Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops
Abstract
:1. Introduction
2. Biosynthesis and Chemical Function
3. Tocopherols in Plants and Mammalians
4. Studying the Plant Tocopherol Biosynthesis Key Genes
5. Breeding for Higher Vitamin E Content
6. Conclusions and Challenges
Acknowledgments
Conflicts of Interest
References
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- DellaPenna, D.; Last, R.L. Progress in the dissection and manipulation of plant vitamin E biosynthesis. Physiol. Plant. 2006, 126, 356–368. [Google Scholar] [CrossRef]
- DellaPenna, D. A decade of progress in understanding vitamin E synthesis in plants. J. Plant Physiol. 2005, 162, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Péter, S.; Friedel, A.; Roos, F.F.; Wyss, A.; Eggersdorfer, M.; Hoffmann, K.; Weber, P. A systematic review of global alpha-tocopherol status as assessed by nutritional intake levels and blood serum concentrations. Int. J. Vitam. Nutr. Res. 2016, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Soll, J.; Douce, R.; Schultz, G. Site of biosynthesis of alpha-tocopherol in spinach chloroplasts. FEBS Lett. 1980, 112, 243–246. [Google Scholar] [CrossRef]
- Vidi, P.A.; Kanwischer, M.; Baginsky, S.; Austin, J.R.; Csucs, G.; Dormann, P.; Kessler, F.; Brehelin, C. Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J. Biol. Chem. 2006, 281, 11225–11234. [Google Scholar] [CrossRef] [PubMed]
- Soll, J.; Schultz, G.; Joyard, J.; Douce, R.; Block, M.A. Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch. Biochem. Biophys. 1985, 238, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Garcia, I.; Rodgers, M.; Lenne, C.; Rolland, A.; Sailland, A.; Matringe, M. Subcellular localization and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterization of the corresponding cDNA. Biochem. J. 1997, 325 Pt 3, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Riewe, D.; Koohi, M.; Lisec, J.; Pfeiffer, M.; Lippmann, R.; Schmeichel, J.; Willmitzer, L.; Altmann, T. A tyrosine aminotransferase involved in tocopherol synthesis in Arabidopsis. Plant J. 2012, 71, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef] [PubMed]
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.; Venkatesh, T.V.; Karunanandaa, B.; Baszis, S.R.; Norris, S.R.; Savidge, B.; et al. The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 2006, 18, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Mene-Saffrane, L.; DellaPenna, D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol. Biochem. 2010, 48, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.R.; Shen, X.; DellaPenna, D. Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 1998, 117, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.R.; Barrette, T.R.; DellaPenna, D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 1995, 7, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, Y.; Shintani, D.K.; DellaPenna, D. Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol. Biochem. 2002, 40, 913–920. [Google Scholar] [CrossRef]
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Savidge, B.; Weiss, J.D.; Wong, Y.H.H.; Lassner, M.W.; Mitsky, T.A.; Shewmaker, C.K.; Post-Beittenmiller, D.; Valentin, H.E. Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2002, 129, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, T.V.; Karunanandaa, B.; Free, D.L.; Rottnek, J.M.; Baszis, S.R.; Valentin, H.E. Identification and characterization of an Arabidopsis homogentisate phytyltransferase paralog. Planta 2006, 223, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, R.; Ito, T.; Kobayashi, M.; Taji, T.; Nagata, N.; Asami, T.; Yoshida, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Functional analysis of the 37 kDa inner envelope membrane polypeptide in chloroplast biogenesis using a Ds-tagged Arabidopsis pale-green mutant. Plant J. 2003, 34, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sattler, S.; Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 2003, 15, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Van Eenennaam, A.L.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering vitamin E content: From Arabidopsis mutant to soy oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Porfirova, S.; Bergmüller, E.; Tropf, S.; Lemke, R.; Dörmann, P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Kanwischer, M.; Porfirova, S.; Bergmuller, E.; Dormann, P. Alterations in tocopherol cyclase activity in transgenic and mutant plants of Arabidopsis affect tocopherol content, tocopherol composition, and oxidative stress. Plant Physiol. 2005, 137, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Cahoon, E.B.; Coughlan, S.J.; DellaPenna, D. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 2003, 132, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Semchuk, N.M.; Lushchak, O.V.; Falk, J.; Krupinska, K.; Lushchak, V.I. Inactivation of genes, encoding tocopherol biosynthetic pathway enzymes, results in oxidative stress in outdoor grown Arabidopsis thaliana. Plant Physiol. Biochem. 2009, 47, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Bergmüller, E.; Porfirova, S.; Dörmann, P. Characterization of an Arabidopsis mutant deficient in γ-tocopherolmethyltransferase. Plant Mol. Biol. 2003, 52, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, D. Tocopherols play a crucial role in low-temperature adaptation and Phloem loading in Arabidopsis. Plant Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef] [PubMed]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [PubMed]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Liebler, D.C. The role of metabolism in the antioxidant function of vitamin E. Crit. Rev. Toxicol. 1993, 23, 147–169. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K.; Tokumura, A.; Ouchi, S.; Tsukatani, H. Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids 1982, 17, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Straight, R.C.; Spikes, J.D. Photosensitized oxidation of biomolecules. In Singlet O2; Frimer, A.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; Volume 4, pp. 91–143. [Google Scholar]
- Lass, A.; Sohal, R.S. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem. Biophys. 1998, 352, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Bolton, E.M.; Burr, J.A.; Liebler, D.C.; Ross, D. The reduction of α-tocopherolquinone by human NAD(P)H: Quinone oxidoreductase: The role of α-tocopherolhydroquinone as a cellular antioxidant. Mol. Pharmacol. 1997, 52, 300–305. [Google Scholar] [PubMed]
- Wu, J.H.; Croft, K.D. Vitamin E metabolism. Mol. Aspects Med. 2007, 28, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Fernández-Martínez, J.; Garcia-Ruiz, R.; Domínguez, J. Genetic and environmental variation for tocopherol content and composition in sunflower commercial hybrids. J. Agric. Sci. 2002, 139, 425–429. [Google Scholar] [CrossRef]
- Bruni, R.; Muzzoli, M.; Ballero, M.; Loi, M.C.; Fantin, G.; Poli, F.; Sacchetti, G. Tocopherols, fatty acids and sterols in seeds of four Sardinian wild Euphorbia species. Fitoterapia 2004, 75, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Monica, H.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Accumulation of tocopherols and tocotrienols during seed development of grape (Vitis vinifera L. cv. Albert Lavallee). Plant Physiol. Biochem. 2006, 44, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.A.; García, J.F. Sorting olive oil based on alpha-tocopherol and total tocopherol content using near-infra-red spectroscopy (NIRS) analysis. J. Food Eng. 2017, 202, 79–88. [Google Scholar] [CrossRef]
- Gotor, A.A.; Farkas, E.; Berger, M.; Labalette, F.; Centis, S.; Daydé, J.; Calmon, A. Determination of tocopherols and phytosterols in sunflower seeds by NIR spectrometry. Eur. J. Lipid Sci. Technol. 2007, 109, 525–530. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [PubMed]
- Arango, Y.; Heise, K.-P. Localization of α-tocopherol synthesis in chromoplast envelope membranes of Capsicum annuum L. fruits. J. Exp. Bot. 1998, 49, 1259–1262. [Google Scholar] [CrossRef]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010, 179, 289–295. [Google Scholar] [CrossRef]
- Falk, J.; Krauß, N.; Dähnhardt, D.; Krupinska, K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. J. Plant Physiol. 2002, 159, 1245–1253. [Google Scholar] [CrossRef]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Bishop, K.S. On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempna, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y. Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Ricciarelli, R.; Zingg, J.M. Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef]
- Zingg, J.M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Folsom, A.R.; Prineas, R.J.; Mink, P.J.; Wu, Y.; Bostick, R.M. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N. Engl. J. Med. 1996, 334, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Wright, M.E.; Lawson, K.A.; Snyder, K.; Mannisto, S.; Taylor, P.R.; Virtamo, J.; Albanes, D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.H. Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Öhrvall, M.; Vessby, B.; Sundlöf, G. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J. Intern. Med. 1996, 239, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.; Mayatepek, E.; Inter, M.; Becker, M.; Pfeiffer, E.; Speer, A.; Hubner, C.; Finckh, B. Treatment of ataxia in isolated vitamin E deficiency caused by alpha-tocopherol transfer protein deficiency. J. Pediatr. 1999, 134, 240–244. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Frei, B.; Beckman, J.S. Vitamin E revisited: Do new data validate benefits for chronic disease prevention? Curr. Opin. Lipidol. 2008, 19, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Do free radicals play causal role in atherosclerosis? Low density lipoprotein oxidation and vitamin E revisited. J. Clin. Biochem. Nutr. 2010, 48, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Many tocopherols, one vitamin E. Mol. Asp. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Meydani, S.N.; Meydani, M.; Zingg, J.M. The rise, the fall and the renaissance of vitamin E. Arch. Biochem. Biophys. 2016, 595, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.; Traber, M.G.; Jacques, P.F.; Cross, C.E.; Hu, Y.; Block, G. Does γ-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J. Am. Coll. Nutr. 2006, 25, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kloz, M.; Pillai, S.; Kodis, G.; Gust, D.; Moore, T.A.; Moore, A.L.; van Grondelle, R.; Kennis, J.T. Carotenoid photoprotection in artificial photosynthetic antennas. J. Am. Chem. Soc. 2011, 133, 7007–7015. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wang, Z.A.; Sun, X.F.; Tang, K.X. Engineering tocopherol biosynthetic pathway in Arabidopsis leaves and its effect on antioxidant metabolism. Plant Sci. 2010, 178, 312–320. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munne-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Molecular mechanism of α-tocopherol action. Free Radic. Biol. Med. 2007, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Mene-Saffrane, L.; Farmer, E.E.; Krischke, M.; Mueller, M.J.; DellaPenna, D. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 2006, 18, 3706–3720. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kumar, K.; Rao, K.P.; Sinha, A.K.; Roitsch, T. Role of α-tocopherol in cellular signaling: α-tocopherol inhibits stress-induced mitogen-activated protein kinase activation. Plant Biotechnol. Rep. 2011, 5, 19–25. [Google Scholar] [CrossRef]
- Denoya, C.D.; Skinner, D.D.; Morgenstern, M.R. A Streptomyces avermitilis gene encoding a 4-hydroxyphenylpyruvic acid dioxygenase-like protein that directs the production of homogentisic acid and an ochronotic pigment in Escherichia coli. J. Bacteriol. 1994, 176, 5312–5319. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ali, S.A.; Nath, P.; Sane, V.A. Activation of ethylene-responsive p-hydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J. Exp. Bot. 2011, 62, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, S.; Wang, X.; Nichelmann, L.; Suppanz, I.; Hadenfeldt, S.; Endrigkeit, J.; Meng, J.; Jung, C. Genetic and functional analysis of tocopherol biosynthesis pathway genes from rapeseed (Brassica napus L.). Plant Breed. 2014, 133, 470–479. [Google Scholar] [CrossRef]
- Falk, J.; Andersen, G.; Kernebeck, B.; Krupinska, K. Constitutive overexpression of barley 4-hydroxyphenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Lett. 2003, 540, 35–40. [Google Scholar] [CrossRef]
- Rippert, P.; Scimemi, C.; Dubald, M.; Matringe, M. Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol. 2004, 134, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Sun, X.; Tang, K. Current opinions on the functions of tocopherol based on the genetic manipulation of tocopherol biosynthesis in plants. J. Integr. Plant Biol. 2008, 50, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Karunanandaa, B.; Qi, Q.; Hao, M.; Baszis, S.R.; Jensen, P.K.; Wong, Y.H.; Jiang, J.; Venkatramesh, M.; Gruys, K.J.; Moshiri, F.; et al. Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab. Eng. 2005, 7, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Raclaru, M.; Gruber, J.; Kumar, R.; Sadre, R.; Lühs, W.; Zarhloul, M.; Friedt, W.; Frentzen, M.; Weier, D. Increase of the tocochromanol content in transgenic Brassica napus seeds by overexpression of key enzymes involved in prenylquinone biosynthesis. Mol. Breed. 2006, 18, 93–107. [Google Scholar] [CrossRef]
- Naqvi, S.; Farre, G.; Zhu, C.; Sandmann, G.; Capell, T.; Christou, P. Simultaneous expression of Arabidopsis rho-hydroxyphenylpyruvate dioxygenase and MPBQ methyltransferase in transgenic corn kernels triples the tocopherol content. Transgenic Res. 2011, 20, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kleber-Janke, T.; Krupinska, K. Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta 1997, 203, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Keller, Y.; Bouvier, F.; D’Harlingue, A.; Camara, B. Metabolic compartmentation of plastid prenyllipid biosynthesis—Evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur. J. Biochem. 1998, 251, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Ren, G.; Li, D.; Cahoon, R.E.; Chen, M.; Zhou, Y.; Yu, B.; Cahoon, E.B. Chlorophyll synthase under epigenetic surveillance is critical for vitamin E synthesis, and altered expression affects tocopherol levels in Arabidopsis. Plant Physiol. 2015, 168, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Zbierzak, A.M.; Kanwischer, M.; Dormann, P. A salvage pathway for phytol metabolism in Arabidopsis. J. Biol. Chem. 2006, 281, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Yoshikawa, T.; Kimura, T.; Kaneko, H. Production of tocopherols by cell culture of safflower. Phytochemistry 1987, 26, 2741–2747. [Google Scholar] [CrossRef]
- Almeida, J.; Azevedo Mda, S.; Spicher, L.; Glauser, G.; vom Dorp, K.; Guyer, L.; del Valle Carranza, A.; Asis, R.; de Souza, A.P.; Buckeridge, M.; et al. Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J. Exp. Bot. 2016, 67, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Diepenbrock, C.H.; Kandianis, C.B.; Lipka, A.E.; Magallanes-Lundback, M.; Vaillancourt, B.; Gongora-Castillo, E.; Wallace, J.G.; Cepela, J.; Mesberg, A.; Bradbury, P.; et al. Novel Loci Underlie Natural Variation in Vitamin E Levels in Maize Grain. Plant Cell 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Collakova, E.; DellaPenna, D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2001, 127, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Schledz, M.; Seidler, A.; Beyer, P.; Neuhaus, G. A novel phytyltransferase from Synechocystis sp. PCC 6803 involved in tocopherol biosynthesis. FEBS Lett. 2001, 499, 15–20. [Google Scholar] [CrossRef]
- Sattler, S.E.; Cheng, Z.; DellaPenna, D. From Arabidopsis to agriculture: Engineering improved Vitamin E content in soybean. Trends Plant Sci. 2004, 9, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhao, L.; Wang, Y.; Cui, L.; Tang, Y.; Sun, X.; Tang, K. Overexpression of homogentisate phytyltransferase in lettuce results in increased content of vitamin E. AJB 2011, 10, 14046–14051. [Google Scholar]
- Mene-Saffrane, L.; Davoine, C.; Stolz, S.; Majcherczyk, P.; Farmer, E.E. Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant. Whole-body mapping of malondialdehyde pools in a complex eukaryote. J. Biol. Chem. 2007, 282, 35749–35756. [Google Scholar] [CrossRef] [PubMed]
- Mene-Saffrane, L.; Jones, A.D.; DellaPenna, D. Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 17815–17820. [Google Scholar] [CrossRef] [PubMed]
- Ksas, B.; Becuwe, N.; Chevalier, A.; Havaux, M. Plant tolerance to excess light energy and photooxidative damage relies on plastoquinone biosynthesis. Sci. Rep. 2015, 5, 10919. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, D.; Gogolewski, M.; Nogala-Kałucka, M. Isolation and some properties of plastochromanol-8. Mol. Nutr. Food Res. 1997, 41, 101–104. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, T.; Dellapenna, D. Role of callose synthases in transfer cell wall development in tocopherol deficient Arabidopsis mutants. Front. Plant Sci. 2014, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Raclaru, M.; Schusseler, T.; Gruber, J.; Sadre, R.; Lühs, W.; Zarhloul, K.M.; Friedt, W.; Enders, D.; Frentzen, M.; et al. Characterisation of plant tocopherol cyclases and their overexpression in transgenic Brassica napus seeds. FEBS Lett. 2005, 579, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Zbierzak, A.M.; Kanwischer, M.; Wille, C.; Vidi, P.A.; Giavalisco, P.; Lohmann, A.; Briesen, I.; Porfirova, S.; Brehelin, C.; Kessler, F.; et al. Intersection of the tocopherol and plastoquinol metabolic pathways at the plastoglobule. Biochem. J. 2010, 425, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Yadav, D.K.; Szymanska, R.; Kruk, J.; Sedlarova, M.; Pospisil, P. Singlet oxygen scavenging activity of tocopherol and plastochromanol in Arabidopsis thaliana: Relevance to photooxidative stress. Plant Cell Environ. 2014, 37, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dormann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett. 2002, 524, 145–148. [Google Scholar] [CrossRef]
- Liu, X.; Hua, X.; Guo, J.; Qi, D.; Wang, L.; Liu, Z.; Jin, Z.; Chen, S.; Liu, G. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol. Lett. 2008, 30, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; He, S.; Liu, P.; Zhang, W.; Zhang, J.; Chen, S. The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa). Sci. China Life Sci. 2011, 54, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S. Linking tocopherols with cellular signaling in plants. New Phytol. 2005, 166, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Weiler, E.W.; Alegre, L.; Müller, M.; Düchting, P.; Falk, J. α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 2007, 225, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Schaller, F. Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J. Exp. Bot. 2001, 52, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and Action of Jasmonates in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Botha, C.; Cross, R.; Van Bel, A.; Peter, C. Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath—Vascular parenchyma interface. Protoplasma 2000, 214, 65–72. [Google Scholar] [CrossRef]
- Provencher, L.M.; Miao, L.; Sinha, N.; Lucas, W.J. Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 2001, 13, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Russin, W.A.; Evert, R.F.; Vanderveer, P.J.; Sharkey, T.D.; Briggs, S.P. Modification of a Specific Class of Plasmodesmata and Loss of Sucrose Export Ability in the sucrose export defective1 Maize Mutant. Plant Cell 1996, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Hofius, D.; Hajirezaei, M.R.; Geiger, M.; Tschiersch, H.; Melzer, M.; Sonnewald, U. RNAi-mediated tocopherol deficiency impairs photoassimilate export in transgenic potato plants. Plant Physiol. 2004, 135, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Cela, J.; Chang, C.; Munné-Bosch, S. Accumulation of γ-rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Hass, C.G.; Tang, S.; Leonard, S.; Traber, M.G.; Miller, J.F.; Knapp, S.J. Three non-allelic epistatically interacting methyltransferase mutations produce novel tocopherol (vitamin E) profiles in sunflower. Theor. Appl. Genet. 2006, 113, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Collakova, E.; DellaPenna, D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Endrigkeit, J.; Wang, X.; Cai, D.; Zhang, C.; Long, Y.; Meng, J.; Jung, C. Genetic mapping, cloning, and functional characterization of the BnaX.VTE4 gene encoding a gamma-tocopherol methyltransferase from oilseed rape. Theor. Appl. Genet. 2009, 119, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.C.; Cahoon, E.B. Enhancing vitamin E in oilseeds: Unraveling tocopherol and tocotrienol biosynthesis. Lipids 2007, 42, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, S.; Wang, X.; Li, J.; Stich, B.; Kopisch-Obuch, F.J.; Endrigkeit, J.; Leckband, G.; Dreyer, F.; Friedt, W.; Meng, J.; et al. A candidate gene-based association study of tocopherol content and composition in rapeseed (Brassica napus). Front Plant Sci. 2012, 3, 129. [Google Scholar] [CrossRef] [PubMed]
- Muzhingi, T.; Palacios-Rojas, N.; Miranda, A.; Cabrera, M.L.; Yeum, K.J.; Tang, G. Genetic variation of carotenoids, vitamin E and phenolic compounds in Provitamin A biofortified maize. J. Sci. Food Agric. 2017, 97, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Yoon, M.Y.; He, Q.; Kim, T.S.; Tong, W.; Choi, B.W.; Lee, Y.S.; Park, Y.J. Natural variations in OsgammaTMT contribute to diversity of the alpha-tocopherol content in rice. Mol. Genet. Genomics 2015, 290, 2121–2135. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.J.; Rajcan, I.; Morris, B. Molecular mapping of soybean seed tocopherols in the cross ‘OAC Bayfield’ × ‘OAC Shire’. Plant Breed. 2017, 136, 83–93. [Google Scholar] [CrossRef]
- Graebner, R.C.; Wise, M.; Cuesta-Marcos, A.; Geniza, M.; Blake, T.; Blake, V.C.; Butler, J.; Chao, S.; Hole, D.J.; Horsley, R.; et al. Quantitative Trait Loci Associated with the Tocochromanol (Vitamin E) Pathway in Barley. PLoS ONE 2015, 10, e0133767. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, R. Mapping quantitative trait loci in plants: Uses and caveats for evolutionary biology. Nat. Rev. Genet. 2001, 2, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Li, L.; Fritsche, S.; Endrigkeit, J.; Zhang, W.; Long, Y.; Jung, C.; Meng, J. Unraveling the genetic basis of seed tocopherol content and composition in rapeseed (Brassica napus L.). PLoS ONE 2012, 7, e50038. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.E.; Gore, M.A.; Magallanes-Lundback, M.; Mesberg, A.; Lin, H.; Tiede, T.; Chen, C.; Buell, C.R.; Buckler, E.S.; Rocheford, T.; et al. Genome-wide association study and pathway-level analysis of tocochromanol levels in maize grain. G3 (Bethesda) 2013, 3, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 14015. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Sheoran, R.; Sharma, B. Perspectives of breeding for altering sunflower oil quality to obtain novel oils. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 949–962. [Google Scholar] [CrossRef]
- Rauf, S.; Jamil, N.; Tariq, S.A.; Khan, M.; Kausar, M.; Kaya, Y. Progress in modification of sunflower oil to expand its industrial value. J. Sci. Food Agric. 2017, 97, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Seyis, F.; Snowdon, R.; Luhs, W.; Friedt, W. Molecular characterization of novel resynthesized rapeseed (Brassica napus) lines and analysis of their genetic diversity in comparison with spring rapeseed cultivars. Plant Breed. 2003, 122, 473–478. [Google Scholar] [CrossRef]
- Hasan, M.; Seyis, F.; Badani, A.; Pons-Kühnemann, J.; Friedt, W.; Lühs, W.; Snowdon, R.J. Analysis of genetic diversity in the Brassica napus L. gene pool using SSR markers. Genet. Resour. Crop Evol. 2006, 53, 793–802. [Google Scholar] [CrossRef]
- Quadrana, L.; Almeida, J.; Asis, R.; Duffy, T.; Dominguez, P.G.; Bermudez, L.; Conti, G.; Correa da Silva, J.V.; Peralta, I.E.; Colot, V.; et al. Natural occurring epialleles determine vitamin E accumulation in tomato fruits. Nat. Commun. 2014, 5, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farre, G.; Sudhakar, D.; Naqvi, S.; Sandmann, G.; Christou, P.; Capell, T.; Zhu, C. Transgenic rice grains expressing a heterologous rho-hydroxyphenylpyruvate dioxygenase shift tocopherol synthesis from the gamma to the alpha isoform without increasing absolute tocopherol levels. Transgenic Res. 2012, 21, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Khurana, P. Cloning, functional characterisation and transgenic manipulation of vitamin E biosynthesis genes of wheat. Funct. Plant Biol. 2013, 40, 1129–1136. [Google Scholar] [CrossRef]
- Espinoza, A.; San Martin, A.; Lopez-Climent, M.; Ruiz-Lara, S.; Gomez-Cadenas, A.; Casaretto, J.A. Engineered drought-induced biosynthesis of alpha-tocopherol alleviates stress-induced leaf damage in tobacco. J. Plant Physiol. 2013, 170, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, H.; Zhang, L.; Shi, X.; Chen, X. Tocopherol-deficient rice plants display increased sensitivity to photooxidative stress. Planta 2014, 239, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Daniell, H. Expression of gamma-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol. J. 2014, 12, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yabuta, Y.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Generation of transgenic tobacco plants with enhanced tocotrienol levels through the ectopic expression of rice homogentisate geranylgeranyl transferase. Plant Biotechnol. J. 2015, 32, 233–238. [Google Scholar] [CrossRef]
- Che, P.; Zhao, Z.Y.; Glassman, K.; Dolde, D.; Hu, T.X.; Jones, T.J.; Obukosia, S.; Wambugu, F.; Albertsen, M.C. Elevated vitamin E content improves all-trans beta-carotene accumulation and stability in biofortified sorghum. Proc. Natl. Acad. Sci. USA 2016, 113, 11040–11045. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.; Cázares, P.; Yu, F.; De Luca, V. A picrinine N-methyltransferase belongs to a new family of γ-tocopherol-like methyltransferases found in medicinal plants that make biologically active monoterpenoid indole alkaloids. Plant Physiol. 2016, 170, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Fleta-Soriano, E.; Munne-Bosch, S. Enhanced plastochromanol-8 accumulation during reiterated drought in maize (Zea mays L.). Plant Physiol. Biochem. 2017, 112, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Chen, X.; Wang, M.; Bach, T.J.; Chye, M.L. Improved fruit α-tocopherol, carotenoid, squalene and phytosterol contents through manipulation of Brassica juncea 3-HYDROXY-3-METHYLGLUTARYL-COA SYNTHASE1 in transgenic tomato. Plant Biotechnol. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Demurin, Y.; Skoric, D.; Karlovic, D. Genetic variability of tocopherol composition in sunflower seeds as a basis of breeding improved oil quality. Plant Breed. 1996, 33–36. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Sarin, N.B. Antioxidant value addition in human diets: Genetic transformation of Brassica juncea with gamma-TMT gene for increased alpha-tocopherol content. Transgenic Res. 2007, 16, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Vinutha, T.; Maheswari, C.; Bansal, N.; Prashat, G.R.; Krishnan, V.; Kumari, S.; Dahuja, A.; Sachdev, A.; Rai, R. Expression analysis of γ-tocopherol methyl transferase genes and α-tocopherol content in developing seeds of soybean [Glycine max (L.) Merr.]. IJBB 2015, 52, 267–273. [Google Scholar]
- Tavva, V.S.; Kim, Y.H.; Kagan, I.A.; Dinkins, R.D.; Kim, K.H.; Collins, G.B. Increased alpha-tocopherol content in soybean seed overexpressing the Perilla frutescens gamma-tocopherol methyltransferase gene. Plant Cell. Rep. 2007, 26, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Siles, L.; Cela, J.; Munne-Bosch, S. Vitamin E analyses in seeds reveal a dominant presence of tocotrienols over tocopherols in the Arecaceae family. Phytochemistry 2013, 95, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Matringe, M.; Ksas, B.; Rey, P.; Havaux, M. Tocotrienols, the unsaturated forms of vitamin E, can function as antioxidants and lipid protectors in tobacco leaves. Plant Physiol. 2008, 147, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gonzalez, J.J.; Garvin, D.F. Subgenome-specific assembly of vitamin E biosynthesis genes and expression patterns during seed development provide insight into the evolution of oat genome. Plant Biotechnol. J. 2016, 14, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Marwede, V.; Gul, M.K.; Becker, H.C.; Ecke, W. Mapping of QTL controlling tocopherol content in winter oilseed rape. Plant Breed. 2005, 124, 20–26. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, H.C.; Han, Y.P.; Wu, X.X.; Teng, W.L.; Liu, G.F.; Li, W.B. Identification of QTL underlying vitamin E contents in soybean seed among multiple environments. Theor. Appl. Genet. 2010, 120, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, G.; Wu, D.; Jiang, Z.; Han, Y.; Li, W.; Morris, B. Quantitative trait loci underlying soybean seed tocopherol content with main additive, epistatic and QTL × environment effects. Plant Breed. 2017. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Chen, L.L.; Xie, K. Recent Advances in Genome Editing Using CRISPR/Cas9. Front. Plant Sci. 2016, 7, 703. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Gersbach, C.A. Genome editing: The end of the beginning. Genome Biol. 2015, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Tian, X.; Xu, F.; Liu, J.; Singh, P.K.; Botella, J.R.; Song, C. Genome Editing in Cotton with the CRISPR/Cas9 System. Front. Plant Sci. 2017, 8, 1364. [Google Scholar] [CrossRef] [PubMed]
- Cardi, T.; Neal Stewart, C., Jr. Progress of targeted genome modification approaches in higher plants. Plant Cell Rep. 2016, 35, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Capistrano-Gossmann, G.; Braatz, J.; Sashidhar, N.; Melzer, S. Recent developments in genome editing and applications in plant breeding. Plant Breed. 2017. [Google Scholar] [CrossRef]
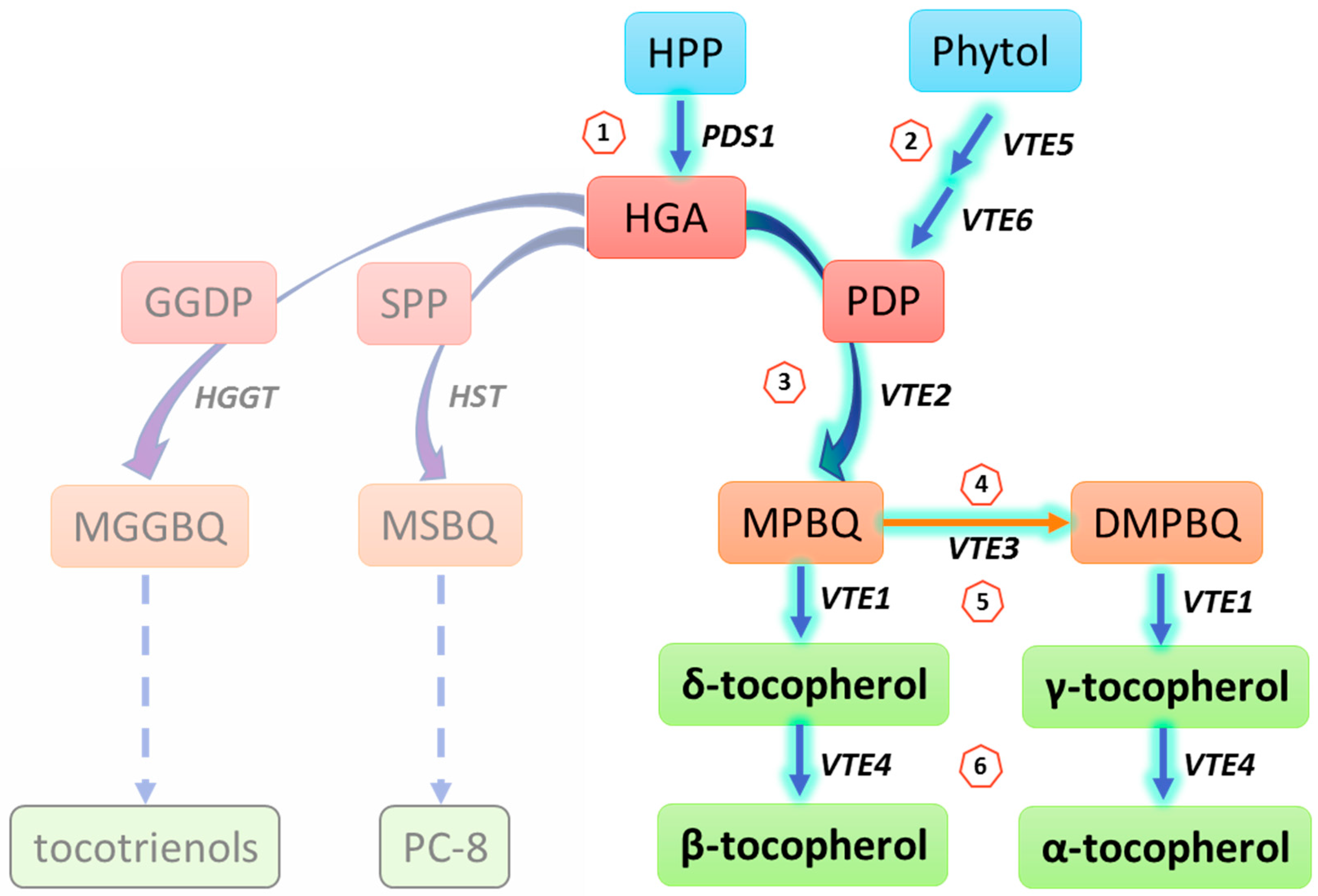
| Biosynthesis Step | Gene | Enzyme | Function | Substrate 1 | Product | At Locus | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PDS1 | HPPD | Head group synthesis | HPP | HGA | AT1G06570 | [16,17,18] |
| 2 | VTE5 | Phytol kinase | Phosphorylation | Phytol + CTP/UTP | Phytyl-P | AT5G04490 | [14] |
| VTE6 | Phytyl -P kinase | Phosphorylation | Phytol-P + CTP | Phytyl-PP(PDP) | AT1G78620 | [13] | |
| 3 | VTE2 | HPT | Phytylation | HGA + PDP | MPBQ | AT2G18950 | [19,20,21,22,23] |
| 4 | VTE3 | MPBQ/MSBQ MT | Methylation | MPBQ/MSBQ | DMPQ | AT3G63410 | [24,25,26] |
| 5 | VTE1 | TC | Cyclization | MPBQ/DMPQ | γ-tocopherol/δ-tocopherol | AT4G32770 | [27,28,29,30] |
| 6 | VTE4 | γ-TMT | Methylation | δ-tocopherol/γ-tocopherol | α-tocopherol/β-tocopherol | AT1G64970 | [30,31,32,33] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritsche, S.; Wang, X.; Jung, C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. https://doi.org/10.3390/antiox6040099
Fritsche S, Wang X, Jung C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants. 2017; 6(4):99. https://doi.org/10.3390/antiox6040099
Chicago/Turabian StyleFritsche, Steffi, Xingxing Wang, and Christian Jung. 2017. "Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops" Antioxidants 6, no. 4: 99. https://doi.org/10.3390/antiox6040099





