A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Technological Maturity
2.3. Sample Preparation for ITV and Glories Methods
2.3.1. ITV (Institut Technique de la Vigne et du Vin) Method
2.3.2. Glories Method
2.4. Statistical Analysis
3. Results and Discussion
3.1. Harvest Dates of Different Cabernet Sauvignon (CS) Plots
3.2. Comparison between ITV and Glories Methods
3.2.1. Phenolic Peaks
3.2.2. Correlation Matrix of ITV and Glories Parameters
3.3. Comparison between Technological and Phenolic Harvest Dates
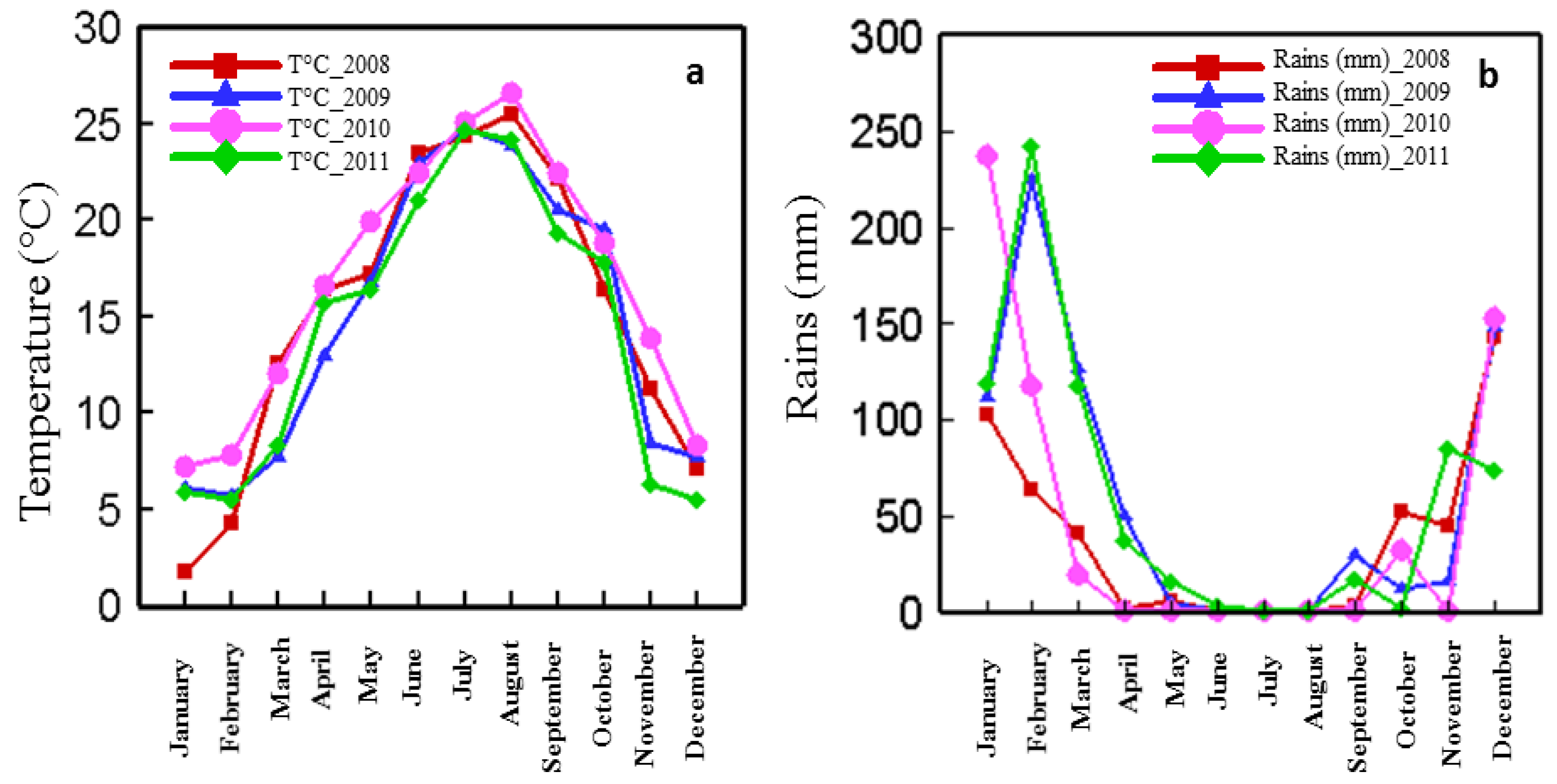
3.4. Evolution of Climate for 2008, 2009, 2010 and 2011 Harvests
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
Author Contributions
References
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, E.; Ortiz, M.C.; Sarabia, L.A.; Íñiguez, M.; Puras, P. Modelling phenolic and technological maturities of grapes by means of the multivariate relation between organoleptic and physicochemical properties. Anal. Chim. Acta 2013, 761, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Boselli, E.; Boulton, R.B.; Thorngate, J.H.; Frega, N.G. Chemical and sensory characterization of DOC red wines from Marche (Italy) related to vintage and grape cultivars. J. Agric. Food Chem. 2004, 52, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. (Eds.) Handbook of Enology. The Chemistry of Wine and Stabilisation and Treatments; John Wiley & Sons: Chichester, UK, 2000; Volume 2, pp. 141–203.
- De Gaulejac, N.S.-C.; Vivas, N.; Glories, Y. Maturation phénolique des raisins rouges. Relation avec la qualité des vins. Comparaison des cépages Merlot et Tempranillo. Le Progrès Agricole et Viticole 1998, 115, 306–318. [Google Scholar]
- Van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar]
- Joutei, K.A.; Bouya, D.; Saucier, C.; Glories, Y. Influence de l’éthylène sur les maturités phénolique et cellulaire des raisins au cours de la vinification. Cah. Agric. 2006, 15, 371–374. [Google Scholar]
- Lamadon, F. Protocole pour l’évaluation de la richesse polyphénolique des raisins. Revue des Œnologues. 1995, 76, 37–38. [Google Scholar]
- Blouin, J.; Guimberteau, G. Maturation et Maturité des Raisins. Available online: http://www.advid.pt/imagens/comunicacoes/14104343276058.pdf (accessed on 6 January 2016).
- Laure, C.; Cottereau, P.; Renord, R. Estimation de la Maturité Phénolique des Raisins Rouges par la Méthode ITV Standard. Available online: http://www.oenologuesdefrance.fr/gestion/fichiers_publications/Cayla_Cott.pdf (accessed on 6 January 2017).
- Ribéreau-Gayon, P.; Stonestree, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar] [PubMed]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Martínez-Cutillas, A.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. Improving colour extraction and stability in red wines: The use of maceration enzymes and enological tannins. Int. J. Food Sci. Tech. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- De La Hera Orts, M.L.; Martínez-Cutillas, A.; Roca, J.L.; Pérez-Prieto, L.J.; Gómez-Plaza, E. Effect d’une irrigation déficitaire sur la teneur en anthocyanes des raisins et des vins issus du cépage Monastrell. J. Int. Sci. Vigne Vin 2005, 39, 47–55. [Google Scholar]
- Anneraud, C.; Vinsonneau, E. Maturité Technologique et Maturité Phénolique des Raisins: Des Références Analytiques Utiles, des Méthodes Simples et des Matériels Pratiques et Prometteurs. Available online: http://www.matevi-france.com/uploads/tx_matevibase/Maturite_technologique_Maturite_phenolique_des_raisins_References_analytiques_Methodes_simples_Materiels.pdf (accessed on 20 October 2016).
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Elsevier: London, UK, 2014. [Google Scholar]
- El Darra, N. Les Composés Phénoliques des Raisins: Étude Du Potentiel Qualitatif et des Procédés émergeants d'extraction. Ph.D. Thesis, Université Saint-Joseph, Beirut, Lebanon, Université de Technologie Compiègne, Compiègne, France, 21 January 2012. [Google Scholar]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Comparison of methods for estimating phenolic maturity in grapes: Correlation between predicted and obtained parameters. Anal. Chim. Acta 2010, 660, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dupuch, V. Maturité phénolique et dates de récoltes: les apports de la méthode «CASV». Journ. Tech. Cinquantenaire ITV Fr. 1998, 8, 3–13. [Google Scholar]
- Lorrain, B.; Chira, K.; Teissedre, P.-L. Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: Comparison to 2006, 2007 and 2008 vintages. Food Chem. 2011, 126, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Carbonneau, A.; Riou, C.; Guyon, D.; Riom, J.; Schneider, C. Agrométéorologie de la vigne en France; Office des Publications Officielles des Communautés Européennes: Luxembourg, Luxembourg, 1992; p. 169. [Google Scholar]
- Coombe, B.G. Distribution of solutes within the developing grape berry in relation to its morphology. Am. J. Enol. Vitic. 1987, 38, 120–127. [Google Scholar]
- Van Leeuwen, C.; Seguin, G. Incidences de l’alimentation en eau de la vigne, appréciée par l’état hydrique du feuillage, sur le développement de l’appareil végétatif et la maturation du raisin (Vitis vinifera variété Cabernet franc, Saint-Emilion, 1990). J. Int. Sci. Vigne Vin 1994, 28, 81–110. [Google Scholar]
- Tregoat, O.; Van Leeuwen, C.; Choné, X.; Gaudillère, J.P. Etude du régime hydrique et de la nutrition azotée de la vigne par des indicateurs physiologiques. Influence sur le comportement de la vigne et la maturation du raisin. J. Int. Sci. Vigne Vin 2002, 36, 133–142. [Google Scholar]
- Morlat, R. Le Terroir Viticole: Contribution à L’étude de sa Caractérisation et de Son Influence sur les vins. Application aux Vignobles Rouges de la Moyenne Vallée de la Loire. Revue française d'oenologie. 1992, 32, S6–S13. [Google Scholar]
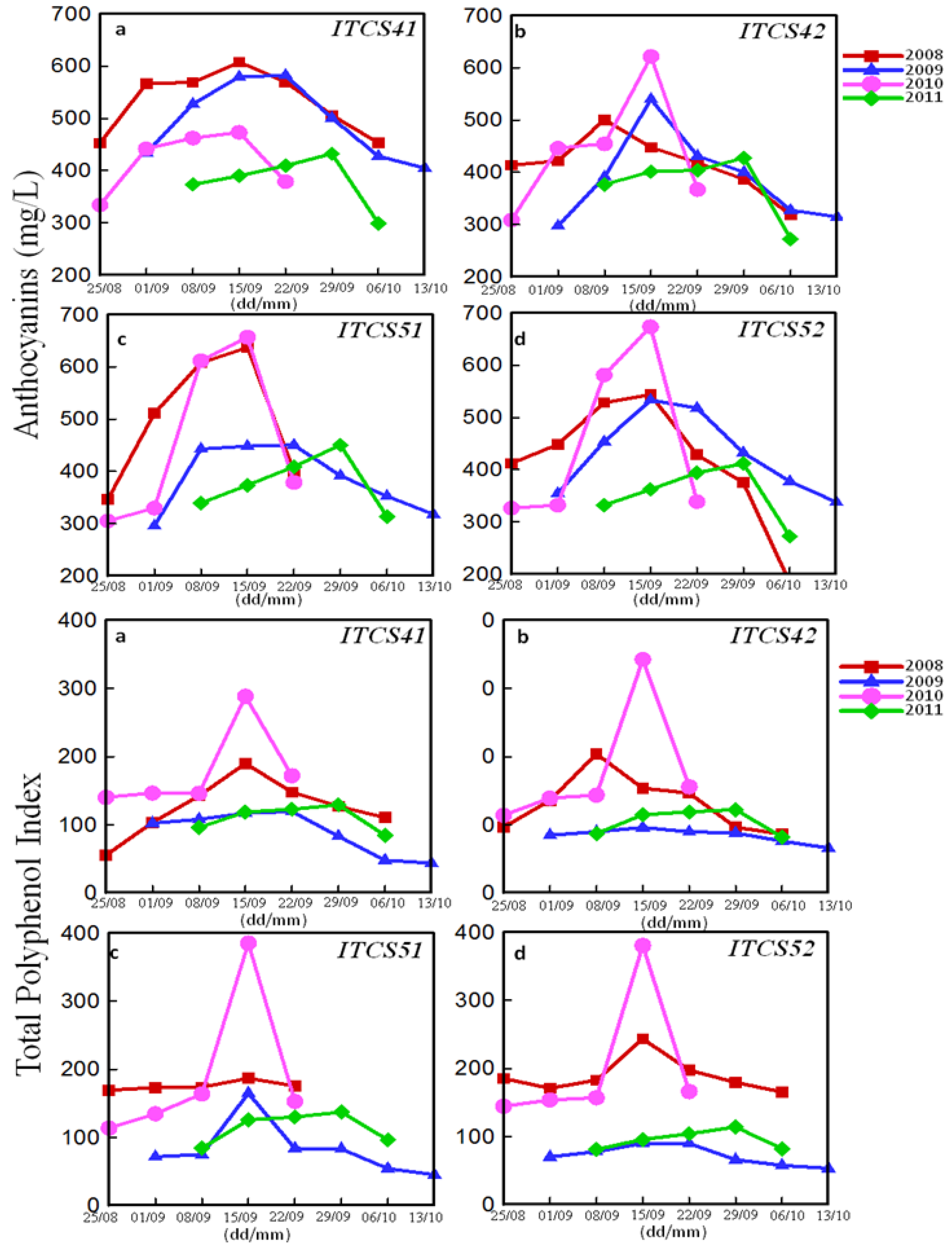
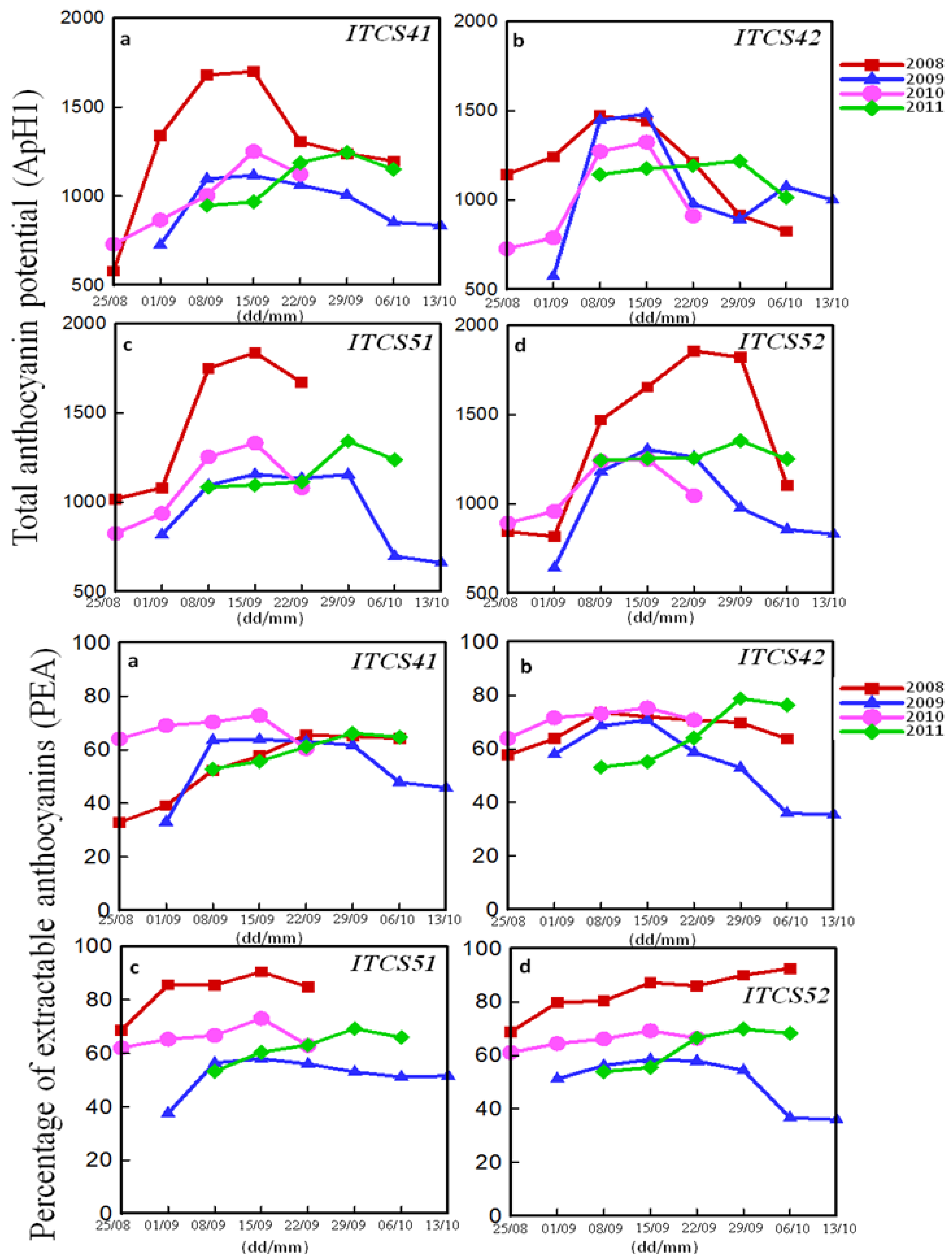
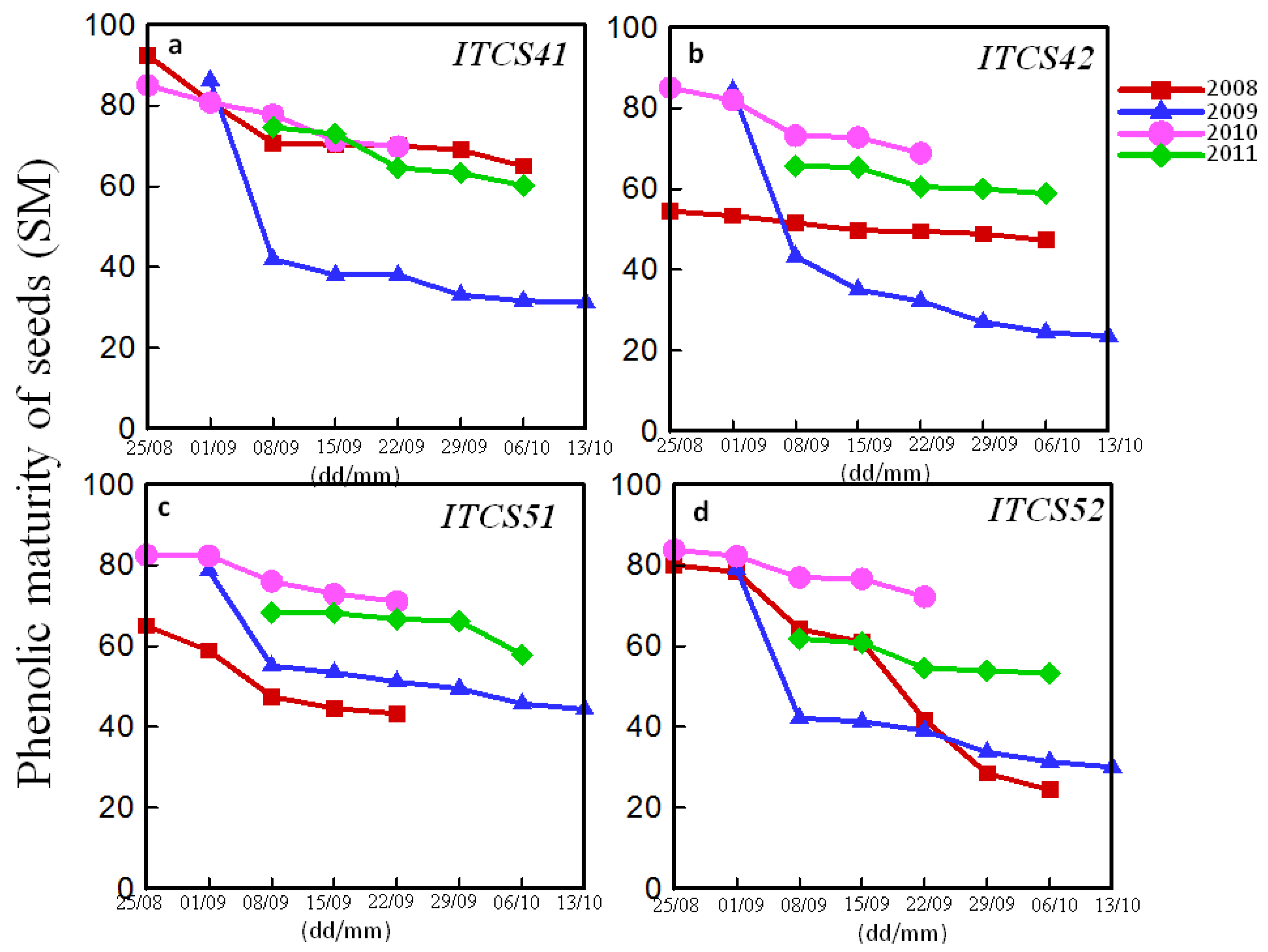
| Domain | Plot | Codex | Peaks of Total Polyphenol Index (mg/L) (ITV Method) | Peaks of Phenolic Richness (RPT) (Glories Method) | ||
|---|---|---|---|---|---|---|
| Harvest 2008 | Harvest 2009 | Harvest 2008 | Harvest 2009 | |||
| Itany | Cabernet 1994-1 | ITCS41 | 15-September | 14-September | 15-September | 14-September |
| Itany | Cabernet 1994-2 | ITCS42 | 8-September | 14-September | 8-September | 14-September |
| Itany | Cabernet 1995-1 | ITCS51 | 15-September | 14-September | 15-September | 14-September |
| Itany | Cabernet 1995-2 | ITCS52 | 15-September | 14-September | 22-September | 14-September |
| Domain | Plot | Codex | Date of Harvest 2008 | |
|---|---|---|---|---|
| Technological | Phenolic | |||
| Mansoura | Cabernet Y. HA. 1995-1 | MVCS51 | 22 to 25-September | 15-September |
| Mansoura | Cabernet K. CH. 1990 | MVCSK0 | 15 to 17-September | 8-September |
| Itany | Cabernet 1994-1 | ITCS41 | 18 to 20-September | 15-September |
| Itany | Cabernet 1994-2 | ITCS42 | 18-September | 8-September |
| Itany | Cabernet 1995-1 | ITCS51 | 20 to 24-September | 15-September |
| Itany | Cabernet 1995-2 | ITCS52 | 19 to 23-September | 15-September |
| Taanayel | Cabernet B6 | TACS6 | 25 to 10-October | 29-September |
| Kanafar | Cabernet 1 | KACS31 | 18 to 19-September | 15-September |
| Kanafar | Cabernet 2 | KACS32 | 18-September | 15-September |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajha, H.N.; Darra, N.E.; Kantar, S.E.; Hobaika, Z.; Louka, N.; Maroun, R.G. A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon. Antioxidants 2017, 6, 8. https://doi.org/10.3390/antiox6010008
Rajha HN, Darra NE, Kantar SE, Hobaika Z, Louka N, Maroun RG. A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon. Antioxidants. 2017; 6(1):8. https://doi.org/10.3390/antiox6010008
Chicago/Turabian StyleRajha, Hiba N., Nada El Darra, Sally El Kantar, Zeina Hobaika, Nicolas Louka, and Richard G. Maroun. 2017. "A Comparative Study of the Phenolic and Technological Maturities of Red Grapes Grown in Lebanon" Antioxidants 6, no. 1: 8. https://doi.org/10.3390/antiox6010008







