Synthesis and Evaluation of the Anti-Oxidant Capacity of Curcumin Glucuronides, the Major Curcumin Metabolites
Abstract
:1. Introduction
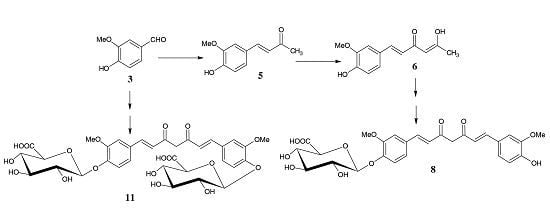
2. Experimental
2.1. Reagents, Instrumentation
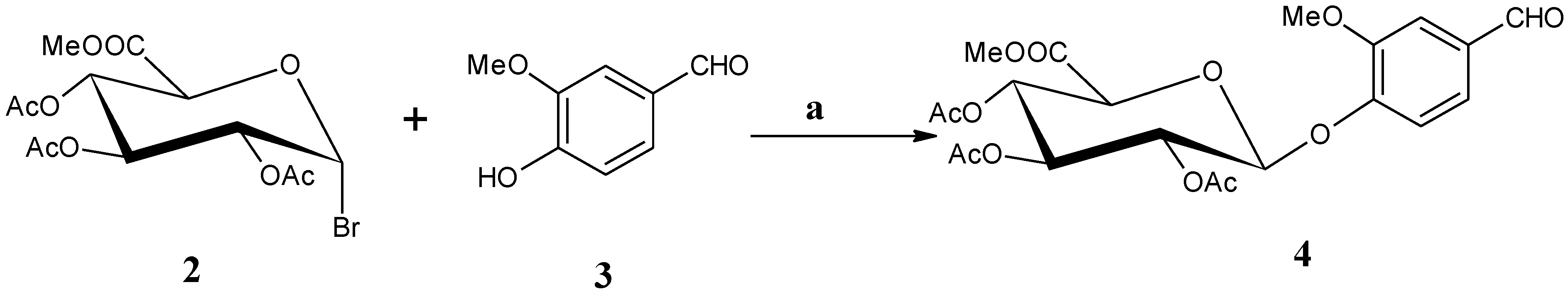
2.1.1. Synthesis of Methyl [1-O-(4′-formyl-2′-methoxyphenyl)-2,3,4-tri-O-acetyl-β-d-glucopyranosiduronate) (4)
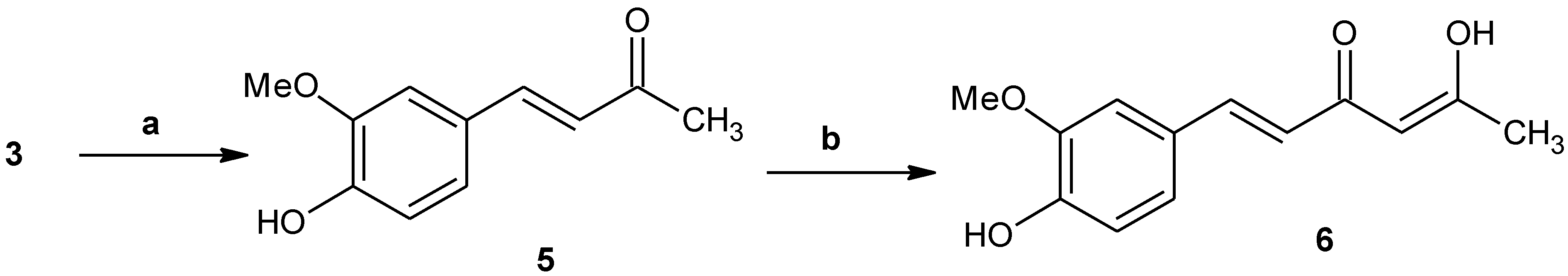
2.1.2. 4-(4-hydroxy-3-methoxyphenyl)-3-buten-2-one (5)
2.1.3. 5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1,4-hexadien-3-one (6)
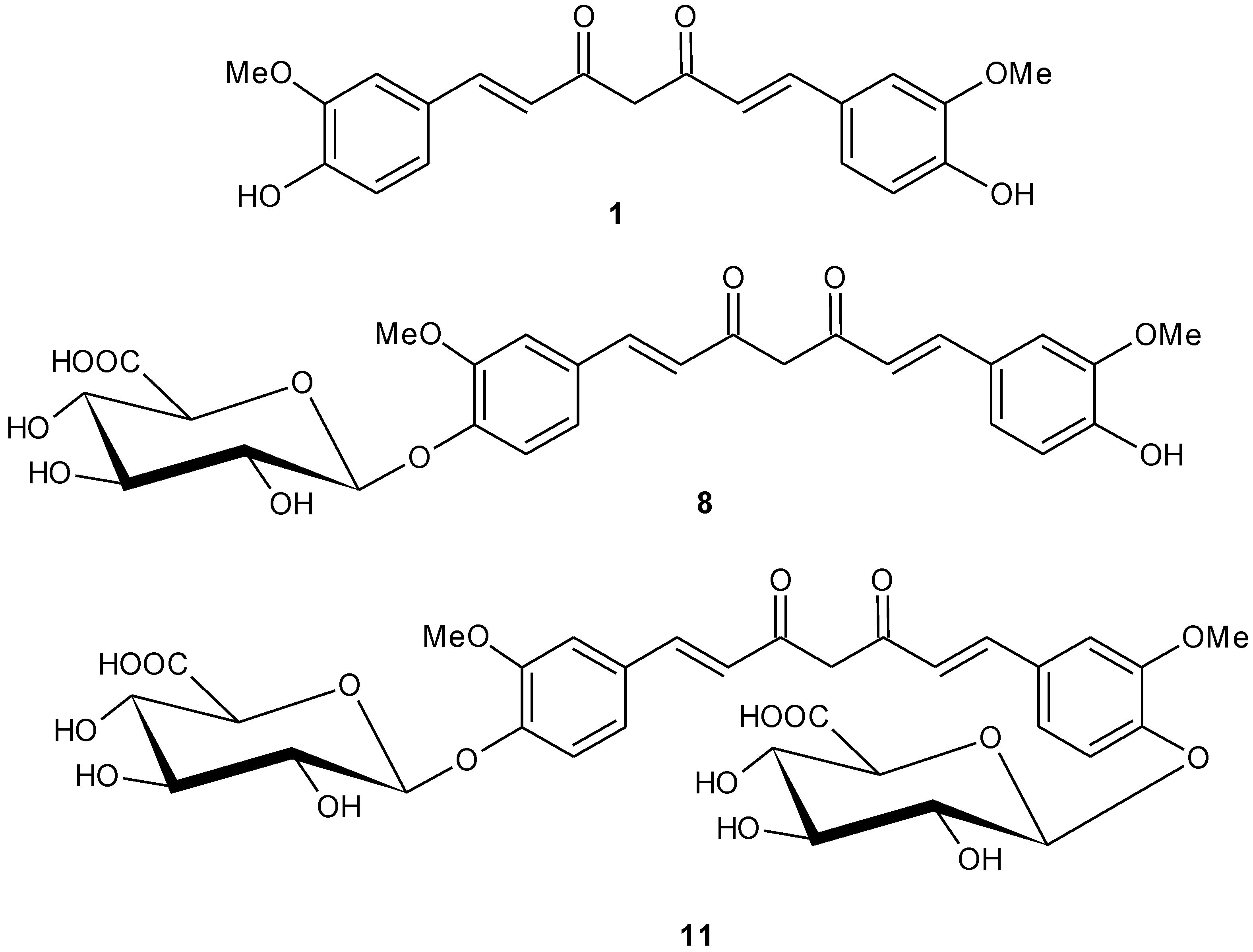
2.1.4. Mono-[methyl 2,3,4-tri-O-acetyl-β-d-glucopyranosiduronate]-curcumin (7)
2.1.5. Mono-(β-d-glucopyranosiduronic acid)-curcumin (curcumin monoglucuronide) (8)
2.1.6. Bis-[methyl 2,3,4-tri-O-acetyl-β-d-glucopyranosiduronate]-curcumin (10)
2.1.7. Bis-(β-d-glucopyranosiduronic acid)-curcumin (curcumin diglucuronide) (11)
2.2. Antioxidant Assay by DPPH [28,29]
| Compounds | Concentration (µg/mL) | DPPH Scavenging Activity (%) | SC 50 (μg/mL) |
|---|---|---|---|
| Curcumin (1) | 3.33 | 72.07 ± 0.19 | 1.58 |
| 1.67 | 50.14 ± 0 | ||
| 0.83 | 27.79 ± 1.93 | ||
| 0.42 | 21.8 ± 0.38 | ||
| 0.21 | 10.08 ± 0 | ||
| Curcumin monoglucuronide (8) | 33.33 | 79.5 ± 0.44 | 15.61 |
| 16.67 | 54.5 ± 0.21 | ||
| 8.33 | 23.6 ± 0.44 | ||
| 4.17 | 6.3 ± 0.22 | ||
| Curcumin diglucuronide(11) | 333.33 | 29.34 ± 0.62 | SC50 was not obtained. Inhibition of 29.77% was obtained at 333 μg/mL |
| 166.67 | 19.5 ± 0.21 | ||
| 83.33 | 9.68 ± 0.62 | ||
| 41.67 | 6.94 ± 0 | ||
| 20.83 | 2.89 ± 0.82 |
Antioxidant Assay by Oxygen Radical Absorbance Capacity (ORAC) [30]
| Compounds | Sample (mg/L) | ORAC Value (µmol TE/g) | Average ORAC Value (µmol TE/g) | SD |
|---|---|---|---|---|
| Curcumin (1) | 0.781 | 15,830.010 | 14,981.34 | 298.41 |
| 1.563 | 15,164.094 | |||
| 3.125 | 14,981.707 | |||
| 6.250 | 14,479.844 | |||
| 12.500 | 14,451.057 | |||
| Curcumin monoglucuronide (8) | 3.125 | 6891.506 | 6891.349 | 340.893 |
| 6.250 | 7132.319 | |||
| 12.500 | 6650.224 | |||
| Curcumin diglucuronide(11) | 6.250 | 2449.353 | 2502.489 | 175.149 |
| 12.500 | 2425.833 | |||
| 25.00 | 2373.853 | |||
| 50.00 | 2760.917 |
3. Results and Discussion
3.1. Synthesis
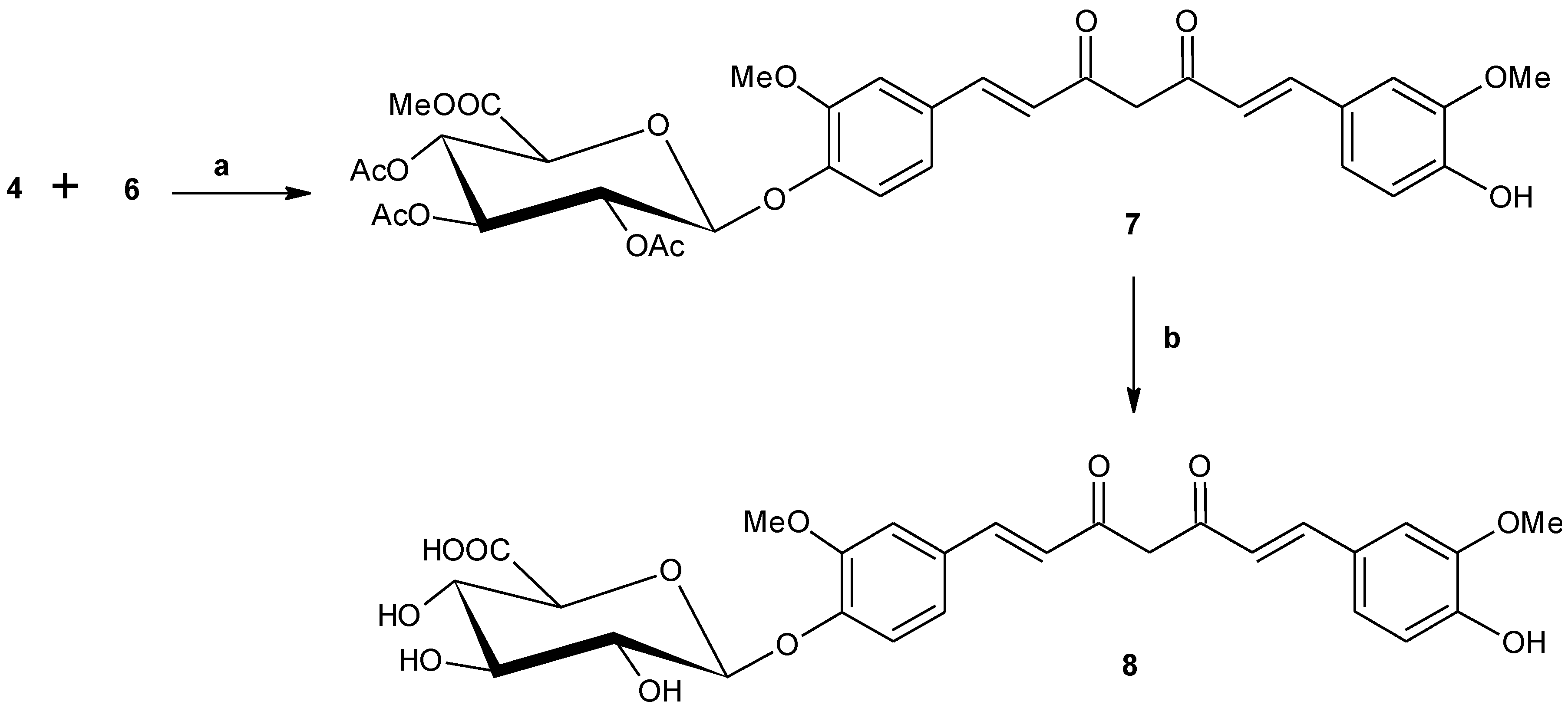
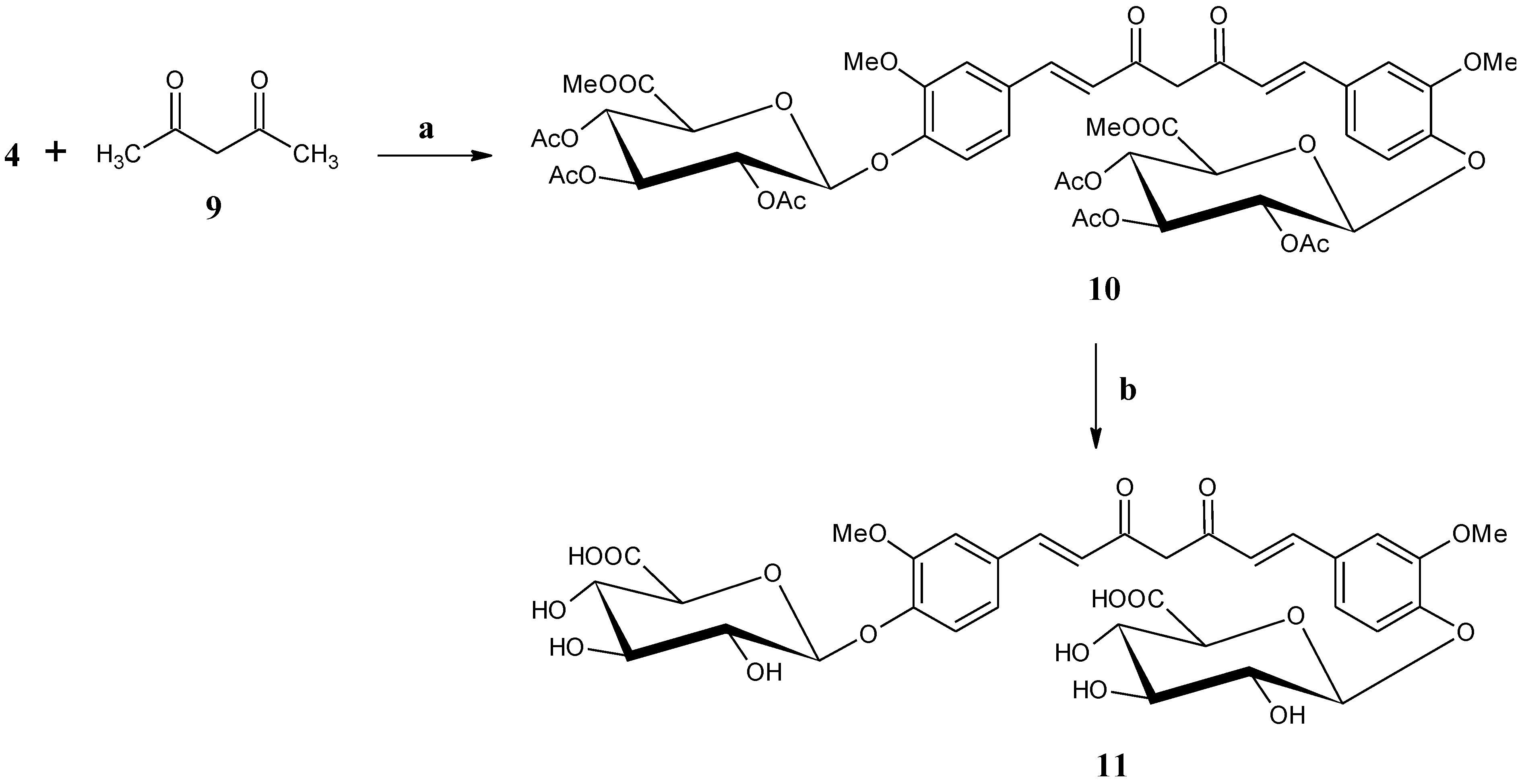
3.2. Anti-Oxidant Studies
3.2.1. Antioxidant Assay by DPPH Scavenging Method [28,29]
3.2.2. Antioxidant Assay by Oxygen Radical Absorbance Capacity (ORAC) [30]
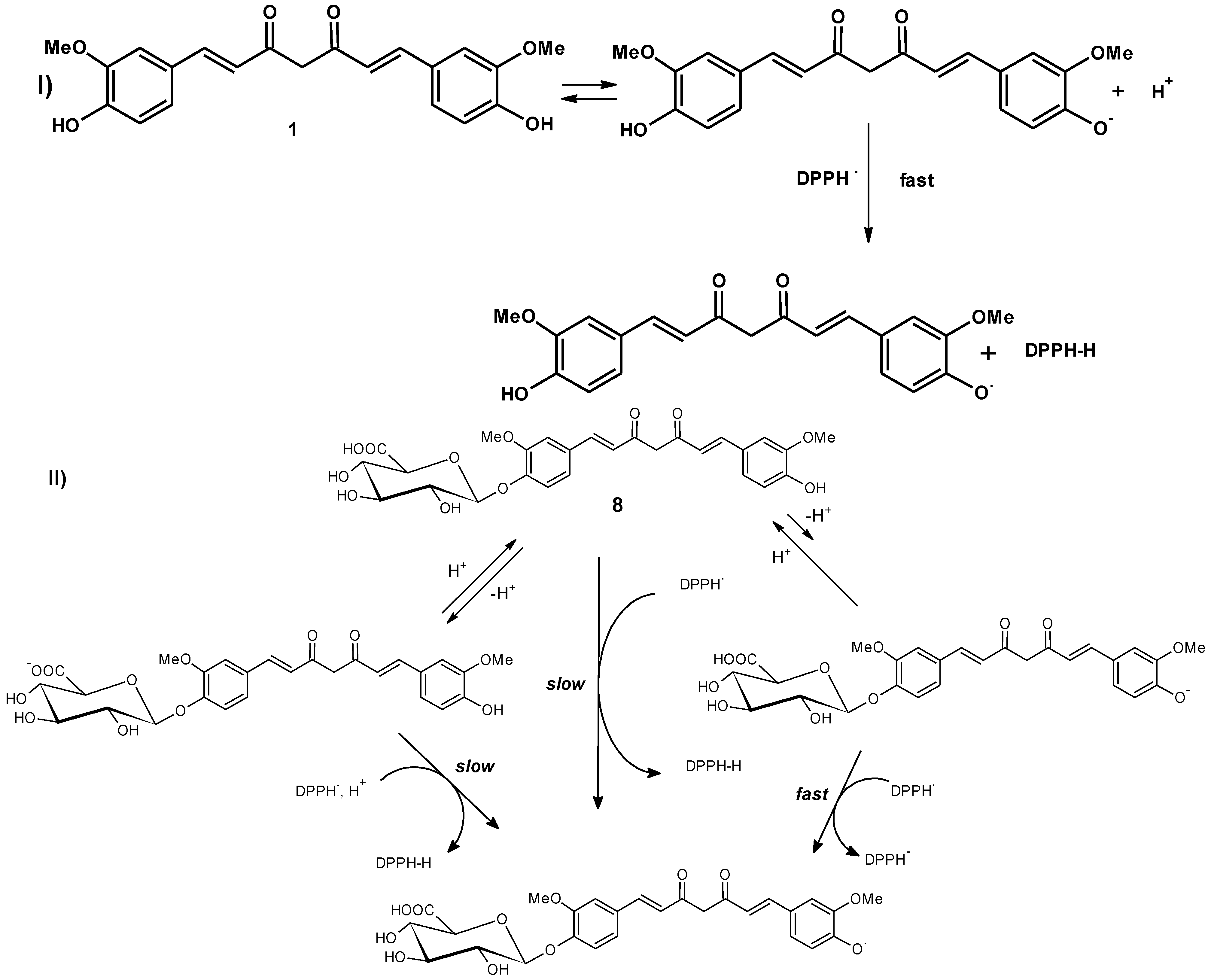
4. Conclusions
Supporting Information
Acknowledgments
Author Contributions
Conflicts of Interest
List of Abbreviations
| DPPH | 1,1-Diphenyl-2-picryl hydrazyl |
| ORAC | Oxygen Radical Absorbance Capacity |
| LC-MS | Liquid chromatography-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| (Bu4N)+Br− | Tetrabutyl ammonium bromide |
| RT | Room Temperature |
| B2O3 | Boric anhydride |
| (sBuO)3B | tri-s-butyl borate |
| n-BuNH2 | n-Butyl amine |
| NaOMe | Sodium methoxide |
| MS (APCI) | Mass Spectrometry (Atmospheric pressure chemical ionization) |
References
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer Potential of Curcumin: Preclinical and Clinical Studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Asouri, M.; Ataee, R.; Ahmadi, A.; Amini, A.A.; Moshaei, M. Antioxidant and Free Radical Scavenging Activities of Curcumin. Asian J. Chem. 2013, 25, 7593–7595. [Google Scholar]
- Jurenka, J.S. Anti-inflammatory Properties of Curcumin, a Major Constituent of Curcuma longa: A Review of Preclinical and Clinical Research. Altern Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed. Res. Int. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and Liver Disease: from Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, N.; Sahebkar, A. Investigation of the Efficacy of Adjunctive Therapy with Bioavailability-Boosted Curcuminoids in Major Depressive Disorder. Phytother. Res. 2015, 29, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Chavan, D.; Pandey, S.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Perspectives on Chemopreventive and Therapeutic Potential of Curcumin Analogs in Medicinal Chemistry. Mini. Rev. Med. Chem. 2010, 10, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Schneider, C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol. Med. 2012, 18, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Low stability remedies the low bioavailability of curcumin. Trends Mol. Med. 2012, 18, 363–364. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Hoehle, S.I.; Pfeiffer, E.; Metzler, M. Glucuronidation of Curcuminoids by Human Microsomal and Recombinant UDP-Glucuronosyltransferases. Mol. Nutr. Food Res. 2007, 51, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Huang, T.-M.; Lin, J.-K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Pfeiffer, E.; Hoehle, S.I.; Walch, S.G.; Riess, A.; Soalyom, A.M.; Metzl, M. Curcuminoids Form Reactive Glucuronides in Vitro. J. Agric. Food Chem. 2007, 55, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid Analysis of Curcumin and Curcumin Metabolites in Rat Biomatrices Using a Novel Ultraperformance Liquid Chromatography (UPLC) Method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Sung, B.; Prasad, B.A.B.; Schuber, P.T., Jr.; Prasad, S.; Aggarwal, B.B.; Bornmann, W.G. Curcumin glucuronides: Assessing the proliferative activity against human cell lines. Bioorg. Med. Chem. 2014, 22, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical Causes Cancer. Nat. Rev. Cancer. 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, S.U.; Patil, P.R.; Mane, S.R. Use of Natural Antioxidants to Scavenge Free Radicals: A Major Cause of Diseases. Int. J. Pharm. Tech. Res. 2010, 2, 1074–1081. [Google Scholar]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant Properties of Honey and Its Role in Preventing Health Disorder. Open Nutraceuticals J. 2010, 3, 6–16. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Riordan, N.H.; Riordan, H.D. Antioxidants as Chemopreventive Agents for Breast Cancer. BioMedicina 1998, 1, 120–127. [Google Scholar]
- Borra, S.K.; Gurumurthy, P.; Mahendra, J.; Joayamathi, K.M.; Cherian, C.N.; Chand, R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J. Med. Plants Res. 2013, 7, 2680–2690. [Google Scholar]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar]
- Acharya, J.D.; Ghaskadbi, S.S. Protective effect of Pterostilbene Against Free Radical Mediated Oxidative Damage. BMC Complement. Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar]
- Caldwell, W.S.; Greene, J.M.; Byrd, G.D.; Chang, K.M.; Uhrig, M.S.; DeBethizy, J.D.; Crooks, P.A.; Bhatti, B.S.; Riggs, R.M. Characterization of the glucuronide conjugate of cotinine: A previously unidentified major metabolite of nicotine in smokers' urine. Chem. Res. Toxicol. 1992, 5, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Esmurziev, A.M.; Reimers, A.; Andreassen, T.; Simic, N.; Sundby, E.; Hoff, B.H. Benzoylated Uronic Acid Building Blocks and Synthesis of N-Uronate Conjugates of Lamotrigine. Molecules 2012, 17, 820–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smarrito, C.M.; Wong, C.C.; Meinl, W.; Glatt, H.; Fumeaux, R.; Munari, C.; Robert, F.; Williamson, G.; Barron, D. First Chemical Synthesis and in Vitro Characterization of the Potential Human Metabolites 5-O-Feruloylquinic Acid 4′-Sulfate and 4′-O-Glucuronide. J. Agric. Food Chem. 2011, 59, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Shi, Q.; Nyarko, A.K.; Bastow, K.F.; Wu, C.-C.; Su, C.-Y.; Shih, C.C.-Y.; Lee, K.-H. Antitumor Agents. 250. Design and Synthesis of New Curcumin Analogues as Potential Anti-Prostate Cancer Agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [PubMed]
- Ryu, E.K.; Choe, Y.S.; Lee, K.-H.; Choi, Y.; Kim, B.-T. Curcumin and Dehydrozingerone Derivatives: Synthesis, Radiolabeling, and Evaluation for β-Amyloid Plaque Imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-C.; Chern, C.-Y.; Kuo, P.-C.; Wu, Y.-C.; Chan, Y.-Y.; Liao, Y.-R.; Teng, C.-M.; Wu, T.-S. Synthesis of Analogues of Gingerol and Shogaol, the Active Pungent Principles from the Rhizomes of Zingiber officinale and Evaluation of Their Anti-Platelet Aggregation Effects. Int. J. Mol. Sci. 2014, 15, 3926–3951. [Google Scholar] [CrossRef] [PubMed]
- Pabon, H.J.J. A synthesis of curcumin and related compounds. Rec. Trav. Chim. Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Liu, Z.Q. Chemical Methods to Evaluate Antioxidant Ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 2. Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Zhang, H.Y.; Chen, D.Z.; Liu, C.B. Theoretical elucidation on the Antioxidant Mechanism of Curcumin: A DFT Study. Org. Lett. 2002, 4, 2909–2911. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-Y.; Liu, Z.-Q. Phenolic and Enolic Hydroxyl Groups in Curcumin: Which Plays the Major Role in Scavenging Radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, A.K.; Raja, S.; Mahapatra, S.; Nagabhushanam, K.; Majeed, M. Synthesis and Evaluation of the Anti-Oxidant Capacity of Curcumin Glucuronides, the Major Curcumin Metabolites. Antioxidants 2015, 4, 750-767. https://doi.org/10.3390/antiox4040750
Choudhury AK, Raja S, Mahapatra S, Nagabhushanam K, Majeed M. Synthesis and Evaluation of the Anti-Oxidant Capacity of Curcumin Glucuronides, the Major Curcumin Metabolites. Antioxidants. 2015; 4(4):750-767. https://doi.org/10.3390/antiox4040750
Chicago/Turabian StyleChoudhury, Ambar K., Suganya Raja, Sanjata Mahapatra, Kalyanam Nagabhushanam, and Muhammed Majeed. 2015. "Synthesis and Evaluation of the Anti-Oxidant Capacity of Curcumin Glucuronides, the Major Curcumin Metabolites" Antioxidants 4, no. 4: 750-767. https://doi.org/10.3390/antiox4040750