Study of the Properties of Bearberry Leaf Extract as a Natural Antioxidant in Model Foods
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Extraction of BL Extract
2.3. Determination of Total Phenolic Compound (TPC)
2.4. Determination of Antioxidant Activity Using TEAC Assay
2.5. Determination of Radical Scavenging Activity Assay Using Electron Paramagnetic Resonance (EPR)
2.6. Determination of Antioxidant Activity in Food Model
2.6.1. Removal of Tocopherols from Sunflower Oil
2.6.2. Preparation of Emulsion
2.6.3. Determination of Peroxide Value (PV)
2.6.4. Preparation of Gelatin-Based Film with Antioxidant Coating
2.6.5. Thiobarbituric Acid Reacting Substances (TBARS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, Total Phenolic Content (TPC) and Antioxidant Activity
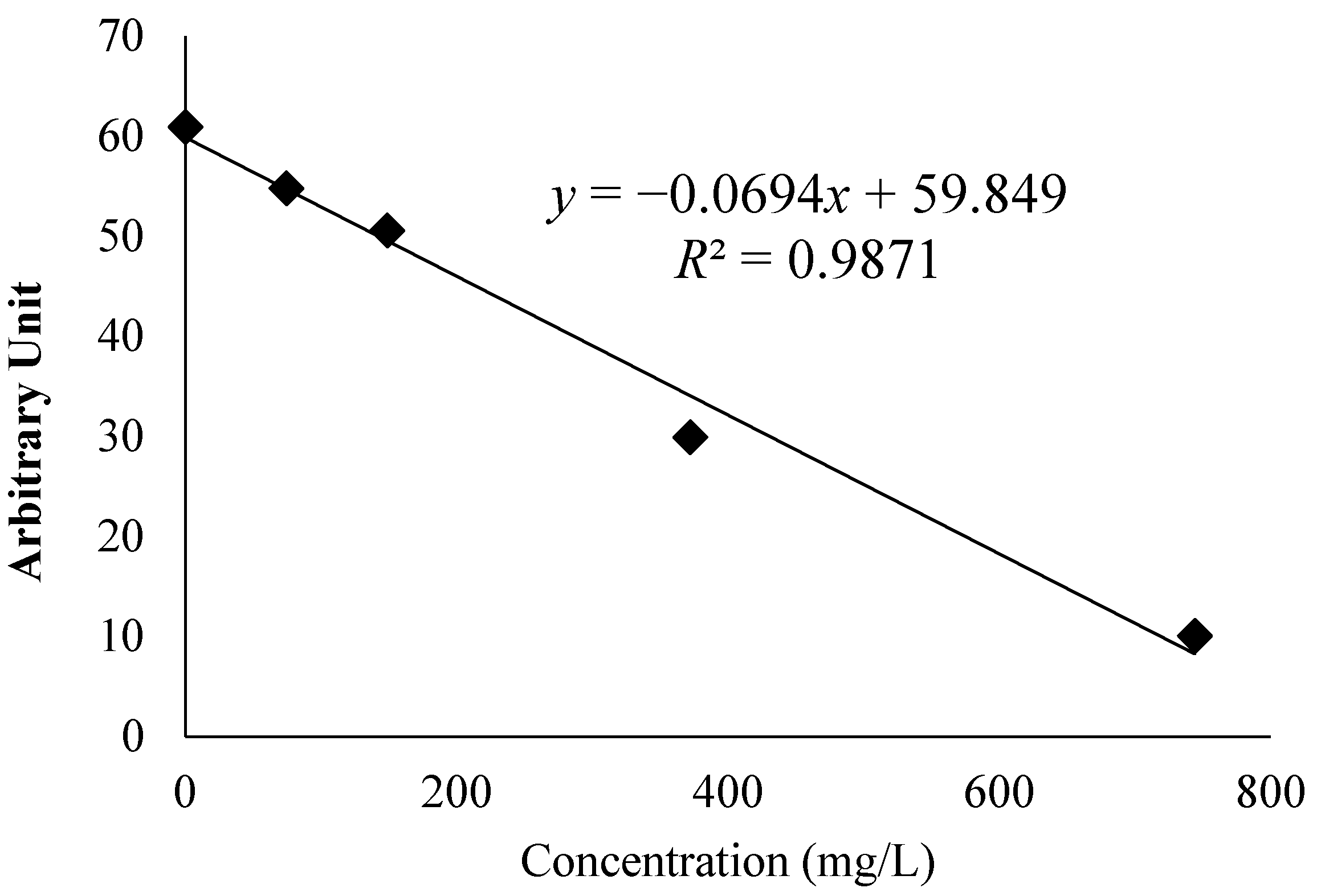
3.2 Analysis of Free Radical Activity Assays
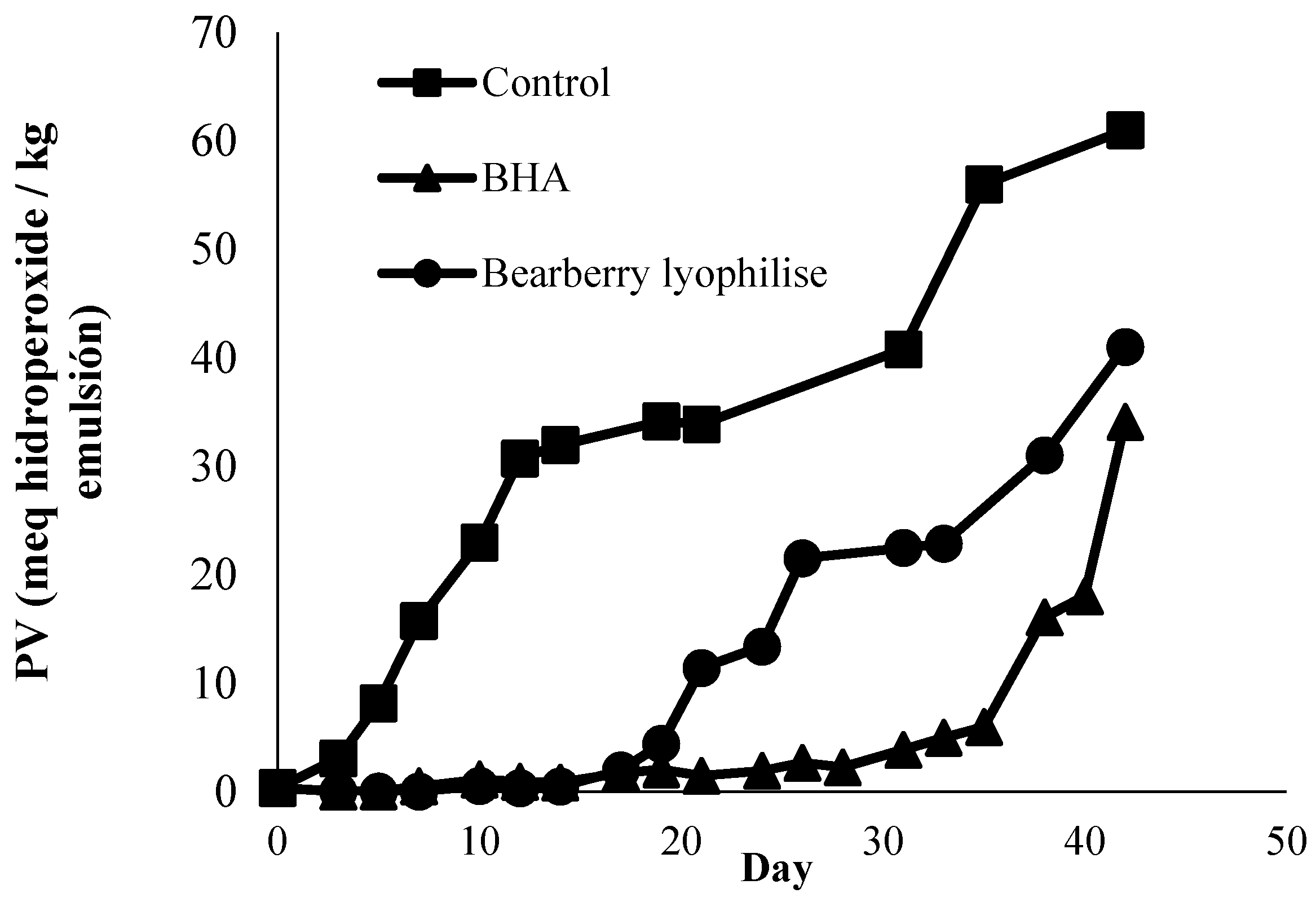
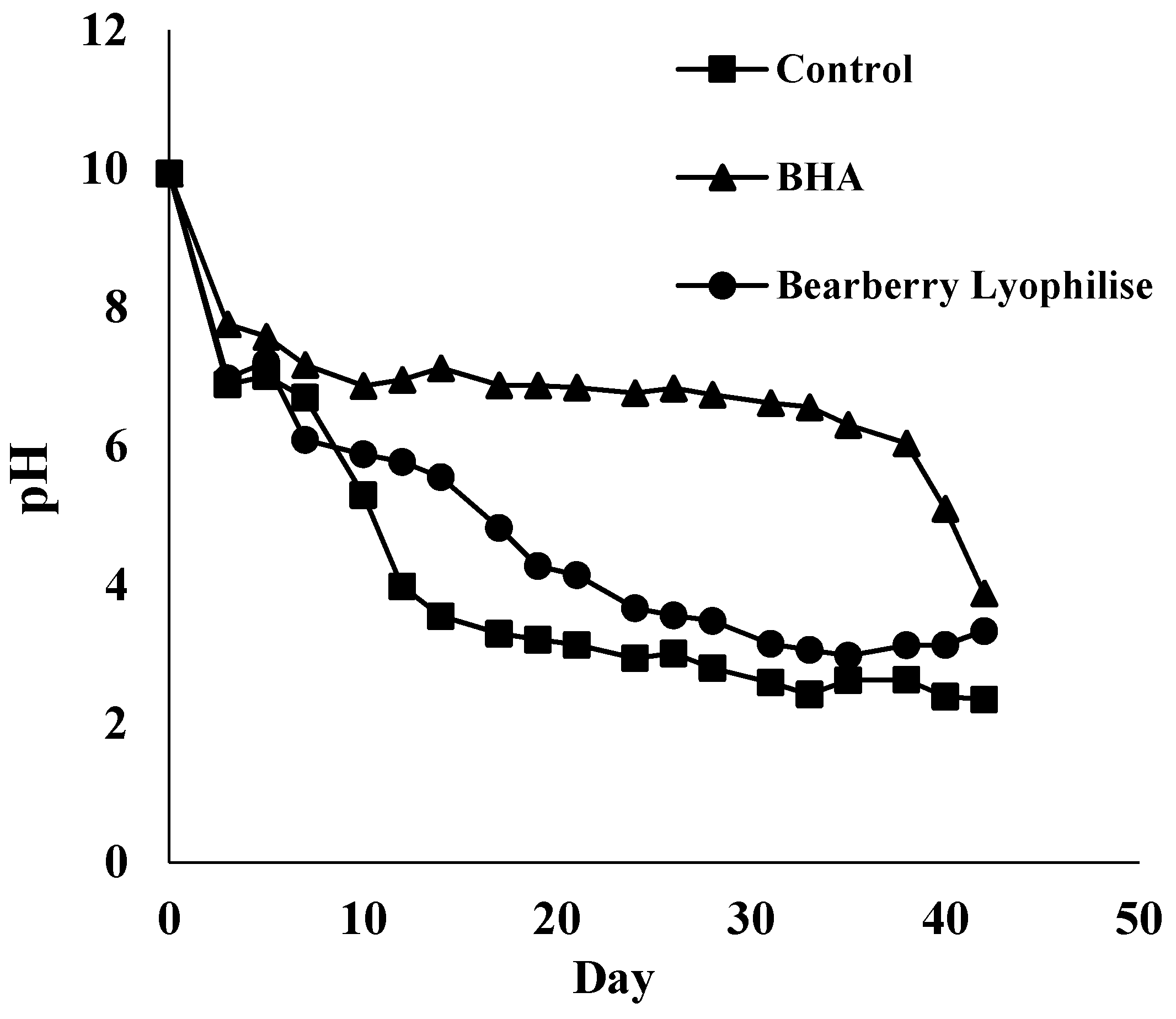
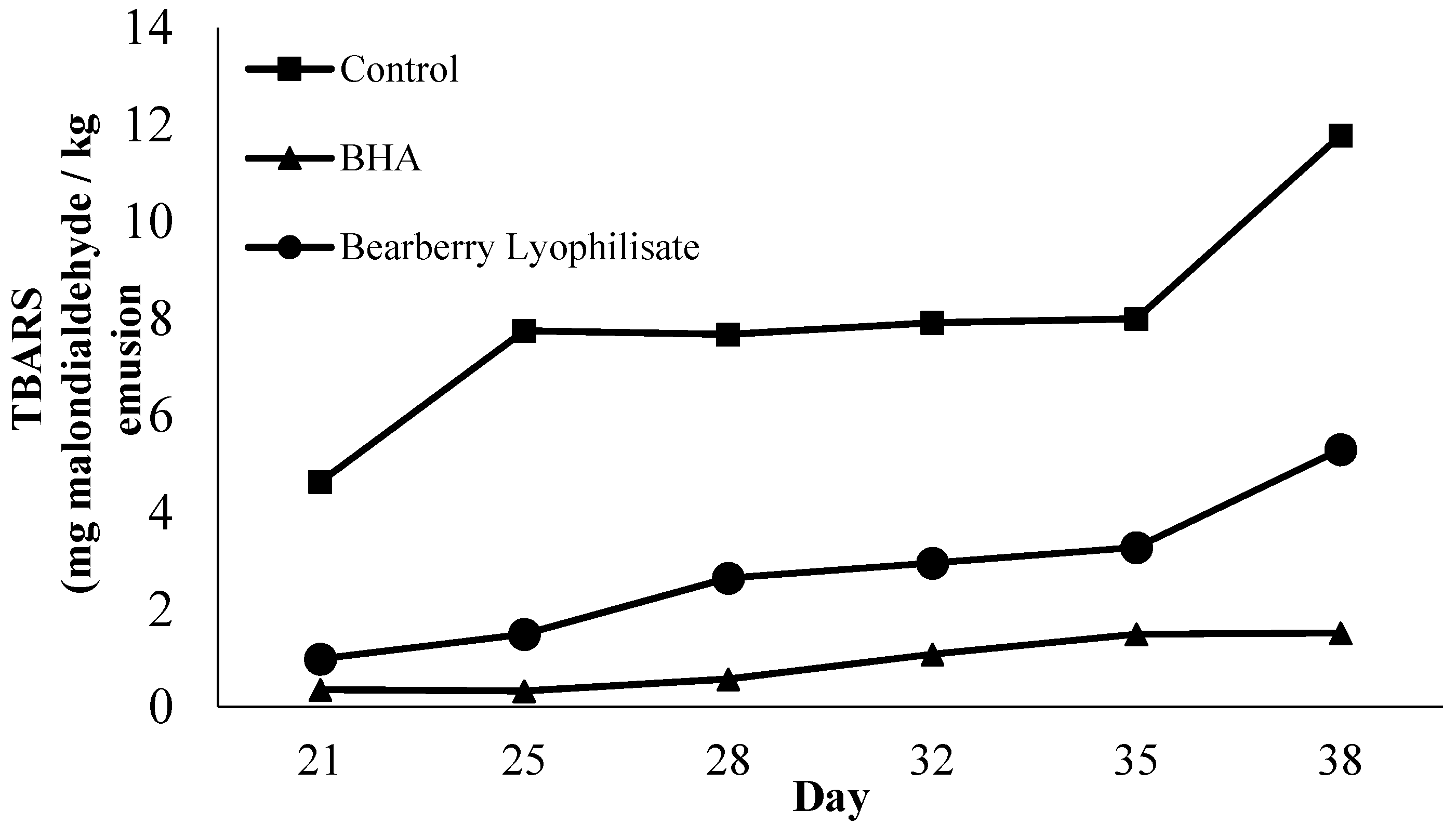
3.3. Antioxidant Effects in Stored o/w Emulsion
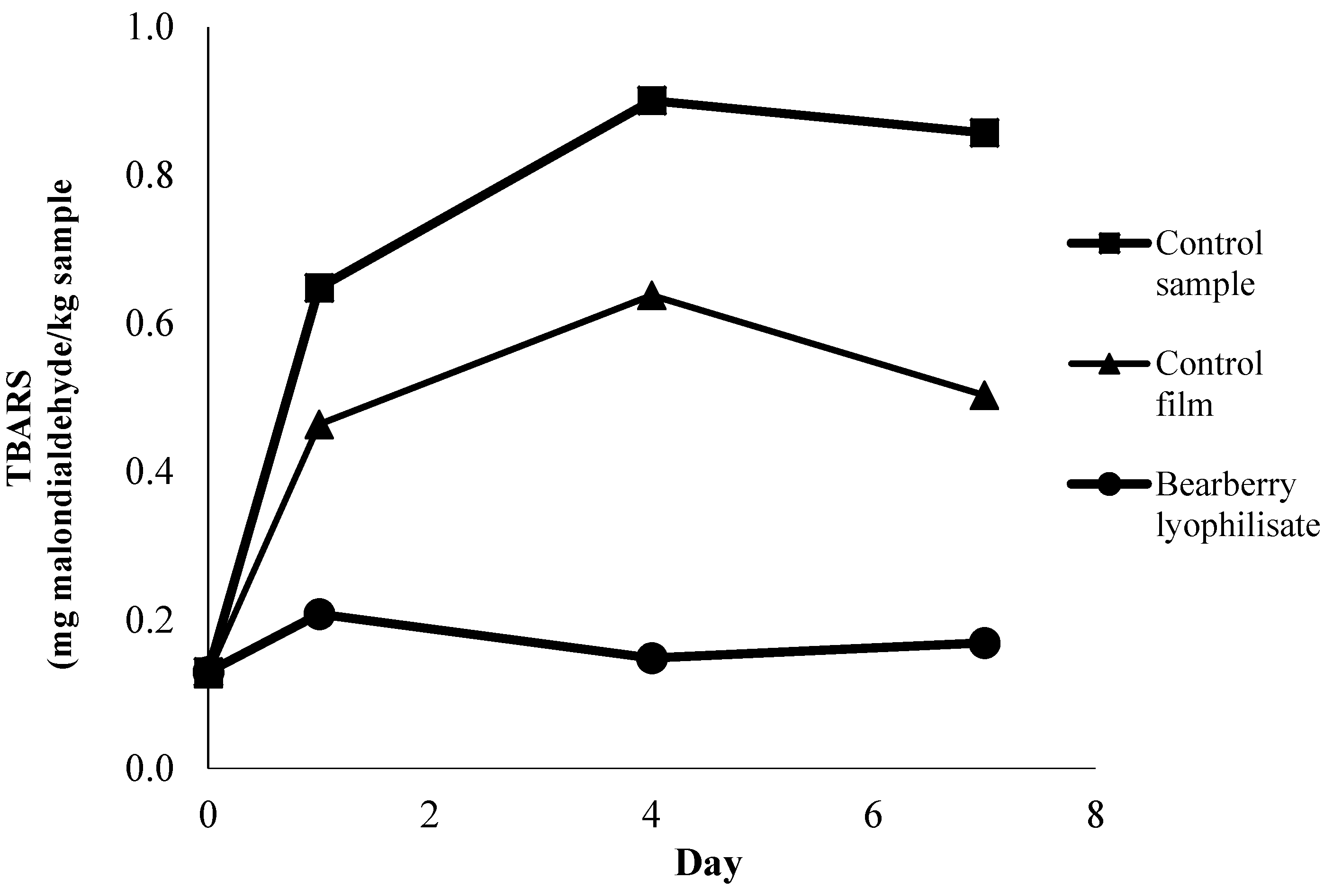
3.4. Antioxidant Effects in Active Film Packaging with Bearberry Coating
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reische, D.W.; Lillard, D.A.; Eitenmiller, R.R. Food Lipid Chemistry, Nutrition and Biotechnology; Akoh, C.C., Min, D.B., Eds.; CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Argoti, J.C.; Salido, S.; Linares-Palomino, P.J.; Ramírez, B.; Insuasty, B.; Altarejos, J. Antioxidant activity and free radical-scavenging capacity of a selection of wild-growing Colombian plants. J. Sci. Food Agric. 2011, 91, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Pegg, R.B.; Amarowicz, R. Protein-precipitating capacity of bearberry-leaf (Arc-tostaphylos uva-ursi L. Sprengel) polyphenolics. Food Chem. 2011, 124, 1507–1513. [Google Scholar] [CrossRef]
- Pegg, R.B.; Amarowicz, R.; Naczk, M. Antioxidant activity of polyphenolics from bearberry leaf (Arctostphylos uva-ursi L. Sprengel) extract in meat systems. ACS Symp. Ser. 2005, 909, 67–82. [Google Scholar]
- Pegg, R.B.; Amarowicz, R.; Naczk, M.; Shahidi, F. PHOTOCHEM® for determination of antioxidant capacity of plant extract. ACS Symp. Ser. 2009, 956, 140–158. [Google Scholar]
- Barl, B.; Loewen, D.; Svendsen, E. Saskatchewan Herb Database; Department of Horticulture Science, University of Saskatchewan: Saskatoon, SK, Canada, 1996. [Google Scholar]
- Kiokias, S.; Varzakas, T.; Oreopoulou, V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Varzakas, T. Innovative applications of food related emulsions. Crit. Rev. Food Sci. Nutr. 2016, in press. [Google Scholar]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Carpenter, R.; O’Grady, M.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Kerry, J.P. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007, 76, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Varzakas, T. Activity of flavonoids and β-carotene during the auto-oxidative deterioration of model food oil-in water emulsions. Food Chem. 2014, 150, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bi, H.; Liu, C. Extraction of bio-active components from Rhodiola sachalinensis under ultrahigh hydrostatic pressure. Sep. Purif. Technol. 2007, 57, 277–282. [Google Scholar] [CrossRef]
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Gallego, G.; Peiró, S.; Almajano, M.P. Antioxidant properties of aqueous and ethanolic extracts of Tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 2014, 94, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Peiró, S.; Fajarí, L.; Julià, L.; Almajano, M.P. Radical scavenging of white tea and its flavonoid constituents by electron paramagnetic resonance (EPR) spectroscopy. J. Agric. Food Chem. 2014, 62, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kajimoto, G.; Emura, S. Antioxidant effects of d-tocopherols at different con-centrations in oils during microwave heating. J. Am. Oil Chem. Soc. 1993, 70, 989–995. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Segovia, F.; Martínez-Farré, X.; Gil, E.; Almajano, M. Screening of antioxidant activity of Gentian lutea root and its application in oil-in-water emulsions. Antioxidants 2014, 3, 455–471. [Google Scholar] [CrossRef] [PubMed]
- American Oil Chemists’ Society. Peroxide value acetic acid-chloroform method. In AOCS Official Method Cd; AOCS: Urbana, IL, USA, 1997; pp. 8–53. [Google Scholar]
- Bodini, R.B.; Sobral, P.J.; Favaro-Trindade, C.S.; Carvalho, R. Properties of gelatin-based films with added ethanol-propolis extract. LWT Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Grau, A.; Guardiola, F.; Boatella, J.; Barroeta, A.; Codony, R. Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: Influence of various parameters. J. Agric. Food Chem. 2000, 48, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Veljkovi, J.N.; Pavlovi, A.N.; Miti, A.N.A.S. Evaluation of individual phenolic compounds and antioxidant properties of black, green, herbal and fruit tea infusions consumed in Serbia: Spectrophotometrical and electrochemical approaches. J. Food Nutr. Res. 2013, 52, 12–24. [Google Scholar]
- Roedig-Penman, A.; Gordon, M.H. Antioxidant properties of catechins and green tea extracts in model food emulsions. J. Agric. Food Chem. 1997, 45, 4267–4270. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (Rosemary, Thyme and Lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Pehlivan, E.; Arslan, G.; Gode, F.; Altun, T.; Özcan, M. Determination of some inorganic metals in edible vegetable oils by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Grasas y Aceites 2008, 59, 239–244. [Google Scholar] [CrossRef]
- Mancuso, J.R.; McClements, D.J.; Decker, E. The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem. 1999, 47, 4112–4116. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Pangloli, P.; Melton, S.L.; Collins, J.L.; Penfield, M.P.; Saxton, A.M. Flavor and storage stability of potato chips fried in cottonseed and sunflower oils and palm olein/sunflower oil blends. J. Food Sci. 2002, 67, 97–103. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldra, F. Handbook of Analysis of Edible Animal By-Products; CRC Press: Gent, Belgium, 2011; p. 471. [Google Scholar]
- Amarowicz, R.; Barl, B.; Pegg, R.B. Potential natural antioxidants from Saskatchewan indigenous plants. J. Food Lipids 1999, 6, 317–329. [Google Scholar] [CrossRef]
- Coutinho de Oliveira, T.L.; de Carvalho, S.M.; de Araújo, R.S.; Andrade, M.A.; Cardoso, M.D.G.; Ramos, E.M. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT Food Sci. Technol. 2012, 45, 204–212. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Sumpavapol, P.; Nirmal, N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012, 155, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
| Activity Bearberry Extract | Extraction Solvent 50:50 (v/v) EtOH:H2O * |
|---|---|
| Extraction yield (%) | 32.1% ± 0.03% |
| Total phenolic content (mg GAE/g DW) | 102.11 ± 7.12 |
| TEAC (mmol of TE/g DW) | 90.42 ± 1.83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Azman, N.A.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Shaarani, S.M.; Almajano Pablos, M.P. Study of the Properties of Bearberry Leaf Extract as a Natural Antioxidant in Model Foods. Antioxidants 2016, 5, 11. https://doi.org/10.3390/antiox5020011
Mohd Azman NA, Gallego MG, Segovia F, Abdullah S, Shaarani SM, Almajano Pablos MP. Study of the Properties of Bearberry Leaf Extract as a Natural Antioxidant in Model Foods. Antioxidants. 2016; 5(2):11. https://doi.org/10.3390/antiox5020011
Chicago/Turabian StyleMohd Azman, Nurul Aini, Maria Gabriela Gallego, Francisco Segovia, Sureena Abdullah, Shalyda Md Shaarani, and María Pilar Almajano Pablos. 2016. "Study of the Properties of Bearberry Leaf Extract as a Natural Antioxidant in Model Foods" Antioxidants 5, no. 2: 11. https://doi.org/10.3390/antiox5020011







