyom, K.; Sola, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforafane and structurally related synthetic norbornyl isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.H.; Ho, E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin. Cancer Biol. 2007, 17, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforafane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mao, J.; You, Y.; Liu, S. Study on degradation kinetics of sulforaphane in broccoli extract. Food Chem. 2014, 155, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Garlic and Other Alliums: The Lore and the Science; The Royal Society of Chemistry: Cambridge, UK, 2010; ISBN 0-85404-190-7. [Google Scholar]
- Germain, E.; Chevalier, J.; Siess, M.H.; Teyssier, C. Hepatic metabolism of diallyl disulphide in rat and man. Xenobiotica 2003, 33, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Yang, J.J.; Chen, H.W.; Sheen, L.Y.; Lii, C.K. Garlic organosulfur compounds upregulate the expression of the pi class of glutathione S-transferase in rat primary hepatocytes. J. Nutr. 2005, 135, 2560–2565. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Sheen, L.Y.; Chen, H.W.; Kuo, W.W.; Tsai, S.J.; Lii, C.K. Differential effects of garlic oil and its three major organosulfur components on the hepatic detoxification system in rats. J. Agric. Food Chem. 2002, 50, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Hosono, T.; Misawa, S.; Seki, T.; Ariga, T. The effects of allyl sulfides on the induction of phase II detoxification enzymes and liver injury by carbon tetrachloride. Food Chem. Toxicol. 2004, 42, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lemar, K.M.; Aon, M.A.; Cortassa, S.; O’Rourke, B.; Müller, C.T.; Lloyd, D. Diallyl disulphide depletes glutathione in Candida albicans: Oxidative stress-mediated cell death studied by two-photon microscopy. Yeast 2007, 24, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Urig, S.; Koncarevic, S.; Wu, X.; Fischer, M.; Rahlfs, S.; Mersch-Sundermann, V.; Becker, K. Glutathione- and thioredoxin-related enzymes are modulated by sulfur-containing chemopreventive agents. Biol. Chem. 2007, 388, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Kwon, H.; Park, K.H.; Ko, J.K.; Kim, J.H.; Hwang, M.S.; Yum, Y.N.; Kim, O.H.; Kim, J.; Kim, H.T.; et al. Protective effect of diallyl disulfide on oxidative stress-injured neuronally differentiated PC12 cells. Brain Res. Mol. Brain Res. 2005, 133, 17686. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Koh, S.H.; Lee, Y.J.; Lee, K.Y.; Kim, Y.; Kim, S.; Lee, M.K.; Kim, S.H. Differential effects of diallyl disulfide on neuronal cells depend on its concentration. Toxicology 2005, 211, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Marcinek, J.; Mleczko, U.; Włodek, L. Selective effects of diallyl disulfide, a sulfane sulfur precursor, in the liver and Ehrlich ascites tumor cells. Eur. J. Pharmacol. 2007, 569, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Bilska, A.; Ksiazek, L.; Srebro, Z.; Włodek, L. Allyl disulfide as donor and cyanide as acceptor of sulfane sulfur in the mouse tissues. Pharmacol. Rep. 2005, 57, 212–218. [Google Scholar] [PubMed]
- Amonkar, S.V.; Banerji, A. Isolation and characterization of larvicidal principle of garlic. Science 1971, 174, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Tursil, E.; Vitali, C.; Miccolis, V.; Candido, V. Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine 2000, 7, 239–243. [Google Scholar] [CrossRef]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, Y.B.; Wang, X.Y. Effects of preoperatively selected gut decontamination on cardiopulmonary bypass-induced endotoxemia. Chin. J. Traumatol. 2007, 10, 131–137. [Google Scholar] [PubMed]
- Glade, M.J. Food, nutrition, and the prevention of cancer: A global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition 1999, 15, 523–526. [Google Scholar] [PubMed]
- Huang, Z.; Lei, X.; Zhong, M.; Zhu, B.; Tang, S.; Liao, D. Bcl-2 small interfering RNA sensitizes cisplatin-resistant human lung adenocarcinoma A549/DDP cell to cisplatin and diallyl disulfide. Acta Biochim. Biophys. Sin. 2007, 39, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Historical, chemical and cardiovascular perspectives on garlic: A review. Pharmacogn. Rev. 2007, 1, 80. [Google Scholar]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [PubMed]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal medicine for the treatment of cardiovascular disease (clinical consideration). Arch. Intern. Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Berthold, H.K.; Sudhop, T.; von Bergmann, K. Effect of a garlic oil preparation on serum lipoproteins and cholesteol metabolism. JAMA 1998, 279, 1900–1902. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.T.; Mulrow, C.D.; Ramirez, G.; Gardner, C.D.; Morbidoni, L.; Lawrence, V.A. Promise for improving some cardiovascular risk factors garlic’s properties. Arch. Intern. Med. 2001, 161, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Vastag, B. Garlic prevents plaque. JAMA 2002, 288, 11. [Google Scholar]
- Nutrition Facts for Raw Garlic USDA National Nutrient Database, Version SR-21. Available online: nutritiondata.com (accessed on 2 November 2014).
- Kinalski, T.; Norena, C.P.Z. Effect of blanching treatments on antioxidant activity and thiosulfinate degradation of garlic (Allium sativum L.). Food Bioproc. Tech. 2014, 7, 2152–2157. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Staba, E.J.; Lash, L.; Staba, J.E. A commentary on the effects of garlic extraction and formulation on product composition. J. Nutr. 2001, 131, 1118–1119. [Google Scholar] [CrossRef]
- Willett, C.W. Diet and health: What should we eat? Science 1994, 264, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, C.Y. Textural change and antioxidant properties of broccoli under different cooking treatments. Food Chem. 2005, 46, 1018–1021. [Google Scholar] [CrossRef]
- Yin, M.C.; Cheng, W.S. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 1991, 63, 23–28. [Google Scholar] [CrossRef]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Weiner, L.; Wilchek, M. A spectrophotometric assay for allicin and alliinase (alliin lyase) activity reaction of 2-nitro-5-thiobenzoate with thiosulfinates. Anal. Biochem. 1998, 265, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. l-ascorbic acid biosynthesis. Vitam. Horm. 2001, 61, 241–266. [Google Scholar] [CrossRef] [PubMed]
- Linster, C.L.; Van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grudpan, K.; Kamfoo, K.; Jakmunee, J. Flow injection spectrophotometric or conductometric determination of ascorbic acid in a vitamin C tablet using permanganate or ammonia. Talanta 1999, 49, 1023–1026. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Birghila, S.; Popescu, V.; Dobrinas, S.; Soceanu, A.; Oprea, C.; Magearu, V. Kinetic study of vitamin C degradation from pharmaceutical products. Rom. J. Phys. 2008, 53, 343–351. [Google Scholar]
- Tola, Y.B.; Ramaswamy, H.S. Temperature and high pressure stability of lycopene and vitamin C of watermelon juice. Afr. J. Food Sci. 2015, 9, 351–358. [Google Scholar] [CrossRef]
- Rahmawati, S.; Bundjali, B. Kinetics of the oxidation of vitamin C. Indones. J. Chem. 2012, 12, 291–296. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Dolinska, B.; Ryszka, F.; Ostrozka-Cieslik, A. The effect of selected antioxidants on the kinetics of changes in the stability of an HTK solution: A technical note. AAPS PharmSciTech 2006, 7, E144–E147. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Facts: Nuts, Walnuts, English Dried per 100 g. Condé Nast. Available online: http://nutritiondata.self.com/facts/nut-and-seed-products/3138/2 (accessed on 4 July 2014).
- Gonabad, M.A.; Noghabi, M.S.; Niazmand, R. Estimation of kinetic parameters of walnut oil using rancimat test. J. Appl. Environ. Biol. Sci. 2015, 4, 28–32. [Google Scholar]
- Gonzaga, F.B.; Pasquini, C.; Rodrigues, C.E.; Meirelles, A.J. Comparison of near-infrared emission spectroscopy and the Rancimat method for the determination of oxidative stability. Eur. J. Lipid Sci. Technol. 2007, 109, 61–65. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L.; Wan, P.J. Temperature effects on the determination of oxidative stability with the Metrohm Rancimat. J. Am. Oil Chem. Soc. 1992, 69, 525–527. [Google Scholar] [CrossRef]
- Campo, P.; Zhao, Y.; Suidan, M.T.; Venosa, A.D.; Sorial, G.A. Biodegradation kinetics and toxicity of vegetable oil triacylglycerols under aerobic conditions. Chemosphere 2007, 68, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Lutterodt, H.; Slavin, M.; Whent, E.; Turner, E.; Yu, L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.R.; Izadyar, M. Gas-phase kinetics and mechanism of diallyl sulphide thermal decomposition. J. Phys. Org. Chem. 2003, 16, 153–157. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R.; Pinto, J.T.; Rivlin, R.S. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2006, 136, 8645–8695. [Google Scholar] [CrossRef]
- Brady, J.F.; Li, D.; Ishizaki, H.; Yang, C.S. Effect of Diallyl sulfide on rat liver microsomal nitrosamine metabolism and other monooxygenase activities. Cancer Res. 1988, 48, 5937–5940. [Google Scholar] [PubMed]
- Mocek, M.; Richardson, P.J. Kinetics and mechanism of quercetin oxidation. J. Inst. Brewing 1972, 78, 459–465. [Google Scholar] [CrossRef]
- Oberoi, D.P.S.; Sogi, D.S. Prediction of lycopene degradation during dehydration of watermelon pomace (cv Sugar Baby). J. Saudi Soc. Agric. Sci. 2017, 16, 97–103. [Google Scholar] [CrossRef]
- Ax, K.; Mayer-Miebach, E.; Link, B.; Schuchmann, H.; Schubert, H. Stability of Lycopene in Oil-in-Water Emulsions. Eng. Life Sci. 2003, 3, 199–201. [Google Scholar] [CrossRef]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs. 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Couto, N.; Malys, N.; Gaskell, S.J.; Barber, J. Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: S proteomic approach. J. Proteome Res. 2013, 12, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Piemonte, F.; Locatelli, M.; Lo Russo, A.; Gaeta, L.M.; Tozzi, G.; Federici, G. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin. Chem. 2001, 47, 1467–1469. [Google Scholar] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annual Review of Biochemistry. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- McIlduff, C.E.; Rutkove, S.B. Critical appraisal of the use of alpha lipoic acid (thioctic acid) in the treatment of symptomatic diabetic polyneuropathy. Ther. Clin. Risk Manag. 2011, 7, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ziegle, D.; Reljanovic, M.; Mehnert, H.; Gries, F.A. α-Lipoic acid in the treatment of diabetic polyneuropathy in Germany: Current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 1999, 107, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Molecules in focus Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Ugur-Altun, B. Melatonin: Therapeutic and clinical utilization. Int. J. Clin. Pract. 2007, 61, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Reiter, R.J.; Acuña-Castroviejo, D.; Tan, D.X.; Burkhardt, S. Free radical-mediated molecular damage, Mechanisms for the protective actions of melatonin in the central nervous system. Ann. N. Y. Acad. Sci. 2001, 939, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered gene for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Neurotoxins: Free radical mechanisms and melatonin protection. Curr. Neuropharmacol. 2010, 8, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlow-Walden, L.R. Melatonin—A highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Marra, M.; Moroni, F.; Recchioni, R.; Marcheselli, F. Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 1994, 55, PL271–PL276. [Google Scholar] [CrossRef]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signaling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Azzi, A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, M.S.; Naoum, J.J.; Arbid, E.J. Vitamin K Dependent Proteins and the Role of Vitamin K2 in the Modulation of Vascular Calcification: A Review. Oman Med. J. 2014, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Czupalla, C.J. Wolburg, Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 2011, 55, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Blood Brain Barrier Johns Hopkins University. Available online: https://www.bme.jhu.edu/about/awards-and-achievements/page/10/ (accessed on 7 May 2013).
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tayarani, I.; Chaudiere, J.; Lefauconnier, J.M.; Bourre, J.M. Enzymatic protection against peroxidative damage in isolated brain capillaries. J. Neurochem. 1987, 48, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Shukla, G.S. Potential role of cerebral glutathione in the maintenance of blood-brain barrier integrity in rat. Neurochem. Res. 1999, 24, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Vatassery, G.T. Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics 1998, 53, S25–S27. [Google Scholar] [PubMed]
- Grammas, P.; Martinez, J.; Miller, B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev. Mol. Med. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D. Effect of aging on the blood-brain barrier. Neurobiol. Aging 1988, 9, 31–39. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Clifford, P.M.; Zarrabi, S.; Siu, G.; Kinsler, K.J.; Koscink, M.C.; Venkataraman, V.; D’Andrea, M.R.; Dinsmore, S.; Nagele, R.G. Aβ peptides can enter the brain through a defective blood-brain barrier and bind selectively to neurons. Brain Res. 2007, 1142, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Munk, B.H.; Burrows, C.J.; Schlegel, H.B. Computational Study of Oxidation of Guanine by Singlet Oxygen (1Δg) and Formation of Guanine: Lysine Cross-Links. Chem. Eur. J. 2017, 23, 5804–5813. [Google Scholar] [CrossRef] [PubMed]
- Scheit, K.; Bauer, G. Direct and indirect inactivation of tumor cell protective catalase by salicylic acid and anthocyanidins reactivates intercellular ROS signaling and allows for synergistic effects. Carcinogenesis 2015, 36, 400–411. [Google Scholar] [CrossRef] [PubMed]
| Τ(i) (Κ) | lnk(i) | Eact(ii) kJmol−1 | ΔΗ≠(iii) kJ−1mol−1 | ΔS≠(iii) JK−1mol−1 | ΔG≠(iii) kJmol−1 | |
|---|---|---|---|---|---|---|
| Case a Thermal injury due to normal body temperature [23] | ||||||
| 314 | −10.360 | 769.52(iii) | 766.89 | 2110.41 | 112.8 at 310 K | |
| 315 | −9.8338 | |||||
| 316 | −8.4168 | |||||
| 317 | −7.4544 | |||||
| 318 | −6.6079 | |||||
| 319 | −6.0185 | |||||
| Case b Degradation kinetics of anthocyanins [29] [blackberry juice and concentrate] | ||||||
| 333 | −11.3732 | 58.90(iii) | 56.01 | −172.01 | 109.33 at 310 K | |
| 343 | −10.7245 | 58.95(b) | ||||
| 353 | −10.0979 | |||||
| 363 | −9.6309 | |||||
| Case c Degradation kinetics of anthocyanins [31] [Guatemala roselle] | ||||||
| 308 | −12.0491 | 39.27(iii) | 36.46 | −226.7 | 106.75 at 310 K | |
| 340.5 | −10.5013 | |||||
| 371.5 | −9.4347 | |||||
| Case c Degradation kinetics of anthocyanins [31] [BRJ] | ||||||
| 308 | −12.7647 | 49.17(iii) | 46.37 | −200.31 | 108.47 at 310 K | |
| 340.5 | −10.7615 | |||||
| 371.5 | −9.4962 | |||||
| Case d Antioxidant activity and carotenoids content [38] | ||||||
| 323 | −14.5426 | 23.34(iii) | 20.53 | −302.48 | 114.31 at 310 K | |
| 333 | −14.2012 | 23.7(d) | ||||
| 353 | −13.7892 | |||||
| Case e Degradation of carotenoid antioxidants [39] (i) cultivar lycopene | ||||||
| 333 | −9.0420 | 4.62(iii) | 1.62 | −315.66 | 99.48 at 310 K | |
| 353 | −8.8620 | 4.63(e) | ||||
| 373 | −8.8049 | |||||
| 393 | −8.7829 | |||||
| (i) cultivar beta-carotene | ||||||
| 333 | −9.0006 | 4.13(iii) | 1.13 | −317.03 | 99.41 at 310 K | |
| 353 | −8.898 | 4.14(e) | ||||
| 373 | −8.8274 | |||||
| 393 | −8.7721 | |||||
| (ii) cultivar lycopene | ||||||
| 333 | −9.5911 | 8.64(iii) | 5.64 | −307.97 | 101.11 at 310 K | |
| 353 | −9.2616 | 8.65(e) | ||||
| 373 | −9.2103 | |||||
| 393 | −9.0852 | |||||
| (ii) cultivar Beta-carotene | ||||||
| 333 | −9.4129 | 4.81(iii) | 1.81 | −318.17 | 100.44 at 310 K | |
| 353 | −9.2271 | 4.81(e) | ||||
| 373 | −9.1616 | |||||
| 393 | −9.1458 | |||||
| Case f Beta-carotene degradation [40] | ||||||
| 333 | −9.6938 | 95.73(iii) | 92.88 | −46.782 | 107.37 at 310 K | |
| 343 | −8.4143 | 95.4(f) | ||||
| 353 | −7.7402 | |||||
| Case g Stability of curcumin during storage [41] With the presence of light | ||||||
| 278 | −8.4068 | 24.213(iii) | 21.752 | −235.8 | 94.912 at 310 K | |
| 306 | −7.3488 | 23.88(g) | ||||
| 318 | −7.1247 | |||||
| Case g Stability of curcumin during storage [41] Without the presence of light | ||||||
| 278 | −9.4980 | 11.803(iii) | 9.342 | −290.3 | 99.32 at 310 K | |
| 306 | −9.3534 | 13.65(g) | ||||
| 318 | −8.74034 | |||||
| Case h, Thermal degradation of polyphenols [51] [grape marc] | ||||||
| 353 | −7.1185 | 5.67(iii) | 2.45 | −298.1 | 94.86 at 310 K | |
| 373 | −6.8831 | 55.98(h) | ||||
| 423 | −6.7709 | |||||
| Case h, Thermal degradation of polyphenols [51] [filtered extract] | ||||||
| 353 | −6.7709 | 9.03(iii) | 5.80 | −285.9 | 94.46 at 310 K | |
| 373 | −6.5583 | 53.196(h) | ||||
| 423 | −6.2520 | |||||
| Case i Degradation kinetics of sulforaphane [55] | ||||||
| pH = 2.2 | 333 | −12.7939 | 85.36(iii) | 82.48 | −103.66 | 114.95 at 310 K |
| 348 | −11.1844 | |||||
| 355 | −10.8479 | |||||
| 363 | −10.2289 | |||||
| pH = 3.0 | 333 | −12.7939 | 93.17(iii) | 90.29 | −79.97 | 115.08at310 K |
| 348 | −11.0021 | |||||
| 355 | −10.5966 | |||||
| 363 | −10.0213 | |||||
| pH = 4 | 333 | −12.1007 | 79.69(iii) | 76.80 | −114.58 | 112.32 at 310k |
| 348 | −10.4913 | |||||
| 355 | −10.1548 | |||||
| 363 | −9.7493 | |||||
| pH = 5 | 333 | −11.4076 | 71.87(iii) | 68.98 | −132.98 | 110.2 at 310 K |
| 348 | −10.1548 | |||||
| 355 | −9.7493 | |||||
| 363 | −9.2675 | |||||
| pH = 6 | 333 | −11.0021 | 69.61(iii) | 66.73 | −136.01 | 108.9 at 310 K |
| 348 | −9.6584 | |||||
| 355 | −9.3281 | |||||
| 363 | −8.9437 | |||||
| Case j Antioxidant activity and thiosulfinate degradation of garlic [80] | ||||||
| Antioxidant activity | 353 | −5.5090 | 89.76(iii) | 86.74 | −46.68 | 101.21 at 310 K |
| 363 | −4.7736 | 89.75(j) | ||||
| 373 | −3.8672 | |||||
| Case j Antioxidant activity and thiosulfinate degradation of garlic [80] | ||||||
| Degradation of thiosulfinates | 353 | −5.5684 | 7.565(iii) | 4.55 | −279.7 | 91.27 at 310 K |
| 363 | −5.5090 | 7.67(j) | ||||
| 373 | −5.4299 | |||||
| Case k, Vitamin C degradation, [91] | ||||||
| Standard ascorbic acid solution (Merck) | 303 | −8.7916 | 36.65(iii) | 34.09 | −205.47 | 97.79 at 310 K |
| 313 | −8.3266 | 36.53(k) | ||||
| Vitamin C 200 mg tablet | 303 | −7.0845 | 27.64(iii) | 25.08 | −221.03 | 93.6 at 310 K |
| 313 | −6.7338 | 27.82(k) | ||||
| Ascovit 100 mg tablet | 303 | −7.1915 | 11.02(iii) | 8.46 | −276.8 | 94.26 at 310 K |
| 313 | −7.0516 | 10.96(k) | ||||
| Vitamin C nose drops | 303 | −7.9778 | 34.89(iii) | 32.33 | −204.53 | 95.73 at 310 K |
| 313 | −7.5351 | 35.10(k) | ||||
| Case l, Temperature and high pressure stability of vitamin C [92] [watermelon] | ||||||
| 343 | −8.4300 | 76.92(iii) | 73.98 | −101.76 | 105.528 at 310 K | |
| 353 | −8.1404 | 76.86(l) | ||||
| 363 | −6.9349 | |||||
| Case l, Temperature and high pressure stability of lycopene [92] [watermelon] | ||||||
| 343 | −11.911 | 97.21(iii) | 94.27 | −71.56 | 116.46 at 310 K | |
| 353 | −11.423 | 97.7(l) | ||||
| 363 | −10.024 | |||||
| Case m, Oxidation of vitamin C [93] | ||||||
| 313 | −12.8992 | 41.65(iii) | 38.89 | −224.4 | 108.47 at 310 K | |
| 323 | −11.5297 | 20.73(m) | ||||
| 333 | −11.1765 | |||||
| 343 | −10.9159 | |||||
| 353 | −10.9823 | |||||
| Case n, Effect of antioxidants [vitamin C, cysteine, fumaric acid] on the stability of an HTK solution [95] HTK | ||||||
| 323 | −13.1563 | 100.48(iii) | 97.75 | −52.15 | 113.92 at 310k | |
| 333 | −12.032 | 100.186(n) | ||||
| HTK + cysteine | 323 | −13.6903 | 92.31(iii) | 89.58 | −81.87 | 114.96 at 310 K |
| 333 | −12.6576 | 91.96(n) | ||||
| HTK + vitamin C | 323 | −13.8494 | 98.20(iii) | 95.47 | −64.98 | 115.61 at 310 K |
| 333 | −12.7508 | 98.127(n) | ||||
| HTK + fumaric acid | 323 | −13.6903 | 89.94(iii) | 87.21 | −89.22 | 114.87 at 310 K |
| 333 | −12.6841 | 89.70(n) | ||||
| Case o, Kinetic parameters of walnut oil [97] | ||||||
| 353 | −9.7921 | 10.04(iii) | 6.99 | −307.8 | 102.4 at 310 K | |
| 363 | −9.7042 | 80.32(o) | 77.27(o) | −55.76(o) | 94.56 at 310 K calculated by us according to the ΔH≠, and ΔS≠ of work [133] | |
| 373 | −9.6146 | |||||
| 383 | −9.5239 | |||||
| Oxygen-free conditions Case p kinetics and mechanism of diallyl disulfide thermal decomposition [102] | ||||||
| 433 | −9.8302 | 123.32(iii) | 119.60 | −52.30 | 135.81 at 310 K | |
| 443 | −8.7702 | |||||
| 453 | −8.1101 | |||||
| 463 | −7.5902 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data. Antioxidants 2018, 7, 35. https://doi.org/10.3390/antiox7030035
Petrou AL, Petrou PL, Ntanos T, Liapis A. A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data. Antioxidants. 2018; 7(3):35. https://doi.org/10.3390/antiox7030035
Chicago/Turabian StylePetrou, Athinoula L., Petros L. Petrou, Theodoros Ntanos, and Antonis Liapis. 2018. "A Possible Role for Singlet Oxygen in the Degradation of Various Antioxidants. A Meta-Analysis and Review of Literature Data" Antioxidants 7, no. 3: 35. https://doi.org/10.3390/antiox7030035




