Protection against Radiotherapy-Induced Toxicity
Abstract
:1. Introduction
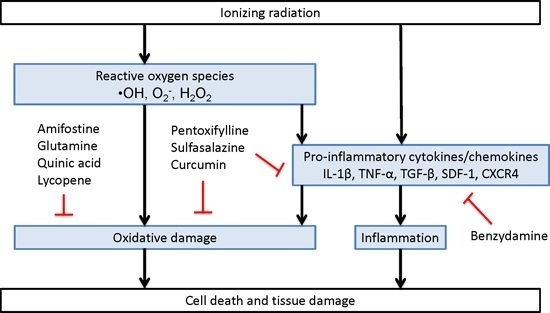
Direct and Indirect Mechanisms of Radiation-Induced Cell Damage
2. Measures to Minimise Radiation Damage
2.1. Clinically Used Radioprotectants
2.1.1. Antioxidants
2.1.1.1. Amifostine
2.1.1.2. Glutamine
2.1.2. Anti-Inflammatory Agents
Benzydamine
2.1.3. Mixed Acting Agents
Pentoxifylline
2.1.4. Sulfasalazine
2.2. Emerging Radioprotectors
2.2.1. Natural Products as Radioprotective Agents
2.2.2. Immunomodulators, Growth Factors and Cytokines
2.2.3. Phyto-Radioprotectors
2.2.4. Polyphenols as Radioprotectant Agents
2.2.5. Lycopene as a Radioprotectant Agent
2.2.6. Other Emerging Radioprotectant Therapies
2.2.6.1. Antioxidants
2.2.6.2 Emerging Anti-Inflammatory Agents
2.2.6.3. Mixed Acting Agents
2.2.6.4. Other Agents
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013, 12, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Furst, C.J. Radiotherapy for cancer. Quality of life. Acta Oncol. 1996, 35 (Suppl. S7), 141–148. [Google Scholar] [CrossRef] [PubMed]
- Berkey, F.J. Managing the adverse effects of radiation therapy. Am. Fam. Phys. 2010, 82, 381–388, 394. [Google Scholar]
- Massie, M.J. Prevalence of depression in patients with cancer. J. Natl. Cancer Inst. Monogr. 2004, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Marsiglia, H.R.; Orecchia, R. Radiotherapy-related fatigue. Crit. Rev. Oncol. Hematol. 2002, 41, 317–325. [Google Scholar] [CrossRef]
- Adams, M.J.; Lipshultz, S.E.; Schwartz, C.; Fajardo, L.F.; Coen, V.; Constine, L.S. Radiation-associated cardiovascular disease: Manifestations and management. Semin. Radiat. Oncol. 2003, 13, 346–356. [Google Scholar] [CrossRef]
- Marks, L.B.; Carroll, P.R.; Dugan, T.C.; Anscher, M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1257–1280. [Google Scholar] [CrossRef]
- Sountoulides, P.; Koletsas, N.; Kikidakis, D.; Paschalidis, K.; Sofikitis, N. Secondary malignancies following radiotherapy for prostate cancer. Ther. Adv. Urol. 2010, 2, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Prise, K.M.; Schettino, G.; Folkard, M.; Held, K.D. New insights on cell death from radiation exposure. Lancet Oncol. 2005, 6, 520–528. [Google Scholar] [CrossRef]
- Ward, J.F. DNA damage as the cause of ionizing radiation-induced gene activation. Radiat. Res. 1994, 138, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.; Menon, V. Influence of ferulic acid on γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology 2006, 228, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.J. Death by protein damage in irradiated cells. DNA Repair 2012, 11, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Beir, V. Health Effects of Exposure to Low Levels of Ionizing Radiation National Research Council, Committee on the Biological Effects of Ionizing Radiations; National Academy of Sciences, National Academy Press: Washington, DC, USA, 1990. [Google Scholar]
- Liu, C.; Lin, Q.; Yun, Z. Cellular and molecular mechanisms underlying oxygen-dependent radiosensitivity. Radiat. Res. 2015, 183, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Lefaix, J.-L.; Delanian, S. TGF-β1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290. [Google Scholar] [CrossRef]
- Ehrhart, E.; Segarini, P.; Tsang, M.; Carroll, A.G.; Barcellos-Hoff, M.-H. Latent transforming growth factor beta1 activation in situ: Quantitative and functional evidence after low-dose gamma-irradiation. FASEB J. 1997, 11, 991–1002. [Google Scholar] [PubMed]
- Graves, P.R.; Siddiqui, F.; Anscher, M.S.; Movsas, B. Radiation pulmonary toxicity: From mechanisms to management. Semin. Radiat. Oncol. 2010, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candeias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2016, 5, 12–33. [Google Scholar] [CrossRef]
- Choi, N.C. Radioprotective effect of amifostine in radiation pneumonitis. Semin. Oncol. 2003, 30, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhu, S.; Li, X.; Wu, H.; Li, Y.; Hua, F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis based on randomized controlled trials. PLoS ONE 2014, 9, e95968. [Google Scholar] [CrossRef] [PubMed]
- Antonadou, D.; Pepelassi, M.; Synodinou, M.; Puglisi, M.; Throuvalas, N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 739–747. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Uzal, C.; Durmus-Altun, G.; Caloglu, M.; Ergulen, A.; Altaner, S.; Yigitbasi, N.O. The protective effect of amifostine on radiation-induced acute pulmonary toxicity: Detection by 99mTc-DTPA transalveolar clearances. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 564–569. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Feng, Q.F.; Rabbani, Z.N.; Samulski, T.V.; Anscher, M.S.; Brizel, D.M. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp. Lung Res. 2002, 28, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral glutamine in preventing treatment-related mucositis in adult patients with cancer: A systematic review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Eda, K.; Uzer, K.; Murat, T.; Cenk, U. The effects of enteral glutamine on radiotherapy induced dermatitis in breast cancer. Clin. Nutr. 2016, 35, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Muge, A.; Taner, A.; Sehri, E. Oral glutamine supplementation reduces radiotherapy-induced esophagitis in lung cancer patients. Asian Pac. J. Cancer Prev. 2015, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Yavuz, M.N.; Onal, C.; Yavuz, A.A. Prevention of acute radiation-induced esophagitis with glutamine in non-small cell lung cancer patients treated with radiotherapy: Evaluation of clinical and dosimetric parameters. Lung Cancer 2009, 63, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kozelsky, T.F.; Meyers, G.E.; Sloan, J.A.; Shanahan, T.G.; Dick, S.J.; Moore, R.L.; Engeler, G.P.; Frank, A.R.; McKone, T.K.; Urias, R.E.; et al. Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J. Clin. Oncol. 2003, 21, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casariego, A.; Calleja-Fernandez, A.; Cano-Rodriguez, I.; Cordido, F.; Ballesteros-Pomar, M.D. Effects of oral glutamine during abdominal radiotherapy on chronic radiation enteritis: A randomized controlled trial. Nutrition 2015, 31, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kucuktulu, E.; Guner, A.; Kahraman, I.; Topbas, M.; Kucuktulu, U. The protective effects of glutamine on radiation-induced diarrhea. Support. Care Cancer 2013, 21, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Egehan, I.; Atavci, S.; Kitapci, M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: A double-blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 213–219. [Google Scholar] [CrossRef]
- Koh, W.J.; Stelzer, K.J.; Peterson, L.M.; Staker, B.L.; Ward, W.F.; Russell, K.J.; Griffin, T.W. Effect of pentoxifylline on radiation-induced lung and skin toxicity in rats. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 71–77. [Google Scholar] [CrossRef]
- Okunieff, P.; Augustine, E.; Hicks, J.E.; Cornelison, T.L.; Altemus, R.M.; Naydich, B.G.; Ding, I.; Huser, A.K.; Abraham, E.H.; Smith, J.J.; et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J. Clin. Oncol. 2004, 22, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Wilfert, F.; Uthe, D.; Schmid, K.W.; Knoop, R.; Willich, N.; Schuck, A.; Rube, C. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother. Oncol. 2002, 64, 177–187. [Google Scholar] [CrossRef]
- Sheibani, K.M.; Mafi, A.R.; Moghaddam, S.; Taslimi, F.; Amiran, A.; Ameri, A. Efficacy of benzydamine oral rinse in prevention and management of radiation-induced oral mucositis: A double-blind placebo-controlled randomized clinical trial. Asia-Pac. J. Clin. Oncol. 2015, 11, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, A.; Kamian, S.; Aghili, M.; Hashemi, F.A.; Haddad, P. Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: A double-blind placebo-controlled randomized clinical trial. Eur. J. Cancer Care (Engl.) 2009, 18, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.F.; Yuen, J.K.T. A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nurs. 2006, 29, 423–430. [Google Scholar] [CrossRef]
- Kiliç, D.; Egehan, I.B.; Özenirler, S.; Dursun, A. Double-blinded, randomized, placebo-controlled study to evaluate the effectiveness of sulphasalazine in preventing acute gastrointestinal complications due to radiotherapy. Radiother. Oncol. 2000, 57, 125–129. [Google Scholar] [CrossRef]
- Kilic, D.; Ozenirler, S.; Egehan, I.; Dursun, A. Sulfasalazine decreases acute gastrointestinal complications due to pelvic radiotherapy. Ann. Pharmacother. 2001, 35, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Trifan, A.; Gille, E.; Petreus, T.; Bordeianu, G.; Miron, A. Can phytochemicals be a bridge to develop new radioprotective agents? Phytochem. Rev. 2015, 14, 555–566. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Gomez-Garcia, F.; Garcia Carrillo, N.; Valle-Rodriguez, E.; Xerafin, A.; Vicente-Ortega, V. Radioprotective effects of lycopene and curcumin during local irradiation of parotid glands in sprague dawley rats. Br. J. Oral Maxillofac. Surg. 2016, 54, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Mallaiah, S.H.; Patil, R.K. Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kindekov, I.; Mileva, M.; Krastev, D.; Vassilieva, V.; Raynova, Y.; Doumanova, L.; Aljakov, M.; Idakieva, K. Radioprotective effect of Rapana thomasiana hemocyanin in gamma induced acute radiation syndrome. Biotechnol. Biotechnol. Equip. 2014, 28, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Saha, C.; Dey, S.K. Studies on black tea (Camellia sinensis) extract as a potential antioxidant and a probable radioprotector. Radiat. Environ. Biophys. 2013, 52, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Dhaker, A.; Adhikari, J.; Ivanov, V.; Singh, V.; Chawla, R.; Kumar, R.; Sharma, R.; Karamalakova, Y.; Gadjeva, V.; et al. In vitro studies on radioprotective efficacy of silymarin against gamma-irradiation. Int. J. Radiat. Biol. 2013, 89, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Gravina, G.L.; Festuccia, C.; Popov, V.M.; Colapietro, E.A.; Sanita, P.; Musio, D.; de Felice, F.; Lenzi, A.; Jannini, E.A.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SIRT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Alashkham, A.; Paterson, C.; Rauchhaus, P.; Nabi, G. Can angiotensin-converting enzyme inhibitors reduce the incidence, severity, and duration of radiation proctitis? Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Petruzzi, M.; di Stasio, D.; Lucchese, A. Clinical effectiveness of palifermin in prevention and treatment of oral mucositis in children with acute lymphoblastic leukaemia: A case-control study. Int. J. Oral Sci. 2014, 6, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Smith, T.; Zhang, X.; Goff, J.P.; Franicola, D.; Greenberger, B.; Komanduri, P.; Wang, H.; Greenberger, J.S. Modulation of in utero total body irradiation induced newborn mouse growth retardation by maternal manganese superoxide dismutase-plasmid liposome (MnSOD-Pl) gene therapy. Gene Ther. 2011, 18, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.U.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G.; Heath, E.; Vaishampayan, U.; Cher, M.L.; Andic, F.; Rossi, P.J.; Kucuk, O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr. Cancer 2010, 62, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Krivokrysenko, V.I.; Toshkov, I.A.; Gleiberman, A.S.; Krasnov, P.; Shyshynova, I.; Bespalov, I.; Maitra, R.K.; Narizhneva, N.V.; Singh, V.K.; Whitnall, M.H.; et al. The toll-like receptor 5 agonist entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS ONE 2015, 10, e0135388. [Google Scholar] [CrossRef] [PubMed]
- Mustata, G.; Li, M.; Zevola, N.; Bakan, A.; Zhang, L.; Epperly, M.; Greenberger, J.S.; Yu, J.; Bahar, I. Development of small-molecule puma inhibitors for mitigating radiation-induced cell death. Curr. Top. Med. Chem. 2011, 11, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Maniar, M.; Fornace, A.J., Jr.; Datta, K. Administration of on 01210.Na after exposure to ionizing radiation protects bone marrow cells by attenuating DNA damage response. Radiat. Oncol. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.D.; Cosenza, S.C.; Bonagura, M.; Manair, M.; Reddy, M.V.R.; Reddy, E.P. On01210.Na (Ex-RAD®) mitigates radiation damage through activation of the AKT pathway. PLoS ONE 2013, 8, e58355. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Datta, K.; Chakraborty, K.; Kulkarni, S.S.; Doiron, K.; Fornace, A.J., Jr.; Sree Kumar, K.; Hauer-Jensen, M.; Ghosh, S.P. Gamma tocotrienol, a potent radioprotector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem. Toxicol. 2013, 60, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. Δ-tocotrienol protects mouse and human hematopoietic progenitors from γ-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.; Ney, P.; Graves, J., 3rd; Mullaney, C.; Srinivasan, V. Mechanism of radioprotection by delta-tocotrienol: Pharmacokinetics, pharmacodynamics and modulation of signalling pathways. Br. J. Radiol. 2012, 85, e1093–e1103. [Google Scholar] [CrossRef] [PubMed]
- Connell, P.P.; Weichselbaum, R.R. Small molecule derived from a natural product that mitigates radiation injury. Proc. Natl. Acad. Sci. USA 2013, 110, 18355–18356. [Google Scholar] [CrossRef] [PubMed]
- Ostrau, C.; Hülsenbeck, J.; Herzog, M.; Schad, A.; Torzewski, M.; Lackner, K.J.; Fritz, G. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother. Oncol. 2009, 92, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Hernady, E.; Johnston, C.J.; Reed, C.M.; Fenton, B.; Okunieff, P.; Finkelstein, J.N. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat. Res. 2004, 161, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Wang, J.; Boerma, M.; Fu, Q.; Denham, J.W. Radiation damage to the gastrointestinal tract: Mechanisms, diagnosis, and management. Curr. Opin. Support. Palliat. Care 2007, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Haydont, V.; Bourgier, C.; Pocard, M.; Lusinchi, A.; Aigueperse, J.; Mathé, D.; Bourhis, J.; Vozenin-Brotons, M.-C. Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin. Cancer Res. 2007, 13, 5331–5340. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, M.-H.; Vereycken-Holler, V.; Squiban, C.; Vandamme, M.; Vozenin-Brotons, M.-C.; Benderitter, M. Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat. Res. 2005, 163, 479–487. [Google Scholar] [CrossRef] [PubMed]
- George, K.; Hebbar, S.; Kale, S.; Kesavan, P. Caffeine protects mice against whole-body lethal dose of-irradiation. J. Radiol. Prot. 1999, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, K.J.; Koh, W.-J.; Kurtz, H.; Greer, B.E.; Griffin, T.W. Caffeine consumption is associated with decreased severe late toxicity after radiation to the pelvis. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 411–417. [Google Scholar] [CrossRef]
- Hebbar, S.; Mitra, A.; George, K.; Verma, N. Caffeine ameliorates radiation-induced skin reactions in mice but does not influence tumour radiation response. J. Radiol. Prot. 2002, 22, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76 Pt 3, 626–636. [Google Scholar] [CrossRef]
- Pathak, R.; Cheema, A.K.; Boca, S.M.; Krager, K.J.; Hauer-Jensen, M.; Aykin-Burns, N. Modulation of radiation response by the tetrahydrobiopterin pathway. Antioxidants 2015, 4, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Pawar, S.A.; Fu, Q.; Gupta, P.K.; Berbée, M.; Garg, S.; Sridharan, V.; Wang, W.; Biju, P.G.; Krager, K.J. Characterization of transgenic Gfrp knock-in mice: Implications for tetrahydrobiopterin in modulation of normal tissue radiation responses. Antioxid. Redox Signal. 2014, 20, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Pathak, R.; Zhou, D.; Kumar, K.S.; Hauer-Jensen, M. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: Evidence of a role for tetrahydrobiopterin. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Kalivendi, S.; Hatakeyama, K.; Whitsett, J.; Konorev, E.; Kalyanaraman, B.; Vásquez-Vivar, J. Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide—A role for Gfrp? Free Radic. Biol. Med. 2005, 38, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gesierich, A.; Niroomand, F.; Tiefenbacher, C. Role of human GTP cyclohydrolase I and its regulatory protein in tetrahydrobiopterin metabolism. Basic Res. Cardiol. 2003, 98, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Ishii, M.; Miyasaka, Y.; Wajima, T.; Negoro, T.; Hagiwara, T.; Kiuchi, Y. Possible involvement of hydroxyl radical on the stimulation of tetrahydrobiopterin synthesis by hydrogen peroxide and peroxynitrite in vascular endothelial cells. Int. J. Biochem. Cell Biol. 2005, 37, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, M.F.K.; Welsh, J.; Godoy, M.C.B.; Betancourt, S.L.; Mawlawi, O.R.; Munden, R.F. New era of radiotherapy: An update in radiation-induced lung disease. Clin. Radiol. 2013, 68, e275–e290. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Savarese, D.M.F.; Savy, G.; Vahdat, L.; Wischmeyer, P.E.; Corey, B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat. Rev. 2003, 29, 501–513. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hepgül, G.; Tanrıkulu, S.; Ünalp, H.R.; Akguner, T.; Erbil, Y.; Olgaç, V.; Ademoğlu, E. Preventive effect of pentoxifylline on acute radiation damage via antioxidant and anti-inflammatory pathways. Dig. Dis. Sci. 2010, 55, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Ahnfelt-Rønne, I.; Nielsen, O.H. The antiinflammatory moiety of sulfasalazine, 5-aminosalicylic acid, is a radical scavenger. Agents Actions 1987, 21, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A review of radiation countermeasure work ongoing at the armed forces radiobiology research institute. Int. J. Radiat. Biol. 2012, 88, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Yadav, V.S. Role of cytokines and growth factors in radioprotection. Exp. Mol. Pathol. 2005, 78, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Kuntić, V.S.; Stanković, M.B.; Vujić, Z.B.; Brborić, J.S.; Uskoković-Marković, S.M. Radioprotectors—The evergreen topic. Chem. Biodivers. 2013, 10, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Neta, R.; Oppenheim, J.J.; Schreiber, R.D.; Chizzonite, R.; Ledney, G.D.; MacVittie, T.J. Role of cytokines (interleukin 1, tumor necrosis factor, and transforming growth factor beta) in natural and lipopolysaccharide-enhanced radioresistance. J. Exp. Med. 1991, 173, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Neta, R. Role of cytokines in radioprotection. Pharmacol. Ther. 1988, 39, 261–266. [Google Scholar] [CrossRef]
- Dainiak, N. Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys. 2010, 98, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Ramaekers, B.L.T.; van Mastrigt, G.A.P.G.; van den Ende, P.; de Jong, J.; de Ruysscher, D.K.M.; Pijls-Johannesma, M. Erythropoietin as an adjuvant treatment with (chemo) radiation therapy for head and neck cancer. Cochrane Database Syst. Rev. 2009, 3. [Google Scholar] [CrossRef]
- Gonzalez, J.; Kumar, A.J.; Conrad, C.A.; Levin, V.A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Yamini, K.; Gopal, V. Natural radioprotective agents against ionizing radiation—An overview. Int. J. PharmTech Res. 2010, 2, 1421–1426. [Google Scholar]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Kemertelidze, E.P.; Tsitsishvili, V.G.; Alaniya, M.D.; Sagareishvili, T.G. Structure-function analysis of the radioprotective and antioxidant activity of flavonoids. Chem. Nat. Compd. 2000, 36, 54–59. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Xu, J.; Hu, D.; Liu, W.; Zhang, L.; Morrow, G.; Pentland, A.; Ryan, J.L.; Ding, I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mrna expression of inflammatory and fibrogenic cytokines. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Yi, C.O.; Jeon, B.T.; Jeong, Y.Y.; Kang, G.M.; Lee, J.E.; Roh, G.S.; Lee, J.D. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J. Physiol. Pharmacol. 2013, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Prasad, N.R.; Menon, V.P. Protective effect of curcumin on γ-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 611, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.H.; Tawfik, S.S. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur. J. Pharmacol. 2012, 692, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.G.; Soyuer, S.; Saraymen, R.; Eroglu, C. Protective effects of caffeic acid phenethyl ester on radiation induced lung injury in rats. Clin. Investig. Med. 2008, 31, 242–247. [Google Scholar]
- Devipriya, N.; Sudheer, A.R.; Menon, V.P. Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J. Biochem. Mol. Toxicol. 2008, 22, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Srinivasan, M.; Pugalendi, K.; Menon, V.P. Protective effect of ferulic acid on γ-radiation-induced micronuclei, dicentric aberration and lipid peroxidation in human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 603, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Salvi, V.P.; Nair, C.K.K. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol. Cell. Biochem. 2005, 280, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Ehtejab, G. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Cancer Sci. Ther. 2013, 78, 32–37. [Google Scholar]
- Meydan, D.; Gursel, B.; Bilgici, B.; Can, B.; Ozbek, N. Protective effect of lycopene against radiation-induced hepatic toxicity in rats. J. Int. Med. Res. 2011, 39, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Saada, H.N.; Rezk, R.G.; Eltahawy, N.A. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother. Res. 2010, 24, S204–S208. [Google Scholar] [CrossRef] [PubMed]
- Andic, F.; Garipagaoglu, M.; Yurdakonar, E.; Tuncel, N.; Kucuk, O. Lycopene in the prevention of gastrointestinal toxicity of radiotherapy. Nutr. Cancer 2009, 61, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Devipriya, N.; Kalpana, K.; Menon, V.P. Lycopene: An antioxidant and radioprotector against γ-radiation-induced cellular damages in cultured human lymphocytes. Toxicology 2009, 262, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Pillai, K.R.; Kumar, P.R.; Sudhakaran, P.; Menon, V. Lycopene as a natural protector against γ-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim. Biophys. Acta 2007, 1770, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Anoopkumar-Dukie, S.; Conere, T.; Carey, J.B.; Allshire, A. Radical mediators and mitogen-activated protein kinase signaling in oxygen-dependent radiosensitivity of human tumor cell lines. Free Radic. Biol. Med. 2005, 39, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Anoopkumar-Dukie, S.; Conere, T.; Sisk, G.; Allshire, A. Mitochondrial modulation of oxygen-dependent radiosensitivity in some human tumour cell lines. Br. J. Radiol. 2014, 82. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. 2014, 34, e478–e486. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK pathways in radiation responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Howell, R.C.; Jiang, Z.W.; Bryan, R.A.; Gerfen, G.; Chen, C.C.; Mah, D.; Cahill, S.; Casadevall, A.; Dadachova, E. Physico-chemical evaluation of rationally designed melanins as novel nature-inspired radioprotectors. PLoS ONE 2009, 4, e7229. [Google Scholar] [CrossRef] [PubMed]
- Charrier, S.; Michaud, A.; Badaoui, S.; Giroux, S.; Ezan, E.; Sainteny, F.; Corvol, P.; Vainchenker, W. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 2004, 104, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Landauer, M.R.; Mog, S.R.; Barshishat-Kupper, M.; Zins, S.R.; Amare, M.F.; Day, R.M. Timing of captopril administration determines radiation protection or radiation sensitization in a murine model of total body irradiation. Exp. Hematol. 2010, 38, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.C.; Hauer-Jensen, M.; et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Krivokrysenko, V.I.; Tallant, T.C.; Strom, E.; Gleiberman, A.S.; Gupta, D.; Kurnasov, O.V.; Fort, F.L.; Osterman, A.L.; Didonato, J.A.; et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008, 320, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Gleiberman, A.S.; Toshkov, I.; Aygun-Sunar, S.; Bapardekar, M.; Manderscheid-Kern, P.; Bellnier, D.; Krivokrysenko, V.I.; Feinstein, E.; Gudkov, A.V. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: Implications for head-and-neck cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Blijlevens, N.; Sonis, S. Palifermin (recombinant keratinocyte growth factor-1): A pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. 2007, 18, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Gretton, J.E.; Sikora, C.A.; Jefferson, M.; Bernarding, M.; Nie, S.; Greenberger, J.S. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat. Res. 2003, 160, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, J.S. Gene therapy approaches for stem cell protection. Gene Ther. 2008, 15, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Clarke, T.K.; Mog, S.R.; Landauer, M.R. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int. J. Radiat. Biol. 2007, 83, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Mungunsukh, O.; Zins, S.; Day, R.M.; Landauer, M.R. Genistein induces radioprotection by hematopoietic stem cell quiescence. Int. J. Radiat. Biol. 2008, 84, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, L. Puma, a potent killer with or without p53. Oncogene 2008, 27 (Suppl. S1), S71–S83. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Meng, Q.; Xu, J.; Jiao, Y.; Zhao, L.; Zhang, X.; Sarkar, F.H.; Brown, M.L.; Dritschilo, A.; Rosen, E.M. Dim (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18650–18655. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation countermeasure agents: An update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef] [PubMed]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]

| Adverse Effect | Associated Cancer | References |
|---|---|---|
| Depression | Breast, lung, pancreatic, oropharyngeal, brain | [3,4,5] |
| Fatigue | Brain, head and neck, breast, lung, pelvic lymphatic system | [3,5] |
| Dermatitis | Head and neck, breast, prostate, perineal | [3] |
| Cardiovascular disease | Hodgkin lymphoma, breast, lung | [3,6] |
| Pneumonitis | Breast, lung, mediastinal | [3] |
| Xerostomia | Head and neck | [3] |
| Mucositis and esophagitis | Head and neck, thoracic | [3] |
| Enteritis | Abdominal, pelvic | [3] |
| Proctitis | Anal, rectal, cervical, uterine, prostate, bladder, testicular | [3] |
| Emesis | Upper abdominal, craniospinal, pelvic | [3] |
| Cystitis | Prostate, colorectal, bladder, pelvic | [3,7] |
| Erectile dysfunction | Prostate, colorectal | [3] |
| Vaginal dryness and stenosis | Cervical, endometrial, vaginal | [3] |
| Infertility and teratogenicity | Cervical, pelvic, testicular | [3] |
| Compound | Mechanism of Action | References |
|---|---|---|
| Clinically used radioprotectants | ||
| Amifostine | Antioxidant | [23,24,25,26,27,28] |
| Glutamine | Antioxidant | [29,30,31,32,33,34,35] |
| Pentoxifylline | Antioxidant, anti-inflammatory | [36,37,38,39] |
| Benzydamine | Anti-inflammatory | [40,41,42] |
| Sulfasalazine | Anti-inflammatory, antioxidant | [43,44] |
| Emerging Radioprotectants—Natural Products | ||
| Curcumin (Curcuma longa) | Antioxidant, anti-inflammatory, antiproliferatiive | [45] |
| Quinic acid (Coffee, cocoa) | Antioxidant, decreases DNA damage | [46] |
| lycopene (Lycopersicon esculentum) | Antioxidant, peroxidation inhibitor, free radical scavenger | [47] |
| Rutin (bioflavonoid) | Antioxidant | [48] |
| Hemocyanin (Rapana thomasiana) | Radiomitigator | [49] |
| Black tea extract (Camellia sinensis) | Free radical scavenger | [50] |
| Silymarin (Silybum marianum) | Anti-apoptotic agent, Reduces DNA damage | [51] |
| Other Emerging Therapies | ||
| Vitamin D | Protection of endothelial cells by modulating MAPK/SirT1 axis | [52] |
| Melanin nanoparticles | Protection of haematopoietic cells by preventing generation of free radicals and of free radical scavenging | [53] |
| Angiotensin-I-Converting Enzyme (ACE) inhibitors -Captopril | Haematopoietic radioprotection mediated by angiotensin II pathway through AT1 receptors | [54] |
| Palifermin | Stimulation of cell proliferation and inhibition of cell apoptosis | [55] |
| Manganese superoxide dismutase-plasmid liposome (MnSOD-PL) gene therapy | Antioxidant, decreases free radical production and inflammatory cytokine release | [56] |
| Genistein | Antioxidant and anti-inflammatory | [57] |
| Entolimod | Apoptosis modulation | [58] |
| PUMA inhibitors | Apoptosis modulation | [59] |
| Recilisib | Intracellular signalling and DNA damage repair pathways P53 down-regulation and up-regulation of AKT pathways | [60,61] |
| γ-Tocotrienol | Antioxidant, free-radical scavenger, HMG-CoA reductase inhibitor | [62,63] |
| δ-Tocotrienol | Antioxidant, enhance haematopoiesis, modulate signalling pathways | [64,65] |
| 3,3′-Diindolylmethane | Induces ATM-dependent DDR-like response, enhances radiation-induced ATM signalling and NF-κB activation | [66] |
| Statins | Anti-inflammatory | [67,68,69,70,71] |
| Caffeine | Antioxidant and anti-inflammatory | [72,73,74,75] |
| Tetrahydrobiopterin | Modulation of free radical-induced damage | [76,77,78,79,80,81] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, S.; Rudrawar, S.; Zunk, M.; Bernaitis, N.; Arora, D.; McDermott, C.M.; Anoopkumar-Dukie, S. Protection against Radiotherapy-Induced Toxicity. Antioxidants 2016, 5, 22. https://doi.org/10.3390/antiox5030022
Hall S, Rudrawar S, Zunk M, Bernaitis N, Arora D, McDermott CM, Anoopkumar-Dukie S. Protection against Radiotherapy-Induced Toxicity. Antioxidants. 2016; 5(3):22. https://doi.org/10.3390/antiox5030022
Chicago/Turabian StyleHall, Susan, Santosh Rudrawar, Matthew Zunk, Nijole Bernaitis, Devinder Arora, Catherine M McDermott, and Shailendra Anoopkumar-Dukie. 2016. "Protection against Radiotherapy-Induced Toxicity" Antioxidants 5, no. 3: 22. https://doi.org/10.3390/antiox5030022