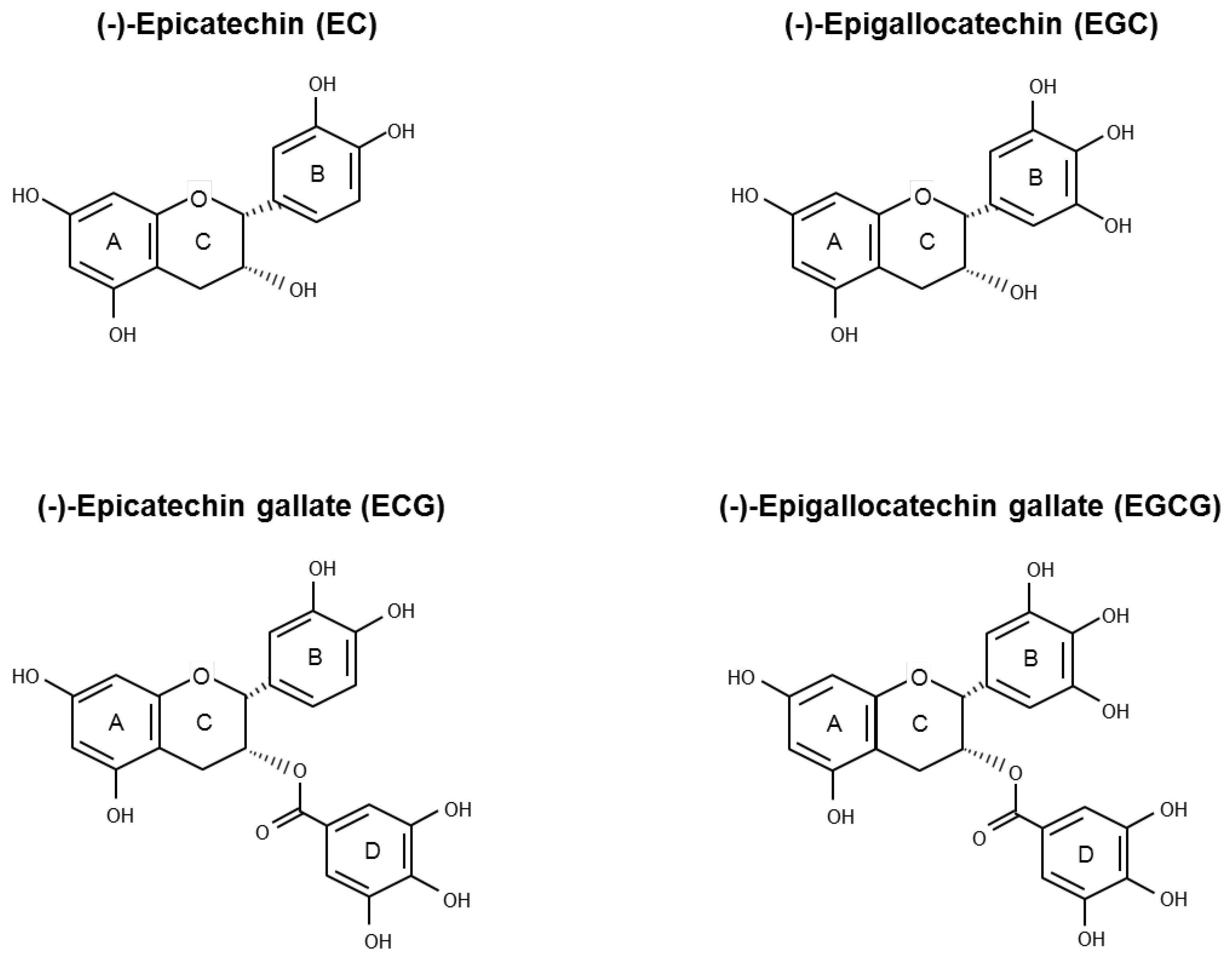
Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges
Abstract
:1. Introduction
2. Effects of GTCs on Cellular Redox Homeostasis and Antioxidant Signaling
2.1. Effects of GTCs on Intracellular ROS Production
2.2. Effects of GTCs on SOD Activity
2.3. Effects of GTCs on -RNS Production
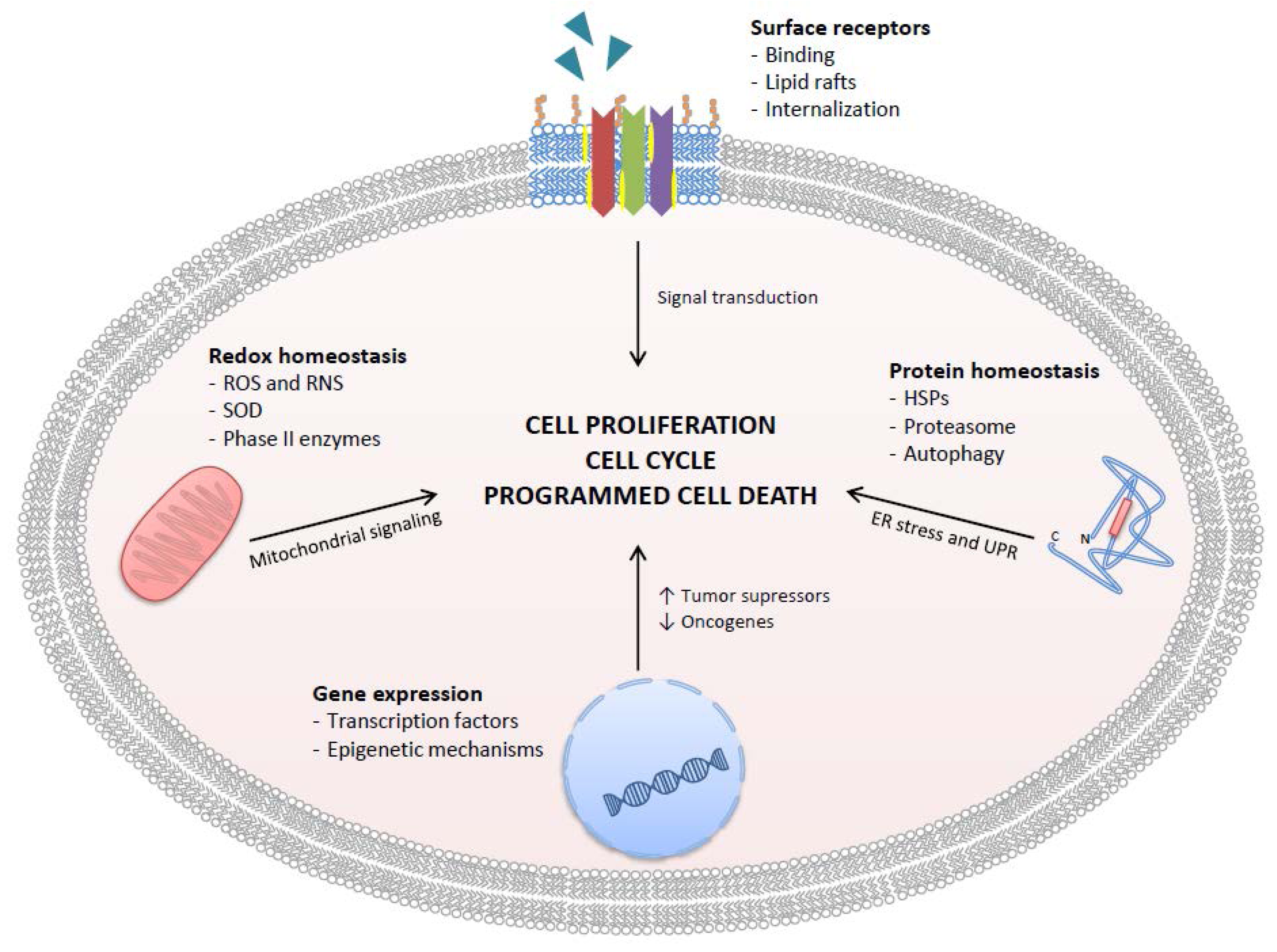
3. Specific Molecular Mechanisms of Action of GTCs
3.1. Cell Surface Receptors
3.2. Effects of GTCs on Regulation of Gene Expression
Epigenetic Mechanisms
3.3. Effects of GTCs on Protein Homeostasis
3.3.1. Heat Shock Proteins
3.3.2. Proteasome Inhibition
3.3.3. Autophagy
4. GTCs in Human PCa Chemoprevention: From Epidemiological Data to Interventional Studies
5. GTCs Bioavailability and Metabolism in Humans
6. Nanotechnology Strategies to Improve GTCs Bioactivity
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Globocan 2012 v1.0, Cancer Incidence and Mortality Worldwide: Iarc Cancerbase No. 11. Available online: http://globocan.iarc.fr (accessed on 1 February 2017).
- Wong, M.C.; Goggins, W.B.; Wang, H.H.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J. Global incidence and mortality for prostate cancer: Analysis of temporal patterns and trends in 36 countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Wang, S.-C.; Ho, C.-J.; Kao, Y.-L.; Hsieh, T.-Y.; Chen, W.-J.; Chen, C.-J.; Wu, P.-R.; Ko, J.-L.; Lee, H.; et al. Prostate cancer mortality-to-incidence ratios are associated with cancer care disparities in 35 countries. Sci. Rep. 2017, 7, 40003. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Youlden, D.R.; Krnjacki, L.J. International epidemiology of prostate cancer: Geographical distribution and secular trends. Mol. Nutr. Food Res. 2009, 53, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Yatani, R.; Henderson, B.E.; Mack, T.M. Cancers of the prostate and breast among japanese and white immigrants in los angeles county. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Demissie, K.; Lu, S.E.; Rhoads, G.G. Cancer incidence among korean-american immigrants in the united states and native koreans in south korea. Cancer Control 2007, 14, 78–85. [Google Scholar] [PubMed]
- Bettuzzi, S.; Rizzi, F.; Belloni, L. Clinical relevance of the inhibitory effect of green tea catechins (GtCs) on prostate cancer progression in combination with molecular profiling of catechin-resistant tumors: An integrated view. Pol. J. Vet. Sci. 2007, 10, 57–60. [Google Scholar] [PubMed]
- Johnson, J.J.; Bailey, H.H.; Mukhtar, H. Green tea polyphenols for prostate cancer chemoprevention: A translational perspective. Phytomedicine 2010, 17, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N. Risk and preventive factors for prostate cancer in Japan: The Japan public health center-based prospective (JPHC) study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Heber, D. Chemopreventive effects of tea in prostate cancer: Green tea versus black tea. Mol. Nutr. Food Res. 2011, 55, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Takehiko, Y.; Mujo, K. Chemistry and Applications of Green Tea; CRC Press: New York, NY, USA, 1997; p. 1e130. [Google Scholar]
- Caporali, A.; Davalli, P.; Astancolle, S.; D’Arca, D.; Brausi, M.; Bettuzzi, S.; Corti, A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis 2004, 25, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem. Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hastak, K.; Ahmad, N.; Lewin, J.S.; Mukhtar, H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. USA 2001, 98, 10350–10355. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Siddiqui, I.A.; Ahmad, N.; Gupta, S.; Mukhtar, H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-i-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004, 64, 8715–8722. [Google Scholar] [CrossRef] [PubMed]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Brausi, M.; Rizzi, F.; Bettuzzi, S. Chemoprevention of human prostate cancer by green tea catechins: Two years later. A follow-up update. Eur. Urol. 2008, 54, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Rizzi, F.; Caporali, A.; Pellacani, D.; Davoli, S.; Bettuzzi, S.; Brausi, M.; D’Arca, D. Anticancer activity of green tea polyphenols in prostate gland. Oxid. Med. Cell. Longev. 2012, 2012, 984219. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Burhans, W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Ghosh, R. Antioxidants for prostate cancer chemoprevention: Challenges and opportunities. Biochem. Pharmacol. 2012, 83, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food. Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Elbling, L.; Weiss, R.M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005, 19, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, M.; Sonda, T.; Nagayama, K.; Tabata, M. Effects of PH and metal ions on antioxidative activities of catechins. Biosci. Biotechnol. Biochem. 2001, 65, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Kirkland, D.; Whitwell, J.; Halliwell, B. Different cytotoxic and clastogenic effects of epigallocatechin gallate in various cell-culture media due to variable rates of its oxidation in the culture medium. Mutat. Res. 2007, 634, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Twum-Ampofo, J.; Fu, D.X.; Passaniti, A.; Hussain, A.; Siddiqui, M.M. Metabolic targets for potential prostate cancer therapeutics. Curr. Opin. Oncol. 2016, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; He, G.Q.; Yu, H.N.; Yang, J.G.; Borthakur, D.; Zhang, L.C.; Shen, S.R.; Das, U.N. Free Zn2+ enhances inhibitory effects of EGCG on the growth of PC-3 cells. Mol. Nutr. Food Res. 2008, 52, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, H.; Sun, S.; Zhang, L.; Das, U.N.; Ruan, H.; He, G.; Shen, S. Mechanism of free Zn2+ enhancing inhibitory effects of EGCG on the growth of PC-3 cells: Interactions with mitochondria. Biol. Trace. Elem. Res. 2009, 131, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Yu, H.N.; Sun, S.L.; Zhang, L.C.; He, G.Q.; Das, U.N.; Ruan, H.; Shen, S.R. Epigallocatechin-3-gallate affects the growth of LNCaP cells via membrane fluidity and distribution of cellular zinc. J. Zhejiang Univ. Sci. B 2009, 10, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Amatangelo, M.D.; Varma, D.; Sell, C.; Goodyear, S.M. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: Inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am. J. Pathol. 2010, 177, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y.; Cheung, T.C.; Kong, S.K.; Fung, K.P.; Choy, Y.M.; Chan, Z.Y.; Kwok, T.T. Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci. 2001, 68, 1207–1214. [Google Scholar] [CrossRef]
- Kanwal, R.; Pandey, M.; Bhaskaran, N.; Maclennan, G.T.; Fu, P.; Ponsky, L.E.; Gupta, S. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol. Carcinog. 2014, 53, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Naponelli, V.; Silva, A.; Modernelli, A.; Ramazzina, I.; Bonacini, M.; Tardito, S.; Gatti, R.; Uggeri, J.; Bettuzzi, S. Polyphenon e®, a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 2014, 35, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Candas, D.; Li, J.J. Mnsod in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal 2014, 20, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Holly, J.M.; Persad, R.; Bahl, A.; Perks, C.M. Green tea extract (epigallocatechin-3-gallate) reduces efficacy of radiotherapy on prostate cancer cells. Urology 2011, 78, 475.e15–475.e21. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.; Sheridan, J.; Mulcahy, H.; Tenniswood, M.; Morrissey, C. The effect of green tea on oxidative damage and tumour formation in lobund-wistar rats. Eur. J. Cancer Prev. 2008, 17, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Nyska, A.; Suttie, A.; Bakshi, S.; Lomnitski, L.; Grossman, S.; Bergman, M.; Ben-Shaul, V.; Crocket, P.; Haseman, J.K.; Moser, G.; et al. Slowing tumorigenic progression in TRAMP mice and prostatic carcinoma cell lines using natural anti-oxidant from spinach, NAO—A comparative study of three anti-oxidants. Toxicol. Pathol. 2003, 31, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.N.; Nyska, A.; Maronpot, R.R.; Kissling, G.; Lomnitski, L.; Suttie, A.; Bakshi, S.; Bergman, M.; Grossman, S.; Ho, S.M. Differential attenuation of oxidative/nitrosative injuries in early prostatic neoplastic lesions in TRAMP mice by dietary antioxidants. Prostate 2006, 66, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The potential role of nitric oxide in halting cancer progression through chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480–481, 243–268. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-cancer effects of green tea by either anti- or pro- oxidative mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, S.; Porter, A.G. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J. Biol. Chem. 2004, 279, 20096–20107. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.E.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Lamartiniere, C.A. Epigallocatechin-3-gallate suppresses early stage, but not late stage prostate cancer in tramp mice: Mechanisms of action. Prostate 2007, 67, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M. Cellular targets for the beneficial actions of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1642S–1650S. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in ht-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar] [PubMed]
- Shimizu, M.; Adachi, S.; Masuda, M.; Kozawa, O.; Moriwaki, H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol. Nutr. Food Res. 2011, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Nam, K.Y.; Kimura, S.; Fujiki, H.; Imanishi, Y. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 1997, 65, 157–164. [Google Scholar] [CrossRef]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of vegf receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lin-shiau, S.Y.; Chen, C.F.; Lin, J.K. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell. Biochem. 1997, 67, 55–65. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Ermakova, S.; Zheng, D.; Tang, F.; Cho, Y.Y.; Zhu, F.; Ma, W.Y.; Sham, Y.; Rogozin, E.A.; et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in ht29 colon cancer cells. Cancer Res. 2007, 67, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Duhon, D.; Bigelow, R.L.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagao, T.; To, S.; Joe, A.K.; Shimizu, M.; Matsushima-Nishiwaki, R.; Kozawa, O.; Moriwaki, H.; Maxfield, F.R.; Weinstein, I.B. (−)-epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis 2008, 29, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Weinstein, I.B. Modulation of signal transduction by tea catechins and related phytochemicals. Mutat. Res. 2005, 591, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Regad, T. Targeting RTK signaling pathways in cancer. Cancers (Basel) 2015, 7, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.A.; Dashwood, R.H.; Bisson, W.H. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacol. Res. 2010, 62, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.A.; Atreya, H.S. Defining the pathway to insulin-like growth factor system targeting in cancer. Biochem. Pharmacol. 2010, 80, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Pianetti, S.; Guo, S.; Kavanagh, K.T.; Sonenshein, G.E. Green tea polyphenol epigallocatechin-3 gallate inhibits HER-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002, 62, 652–655. [Google Scholar] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Weinstein, I.B. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin. Cancer Res. 2003, 9, 3486–3491. [Google Scholar] [PubMed]
- Shimizu, M.; Deguchi, A.; Lim, J.T.E.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Deguchi, A.; Joe, A.K.; McKoy, J.F.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J. Exp. Ther. Oncol. 2005, 5, 69–78. [Google Scholar] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Tatebe, H.; Nakagawa, T.; Hara, Y.; Weinstein, I.B.; Moriwaki, H. EGCG inhibits activation of the insulin-like growth factor (igf)/igf-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008, 262, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Ueno, T.; Nakamura, T.; Sakamoto, M.; Torimura, T.; Sata, M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line li90. J. Hepatol. 2004, 40, 52–59. [Google Scholar] [CrossRef]
- Larsen, C.A.; Dashwood, R.H. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: Minor role of hydrogen peroxide. Biochem. Biophys. Res. Commun. 2009, 389, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.A.; Bisson, W.H.; Dashwood, R.H. Tea catechins inhibit hepatocyte growth factor receptor (Met kinase) activity in human colon cancer cells: Kinetic and molecular docking studies. J. Med. Chem. 2009, 52, 6543–6545. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008, 283, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCG) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sumida, M.; Kumazoe, M.; Sugihara, K.; Suemasu, Y.; Yamada, S.; Yamashita, S.; Miyakawa, J.; Takahashi, T.; Tanaka, H.; et al. Oligomer formation of a tea polyphenol, EGCG, on its sensing molecule 67 kDa laminin receptor. Chem. Commun. 2017, 53, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Antopolsky, M.; Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar]
- Mocanu, M.M.; Ganea, C.; Georgescu, L.; Varadi, T.; Shrestha, D.; Baran, I.; Katona, E.; Nagy, P.; Szollosi, J. Epigallocatechin 3-O-gallate induces 67 kDa laminin receptor-mediated cell death accompanied by downregulation of ErbB proteins and altered lipid raft clustering in mammary and epidermoid carcinoma cells. J. Nat. Prod. 2014, 77, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Hirotsu, K.; Kumazoe, M.; Goto, Y.; Sugihara, K.; Suda, T.; Tsurudome, Y.; Suzuki, T.; Yamashita, S.; Kim, Y.; et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cdelta and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012, 443, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Umeda, D.; Kiyohara, Y.; Sunada, Y.; Yamada, K.; Tachibana, H. The involvement of the 67 kda laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochem. Biophys. Res. Commun. 2006, 348, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Yamada, K.; Tachibana, H. A lipid raft-associated 67kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on fcepsilonri expression. Biochem. Biophys. Res. Commun. 2005, 336, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Fujimura, Y.; Yamada, K. Tea polyphenol epigallocatechin-3-gallate associates with plasma membrane lipid rafts: Lipid rafts mediate anti-allergic action of the catechin. Biofactors 2004, 21, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.K. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta 2008, 1785, 182–206. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J.; Han, X.; Gross, R.W. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: A shotgun lipidomics study. J. Biol. Chem. 2005, 280, 26796–26804. [Google Scholar] [CrossRef] [PubMed]
- Remacle-Bonnet, M.; Garrouste, F.; Baillat, G.; Andre, F.; Marvaldi, J.; Pommier, G. Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am. J. Pathol. 2005, 167, 761–773. [Google Scholar] [CrossRef]
- Oshikawa, J.; Urao, N.; Kim, H.W.; Kaplan, N.; Razvi, M.; McKinney, R.; Poole, L.B.; Fukai, T.; Ushio-Fukai, M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE 2010, 5, e10189. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Belloni, L.; Caporali, A.; Davalli, P.; Remondini, D.; Rizzi, F.; Astancolle, S.; Corti, A.; Bettuzzi, S. Molecular classification of green tea catechin-sensitive and green tea catechin-resistant prostate cancer in the TRAMP mice model by quantitative real-time PCR gene profiling. Carcinogenesis 2006, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Hun Lee, J.; Shu, L.; Fuentes, F.; Su, Z.Y.; Tony Kong, A.N. Cancer chemoprevention by traditional Chinese herbal medicine and dietary phytochemicals: Targeting Nrf2-mediated oxidative stress/anti-inflammatory responses, epigenetics, and cancer stem cells. J. Tradit. Complement. Med. 2013, 3, 69–79. [Google Scholar] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Connors, S.K.; Chornokur, G.; Kumar, N.B. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr. Cancer 2012, 64, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Vayalil, P.K.; Katiyar, S.K. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate 2004, 59, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hastak, K.; Gupta, S.; Ahmad, N.; Agarwal, M.K.; Agarwal, M.L.; Mukhtar, H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene 2003, 22, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Shukla, Y.; Adhami, V.M.; Sarfaraz, S.; Asim, M.; Hafeez, B.B.; Mukhtar, H. Suppression of NFkappaB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharm. Res. 2008, 25, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Sartor, L.; Pezzato, E.; Dona, M.; Dell’Aica, I.; Calabrese, F.; Morini, M.; Albini, A.; Garbisa, S. Prostate carcinoma and green tea: (−)epigallocatechin-3-gallate inhibits inflammation-triggered MMP-2 activation and invasion in murine TRAMP model. Int. J. Cancer 2004, 112, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Nandy, S.K.; Chowdhury, A.; Chakraborti, T.; Chakraborti, S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomedi. Pharmacother. 2016, 84, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Shukla, S.; Gupta, S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int. J. Cancer 2010, 126, 2520–2533. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (−)-epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Yoo, C.B.; Kwan, J.M.; Li, T.W.; Liang, G.; Yang, A.S.; Jones, P.A. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2′-deoxycytidine. Mol. Cancer Ther. 2005, 4, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Morey Kinney, S.R.; Zhang, W.; Pascual, M.; Greally, J.M.; Gillard, B.M.; Karasik, E.; Foster, B.A.; Karpf, A.R. Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate. Cancer Prev. Res. 2009, 2, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Said, J.; Magyar, C.; Castor, B.; Doan, N.; Tosity, C.; Moro, A.; Gao, K.; Li, L.; et al. Polyphenols in brewed green tea inhibit prostate tumor xenograft growth by localizing to the tumor and decreasing oxidative stress and angiogenesis. J. Nutr. Biochem. 2012, 23, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Peran, E.; Cabezas-Herrera, J.; Campo, L.S.; Rodriguez-Lopez, J.N. Effects of folate cycle disruption by the green tea polyphenol epigallocatechin-3-gallate. Int. J. Biochem. Cell Biol. 2007, 39, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 351–354. [Google Scholar] [PubMed]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, Y.-P.; Chen, H.-Y.; Li, J. Histone deacetylases function as novel potential therapeutic targets for cancer. Hepatol. Res. 2017, 47, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.S.; Gupta, K.; Gupta, S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class i histone deacetylases. Int. J. Oncol. 2012, 41, 353–361. [Google Scholar] [PubMed]
- Rivera-Barahona, A.; Pérez, B.; Richard, E.; Desviat, L.R. Role of mirnas in human disease and inborn errors of metabolism. J. Inherit. Metab. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ribas, J.; Ni, X.; Haffner, M.; Wentzel, E.A.; Salmasi, A.H.; Chowdhury, W.H.; Kudrolli, T.A.; Yegnasubramanian, S.; Luo, J.; Rodriguez, R.; et al. miR-21: An androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009, 69, 7165–7169. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2010, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Schonthal, A.H. Targeting endoplasmic reticulum stress for cancer therapy. Front. Biosci. (Schol. Ed.) 2012, 4, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A.; Juckstock, J. Misfolded proteins: From little villains to little helpers in the fight against cancer. Front. Oncol. 2015, 5, 47. [Google Scholar] [PubMed]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014, 2, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Fanelli, M.A.; Cuello-Carrion, F.D.; Castro, G.N. Heat shock proteins in prostate cancer: From tumorigenesis to the clinic. Int. J. Hyperth. 2010, 26, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Xu, J.; Shi, J.; Jiang, L.; Yao, N.; Ye, W. Discovery and development of natural heat shock protein 90 inhibitors in cancer treatment. Acta Pharm. Sin. B 2012, 2, 238–245. [Google Scholar] [CrossRef]
- Moses, M.A.; Henry, E.C.; Ricke, W.A.; Gasiewicz, T.A. The heat shock protein 90 inhibitor, (−)-epigallocatechin gallate, has anticancer activity in a novel human prostate cancer progression model. Cancer Prev. Res. (Phila) 2015, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Smith, D.M.; Dou, Q.P. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J. Biol. Chem. 2001, 276, 13322–13330. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Landis-Piwowar, K.; Chan, T.H.; Dou, Q.P. Green tea polyphenols as proteasome inhibitors: Implication in chemoprevention. Curr. Cancer Drug Targets 2011, 11, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.; Wang, Z.; Kumar, N.; Falsetti, S.C.; Chan, T.H.; Dou, Q.P. Structure-activity relationships of synthetic analogs of (−)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer Res. 2004, 24, 943–954. [Google Scholar] [PubMed]
- Smith, D.M.; Wang, Z.; Kazi, A.; Li, L.H.; Chan, T.H.; Dou, Q.P. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol. Med. 2002, 8, 382–392. [Google Scholar] [PubMed]
- Modernelli, A.; Naponelli, V.; Giovanna Troglio, M.; Bonacini, M.; Ramazzina, I.; Bettuzzi, S.; Rizzi, F. EGCG antagonizes bortezomib cytotoxicity in prostate cancer cells by an autophagic mechanism. Sci. Rep. 2015, 5, 15270. [Google Scholar] [CrossRef] [PubMed]
- Naponelli, V.; Modernelli, A.; Bettuzzi, S.; Rizzi, F. Roles of autophagy induced by natural compounds in prostate cancer. Biomed. Res. Int. 2015, 2015, 121826. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Rizzi, F.; Caldara, G.F.; Davoli, S.; Corti, A.; Silva, A.; Astancolle, S.; Vitale, M.; Bettuzzi, S.; Arcari, M.; et al. Chronic administration of green tea extract to TRAMP mice induces the collapse of Golgi apparatus in prostate secretory cells and results in alterations of protein post-translational processing. Int. J. Oncol. 2011, 39, 1521–1527. [Google Scholar] [PubMed]
- Kikuchi, N.; Ohmori, K.; Shimazu, T.; Nakaya, N.; Kuriyama, S.; Nishino, Y.; Tsubono, Y.; Tsuji, I. No association between green tea and prostate cancer risk in Japanese men: The Ohsaki Cohort Study. Br. J. Cancer 2006, 95, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Sauvaget, C.; Roddam, A.W.; Appleby, P.; Nagano, J.; Suzuki, G.; Key, T.J.; Koyama, K. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control 2004, 15, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar] [PubMed]
- Kurahashi, N.; Sasazuki, S.; Iwasaki, M.; Inoue, M. Green tea consumption and prostate cancer risk in Japanese men: A prospective study. Am. J. Epidemiol. 2007, 167, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Xie, L.P.; Lee, A.H.; Binns, C.W. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Int. J. Cancer 2004, 108, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, T.; Nagata, Y.; Mori, M.; Miyanaga, N.; Takashima, N.; Okumura, K.; Goto, K.; Naito, S.; Fujimoto, K.; Hirao, Y.; et al. A case-control study of diet and prostate cancer in Japan: Possible protective effect of traditional Japanese diet. Cancer Sci. 2004, 95, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Siddiqui, I.A.; Sarfaraz, S.; Khwaja, S.I.; Hafeez, B.B.; Ahmad, N.; Mukhtar, H. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin. Cancer Res. 2009, 15, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.; Ellison, N.; Burch, P.A.; Sloan, J.A.; Dakhil, S.R.; Novotny, P.; Tan, W.; Fitch, T.R.; Rowland, K.M.; Young, C.Y.; et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer 2003, 97, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- McLarty, J.; Bigelow, R.L.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. (Phila) 2009, 2, 673–682. [Google Scholar] [PubMed]
- Nguyen, M.M.; Ahmann, F.R.; Nagle, R.B.; Hsu, C.H.; Tangrea, J.A.; Parnes, H.L.; Sokoloff, M.H.; Gretzer, M.B.; Chow, H.H. Randomized, double-blind, placebo-controlled trial of polyphenon e in prostate cancer patients before prostatectomy: Evaluation of potential chemopreventive activities. Cancer Prev. Res. (Phila) 2012, 5, 290–298. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd G, J.; Miller, M.C.; Orozco, R.; Veltri, R.W. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology 2000, 55, 553–559. [Google Scholar] [CrossRef]
- Kumar, N.B.; Pow-Sang, J.; Spiess, P.E.; Park, J.; Salup, R.; Williams, C.R.; Parnes, H.; Schell, M.J. Randomized, placebo-controlled trial evaluating the safety of one-year administration of green tea catechins. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, S.; Zhao, C.; Miyamoto, H. Histopathologic features of atypical glands on prostate biopsy: Nucleolar size is a predictor of subsequent detection of prostatic adenocarcinoma. Prostate 2013, 73, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, P.; Wolski, Z.; Butkiewicz, R.; Nussbeutel, J.; Drewa, T. Significance of atypical small acinar proliferation and extensive high-grade prostatic intraepithelial neoplasm in clinical practice. Cent. Eur. J. Urol. 2014, 67, 136–141. [Google Scholar]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase i pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 53–58. [Google Scholar] [PubMed]
- Henning, S.M.; Aronson, W.; Niu, Y.; Conde, F.; Lee, N.H.; Seeram, N.P.; Lee, R.P.; Lu, J.; Harris, D.M.; Moro, A.; et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J. Nutr. 2006, 136, 1839–1843. [Google Scholar] [PubMed]
- Wang, P.; Aronson, W.J.; Huang, M.; Zhang, Y.; Lee, R.P.; Heber, D.; Henning, S.M. Green tea polyphenols and metabolites in prostatectomy tissue: Implications for cancer prevention. Cancer Prev. Res. 2010, 3, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; De, R.; Begun, J.; Popat, A. Cancer therapeutics with epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. Int. J. Pharm. 2017, 518, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pintus, G.; Roggio, A.M.; Punzoni, S.; Posadino, A.M.; Arca, A.; Marceddu, S.; Bandiera, P.; Uzzau, S.; Sechi, M. Targeted biocompatible nanoparticles for the delivery of (−)-epigallocatechin 3-gallate to prostate cancer cells. J. Med. Chem. 2011, 54, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2013, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Shafiei, S.S.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Kalantarinejad, R.; Asadi-Eydivand, M.; Abu Osman, N.A. Epigallocatechin gallate/layered double hydroxide nanohybrids: Preparation, characterization, and in vitro anti-tumor study. PLoS ONE 2015, 10, e0136530. [Google Scholar]
- Tsai, L.-C.; Hsieh, H.-Y.; Lu, K.-Y.; Wang, S.-Y.; Mi, F.-L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine 2016, 11, 9–30. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants 2017, 6, 26. https://doi.org/10.3390/antiox6020026
Naponelli V, Ramazzina I, Lenzi C, Bettuzzi S, Rizzi F. Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants. 2017; 6(2):26. https://doi.org/10.3390/antiox6020026
Chicago/Turabian StyleNaponelli, Valeria, Ileana Ramazzina, Chiara Lenzi, Saverio Bettuzzi, and Federica Rizzi. 2017. "Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges" Antioxidants 6, no. 2: 26. https://doi.org/10.3390/antiox6020026





